Abstract
Background
Bevacizumab and erlotinib have been demonstrated to prolong overall survival in patients with non-squamous non-small cell lung cancer (NSCLC). We designed a four-arm Phase III trial to evaluate the efficacy and toxicity of the combination of docetaxel, carboplatin, bevacizumab, and erlotinib in the first-line treatment of patients with NSCLC.
Methods
A total of 229 patients with stage IIIb/IV non-squamous NSCLC were treated with two cycles of carboplatin (area under the concentration-time curve 5.5) and docetaxel 100 mg/mCitation2 as chemotherapy. After completion of two treatment cycles, patients were evaluated for response and divided into four groups: 61/229 continued with four more cycles of chemotherapy (control group), 52/229 received chemotherapy plus erlotinib 150 mg daily, 56/229 received chemotherapy plus bevacizumab 7.5 mg/kg, and 60/229 were treated with the combination of chemotherapy, erlotinib, and bevacizumab until disease progression. The primary endpoint was overall survival.
Results
Over 4 years of follow-up, there was no statistically significant difference in survival and time to progression between the four treatment groups. After two cycles of chemotherapy, responders and nonresponders were divided according to their response in order to examine the role of initial response as an independent factor in survival and response when a biological agent is combined with chemotherapy. Nonresponders, who received additional therapy with bevacizumab or combination therapy, had a survival benefit [657 days (95% confidence interval 349–970) and 681 days (95% confidence interval 315–912), respectively], which was statistically significant compared with continuation of cytotoxic chemotherapy (P < 0.001). The combination therapy had a safety profile comparable with that of bevacizumab and erlotinib taken individually.
Conclusion
Administration of bevacizumab and erlotinib in combination with first-line chemotherapy, followed by bevacizumab and erlotinib monotherapy as maintenance, showed promising results in patients with NSCLC, with reduced toxicity as compared with chemotherapy alone, but did not translate into longer overall survival.
Introduction
The prognosis for patients with advanced non-small cell lung cancer (NSCLC) remains poor. While platinum-based combination chemotherapy has reached an efficacy plateau, preclinical and clinical data support the hypothesis that inhibiting multiple biological pathways that mediate tumor growth may be an effective therapeutic strategy.Citation1 Progress in understanding cancer biology and mechanisms of oncogenesis has allowed the development of treatment against specific molecular targets, such as epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF), which are of special interest in NSCLC.
Angiogenesis is considered to be an absolute prerequisite for malignant tumor growth and dissemination.Citation2 VEGF is a key molecule in the upregulation of tumor angiogenesis. Targeting VEGF has led to major advances in treating different tumors. Bevacizumab, a recombinant humanized monoclonal antibody against VEGF, has shown relevant clinical activity in different types of human cancer, particularly in NSCLC.Citation3 Two Phase III trials were designed for NSCLC patients with non-squamous cell tumors, comparing standard chemotherapy alone or treatment with bevacizumab. A survival benefit was demonstrated in the E4599 study and a benefit in progression-free survival in both studies for the combination arm.Citation4,Citation5
Activation of the EGFR pathway initiates a process that promotes tumor cell proliferation, angiogenesis, decreased apoptosis, and metastasis.Citation6 EGFR has emerged as an attractive therapeutic target for patients with NSCLC. Erlotinib inhibits the tyrosine kinase activity of EGFR and has been studied extensively in randomized Phase III trials,Citation7,Citation8 yielding promising results, especially as second-line, third line, and maintenance therapy, and in patients with activating mutations of the EGFR receptor.Citation9
Because tumor progression, metastasis, and angiogenesis depend on activation of multiple growth factor pathways and genetic alterations,Citation10 it has been suggested that simultaneous blockade of several signaling pathways may improve treatment efficacy. This is the rationale for the combination of bevacizumab and erlotinib in NSCLC, which has proven to be well tolerated even when both are administered at their recommended Phase II dose.Citation1 On this basis, a dual-center Phase I/II study was conducted to examine the combination of bevacizumab and erlotinib in patients with stage IIIb/IV or recurrent non-squamous NSCLC, with promising results.Citation11 Another Phase II trial evaluated the safety of combining bevacizumab with either chemotherapy or erlotinib versus chemotherapy alone, and results for progression-free survival and overall survival favored the combination of bevacizumab with either chemotherapy or erlotinib over chemotherapy alone in the second-line setting.Citation12 In contrast, more recently, the BeTa (Bevacizumab/Tarceva) trial, investigating the benefits of addition of bevacizumab to erlotinib for second-line treatment of advanced NSCLC, showed a doubling of progression-free survival with combination therapy (3.4 months) as compared with erlotinib monotherapy (1.7 months, P < 0.001) but no benefit in terms of overall survival.Citation13 In this trial, we aimed to compare each targeted therapy alone (bevacizumab, erlotinib) with their combination and cytotoxic chemotherapy alone in previously untreated and advanced non-squamous NSCLC, following by administration of these agents as maintenance therapy. Moreover, in this study, we evaluated the role of radiological response of patients to the initial chemotherapy as a predictive factor, although this was not taken into consideration for the division of patients in subgroups.
Materials and methods
For this study, we enrolled patients with histologically or cytologically confirmed newly diagnosed stage IIIb or stage IV non-squamous NSCLC. Other inclusion criteria were age ≥ 18 years, an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate hematologic, hepatic, and renal function (including urinary excretion of ≤500 mg of protein per day). Exclusion criteria included hemoptysis, a history of documented hemorrhagic diathesis or coagulopathy, therapeutic anticoagulation, radiation therapy within 21 days before enrolment or major surgery within 28 days before enrolment, clinically significant cardiovascular disease, medically uncontrolled hypertension, prior systemic chemotherapy for NSCLC, and symptomatic or untreated brain metastases. Patients with tumors invading or abutting major blood vessels (based on radiologist assessment) were also excluded.
Study design
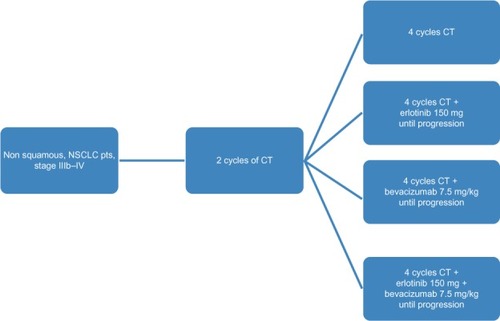
Patients were randomly allocated to receive docetaxel and carboplatin chemotherapy alone (control group