Abstract
Fluorescence diagnosis is a fast, easy, noninvasive, selective, and sensitive diagnostic tool for estimation of treatment results in oncology. In clinical practice the use of photodynamic diagnosis is focused on five targets: detection for prevention of malignant transformation precancerous changes, detection of neoplasmatic tissue in the early stages for fast removal, prevention of expansion and detection of recurrence of the cancer, monitoring therapy, and the possibility of excluding neoplasmatic disease. In this article, selected applications of fluorescence diagnosis at the Center for Laser Diagnostics and Therapy in Bytom, Poland, for each of these targets are presented.
Introduction
Fluorescence diagnosis is a fast and noninvasive method for imaging of precancerous and cancerous tissues in many locations. Many applications of this method have been discussed in the wide fields of oncology, dermatology, laryngology, pulmonology, gynecology, and gastroenterology.Citation1–Citation3 In many studies, this method has been described as very sensitive and specific especially in diagnostics of small changes that are not visible using white light endoscopic procedures.Citation2–Citation4
In endoscopic fluorescence diagnosis we can distinguish two major directions:Citation5–Citation10 (1) autofluorescence and exogenous (fluorophore-enhanced) fluorescence, depending on the origin of the fluorescent signal detected; and (2) point measurements and multidimensional imaging (tomography), depending on the signal detected.
Autofluorescence diagnosis
Autofluorescence is generated by endogenous molecules, such as aromatic amino acids, nicotinamide adenine dinucleotide, or porphyrins.Citation10,Citation11 This method has been described as very sensitive and specific especially in diagnosing small changes that are not visible using white light endoscopic procedures.Citation6,Citation11,Citation12 Red fluorescence is stronger in the tumor tissue, in contrast to normal tissue, whose fluorescence is mainly green. As autofluorescence may be disturbed by false positive effects due to inflammation or neovascularization, many authors use the intensity ratios red–to–green, red–to–blue, or vice versa to evaluate neoplastic changes in the tissues.Citation13,Citation14 The red:green ratio is used as a basis of Xillix® Laser-Induced Fluorescence Endoscopy (LIFE) (Xillix Technologies Corporation, Richmond, BC, Canada) as a technique for detection of cancerous lesions.Citation8,Citation10 This system uses blue light excitation and can detect both green and red tissue autofluorescence, applying band-pass filters before two image intensifier cameras for observation of native fluorescence in the green and red spectral ranges, which are fused to create a real time red–green image of the pathological changes.Citation10,Citation14 As a result of numerical analysis we can transform the autofluorescence image into a spatial map of Numerical Color Value (NCV) to establish the quality of imaging and to show places with the highest red:green ratio where the biopsy specimen was taken.Citation15
Exogenous fluorescence diagnosis
Exogenous fluorescence diagnosis is based on the use of exogenous fluorophores revealing certain tumor selectivity, such as porphyrins, chlorins, phthalocyanines, or 5-aminolevulinic acid (ALA), a natural precursor of protoporphyrin IX which selectively accumulates in tumor tissues showing red fluorescence.Citation10,Citation16 The advantages of this modality are good visualization of the tumor area, with a strong fluorescent signal, less ambiguity relative to autofluorescence, as well as simpler (and sometimes cheaper) instrumentation.Citation12 Another advantage is related to a priori knowledge of the optical properties of exogenous fluorophores; their excitation and emission spectra are well known, and their applicability is related mainly to selective localization within tissues of interest, mode of administration, and few side effects for the patients.Citation10 On the other hand, the costs related to the process of registration and approval of such exogenous fluorophores is a significant drawback for faster introduction of such fluorescence systems in clinical practice. In many research reports both autofluorescence and exogenous fluorescence are applied simultaneously for better diagnosis of tumors, particularly in their early stages.Citation13,Citation16
Point measurement provides fluorescence spectral data from a single illuminated point, usually in terms of the signal intensity versus wavelength of fluorescence, while in the case of multidimensional imaging, multiple points of the tissue boundaries are illuminated in a time-sharing fashion to form a fluorescent color map enabling determination and specification of pathological lesions.Citation10,Citation17
Targets of fluorescence diagnosis
In clinical practice we can distinguish five targets of fluorescence diagnosis:Citation4,Citation5 (1) detecting precancerous changes in order to prevent malignant transformation; (2) detecting neoplasmatic tissue in its early stages for fast removal; (3) preventing expansion, and detecting the recurrence of cancer; (4) monitoring therapy; and (5) possibly excluding neoplasmatic disease.
All present targets of fluorescence diagnosis became a part of wide oncologic prophylaxis performed at the Center for Laser Diagnostics and Therapy in Bytom. Our clinical experience has led us to use the Xillix Onco LIFE system which allows us to perform two-modal (fluorescence and optical RGB [red-green-blue] color) imaging, in real time during endoscopic procedures.Citation5,Citation8 All images reproduced in this article were made at the Center in Bytom.
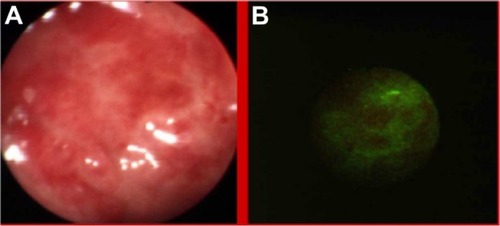
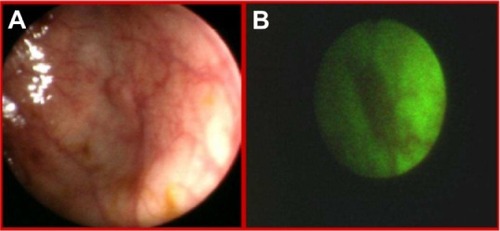
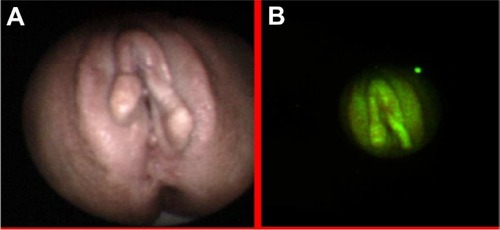
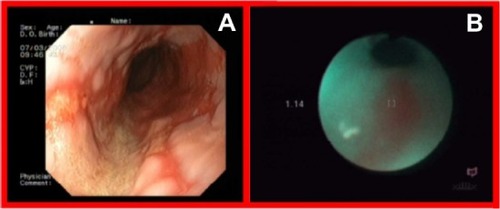
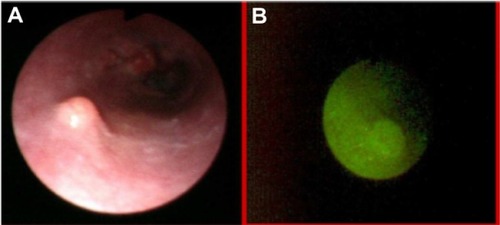
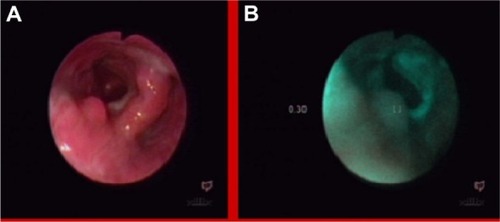
Autofluorescence imaging allowed us to detect some premalignant changes which were not visible in white light, such as low grade dysplasias in Barrett’s esophagus (), ulcerative colitis (), high grade dysplasia in lung tissue (), tubular adenoma (), or lichen sclerosus (). This early detection of neoplasmatic tissue allows us to prevent malignant transformation.Citation18–Citation21
Figure 1 Low grade dysplasias in Barrett’s esophagus in white light endoscopy (A) and autofluorescence (B) imaging.
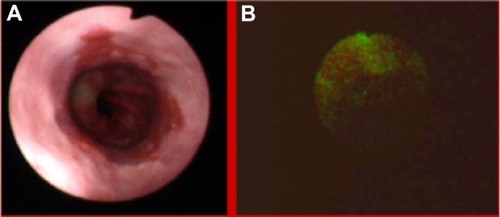
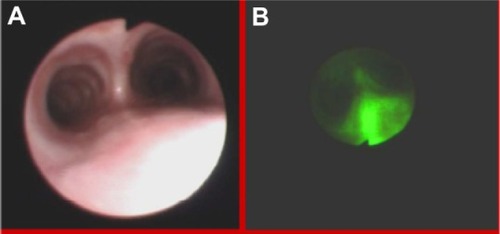
Figure 3 High grade dysplasia in lung tissue in white light bronchoscopy (A) and autofluorescence (B) imaging.
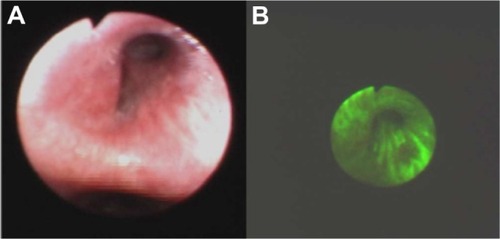
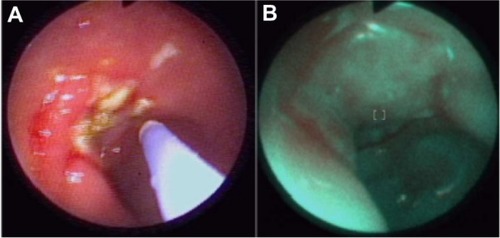
Detection of early stages of neoplasmatic tissues also enables their fast removal. Mainly in our Clinic, autofluorescence and exogenous fluorescence examination allows us to detect the early stages of precancer and cancer not only in endoscopic procedures but also on the skin and the genital organs. Histopathological examination of biopsies excised from the esophagus of a 71-year-old patient, did not detect the presence of malignant tissues, while ALA diagnosis and RGB color imaging showed the esophageal tumor ( and ). In biopsies excised under fluorescence navigation, histopathological analysis confirmed the development of malignant changes. Similarly, in the patient with Barrett’s esophagus, who 3 years ago was treated with ALA-photodynamic therapy (PDT), but who subsequently complained of dysphagia and heartburn for 4 months, malignant changes in the esophagus were localized using fluorescence and autofluorescence methods. The photodiagnosis method also allowed us to detect sunken colon cancer ( and ) and squamous cell carcinoma in lung tissue ( and ).Citation14–Citation23
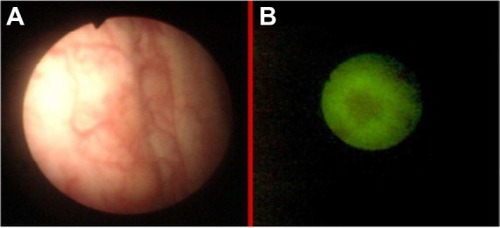
Figure 7 Sunken colon cancer in white light colonoscopy (A) and autofluorescence (B) imaging.
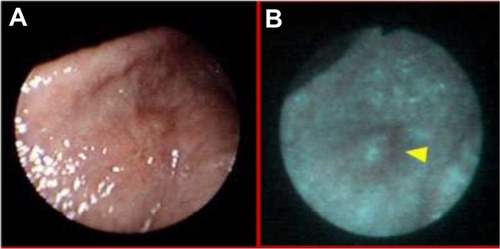
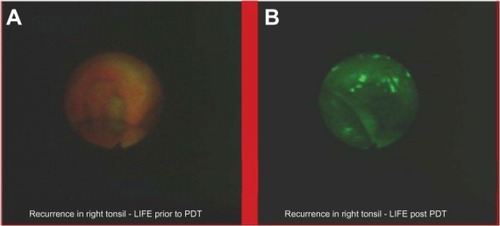
Autofluorescence diagnosis allows us to prevent cancer expansion and recurrence. One of its applications is estimation of radicality of polypectomy, one of the most common procedures performed in the upper and lower digestive tract. The presence of fluorescence may reveal that polypectomy is not radical, and the patient could qualify for surgery (). Apart from this, the autofluorescence method can show the recurrence of cancer eg, in urologyCitation24 () or in otolaryngology ().Citation25
Figure 9 Estimation of radicality of polypectomy in white light (A) and autofluorescence (B) imaging.
Real time autofluorescence spectroscopy
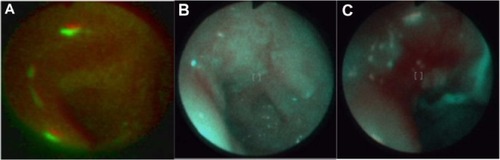
With the use of real time autofluorescence spectroscopy, we can observe changes in the photobleaching of a photosensitizer during therapy and after. Measurements of fluorescence intensity, numerical color value, red and green color intensity, and three-dimensional imaging have allowed us to observe ALA administration in treated cancers and, allowed us to target the therapy on places with the highest fluorescence intensity after ALA administration.Citation7 This method allows us to observe if an applied treatment is effective, or not. For example, in a patient with basal cell carcinoma, autofluorescence ( and ) autofluorescence was effective, while in patients with acuminate condyloma in the glans, no response to the treatment was observed ( and ). Autofluorescence diagnosis also allows for monitoring the effect of ulceration treatment with ALA-PDT (, and ) and with argon laser coagulation ( and ).Citation20.
Figure 12 Monitoring of basal cell carcinoma treatment with autofluorescence method: before (A) during (B) and after (C) treatment.
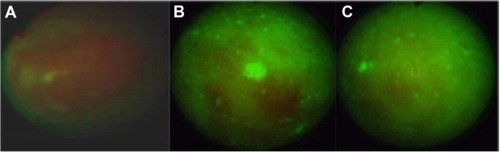
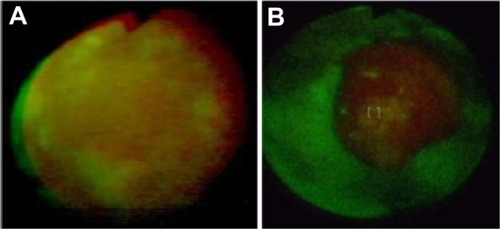
Figure 13 Monitoring of resistant to treatment acuminate condyloma in glans with autofluorescence method before (A) and during (B) treatment (until now no response).
Autofluorescence NCV diagnosis
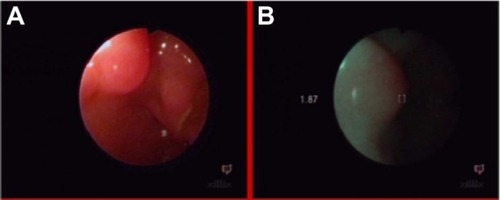
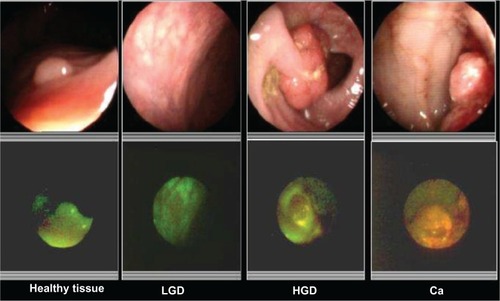
Autofluorescence NCV diagnosis is an effective tool in differentiating benign and malignant lesions.Citation6,Citation8 The red–to– green ratio increases with the advance of histological stage, allowing us to establish the grade of malignancy, which is difficult to do, using white light endoscopy (). We estimated that changes showing an NCV lower than 1.0 are commonly benign, but NCVs between 0.5 to 1.0 can exclude neoplasmatic disease. This method allows us to show small changes which are not visible in white light, such as polyps in the esophagus (), glycogen acanthosis (), or pharyngeal lipoma ().Citation8,Citation18
Figure 16 Differentiation of histological stages of malignancy using white light colonoscopy and fluorescence imaging.
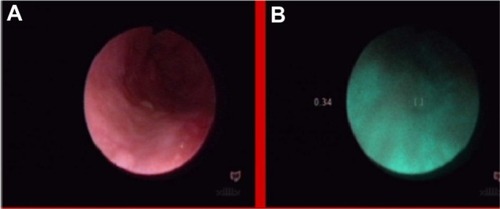
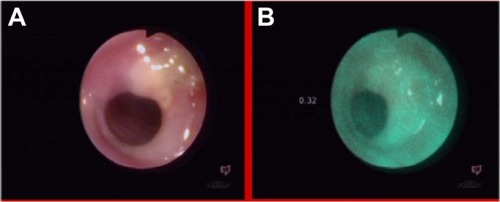
A 71-year-old patient, who was difficult to interview, suffered from dysphagia and suspicion of esophageal tumor, without histologic confirmation in taken biopsy specimens and without dysplasia in photodynamic diagnosis (). The NCV method revealed a benign lesion (). A later interview with the patient’s family established a nitro solvent chemical burn about 4 years previously; thus, the observed change was probably a scar after the burn. This scar qualified for endoscopic dilatation.
Figure 20 Esophagus of patient after nitro solvent chemical burn, in white light endoscopy (A) and autofluorescence (B) imaging.
These results show that fluorescence diagnosis is a fast, easy, noninvasive and sensitive diagnostic tool for estimation of treatment results in oncology. On the other hand, because knowledge about the usefulness of photodiagnosis is still not widespread enough in clinical practice, it is necessary to promote the advantages of this diagnostic method among clinicians.
Disclosure
The authors report no conflicts of interest in this work.
References
- StringerMMoghissiKPhotodiagnosis and fluorescence imaging in clinical practicePhotodiagnosis Photodyn Ther20041912
- MoghissiKStringerMRDixonKFluorescence photodiagnosis in clinical practicePhotodiagnosis Photodyn Ther2008523523719356662
- AllisonRRSibataCHPhotodiagnosis for cutaneous malignancy: a brief clinical and technical reviewPhotodiagnosis Photodyn Ther2008524725019356664
- MittonDAckroydRA brief overview of photodynamic therapy in EuropePhotodiagnosis Photodyn Ther2008510311119356640
- SierońAKwiatekSTwenty years of experience with PDD and PDT in Poland – ReviewPhotodiagnosis Photodyn Ther20096737919683204
- de VeldDCGSkurichinaMWitjesMJHDuinRPWSterenborgHJCMRoodenburgJLNClinical study for classification of benign, dysplastic, and malignant oral lesions using autofluorescence spectroscopyJ Biomed Opt2004994095015447015
- SierońAGibińskiPPustelnyTOptical biopsy using spectral camera in BCC and oral leukoplakiaPhotodiagnosis Photodyn Ther2008527127519356670
- Sieroń-StołtnyKKwiatekSLatosWAutofluorescence endoscopy with “real-time” digital image processing in differential diagnostics of selected benign and malignant lesions in the oesophagusPhotodiagnosis Photodyn Ther2012951022369723
- WagnièresGAStarWMWilsonBCln vivo fluorescence spectros-copy and imaging for oncological applicationsPhotochem Photobiol1998686036329825692
- BorisovaEVladimirovBIvanovaRAvramovLLight-Induced fluorescence techniques for gastrointestinal tumour detectionPascuONew Techniques in Gastrointestinal EndoscopyShanghaiInTech-Rijeka2011231252
- FilipMIordacheSSăftoiuACiureaTAutofluorescence imaging and magnification endoscopyWorld J Gastroenterol20111791421218078
- Roberts-ThomsonICSinghRTeoENguyenNQLidumsIThe future of endoscopyJ Gastroenterol Hepatol2010251051105720594218
- EllCImproving endoscopic resolution and sampling: fluorescence techniquesGut200352Suppl IV3033
- OsadaTArakawaASakamotoNAutofluorescence imaging endoscopy for identification and assessment of inflammatory ulcerative colitisWorld J Gastroenterol2011175110511622171146
- YoshidaTInoueHUsuiSSatodateHFukamiNKudoS-ENarrow-band imaging system with magnifying endoscopy for superficial esophageal lesionsGastrointest Endoscopy200459288295
- Da CostaRSWilsonBCMarconNEFluorescence and spectral imagingScientific World Journal200772046207118167619
- NtziachristosVRipollJWangLVWeisslederRLooking and listening to light: the evolution of whole-body photonic imagingNature Biotechnol20052331332015765087
- NiepsujKNiepsujGCebulaWAutofluorescence endoscopy for detection of high-grade dysplasia in short-segment Barrett’s esophagusGastrointest Endoscopy200358715719
- BiniszkiewiczTOlejekAKozak-DarmasISierońATherapeutic effects of 5-ALA-induced photodynamic therapy in vulvar lichen sclerosusPhotodiagn Photodyn Ther20052157160
- LatosWKawczyk-KrupkaALedwońAThe role of autofluorescence colonoscopy in diagnosis and management of solitary rectal ulcer syndromeProceedings of the SPIE. 6859, Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues VI. Article 685906March 3, 200810.1117/12.762314
- SierońAAdamekMKawczyk-KrupkaAMazurSIlewiczLPhotodynamic therapy (PDT) using topically applied δ-aminolevulinic acid (ALA) for the treatment of oral leukoplakiaJ Oral Pathol Med20033233033612787039
- De VeldDCGWitjesMJHSterenborgHJCMRoodenburgJLNThe status of in vivo autofluorescence spectroscopy and imaging for oral oncologyOral Oncology20054111713115695112
- MoghissiKDixonKStringerMRCurrent indications and future perspective of fluorescence bronchoscopy: a review studyPhotodiagnosis Photodyn Ther2008523824619356663
- SzygulaMWojciechowskiBAdamekMFluorescent diagnosis of urinary bladder cancer – a comparison of two diagnostic modalitiesPhotodiagnosis Photodyn Ther200412326
- BetzCSMehlmannMRickKAutofluorescence imaging and spectroscopy of normal and malignant mucosa in patients with head and neck cancerLasers Surg Med19992532333410534749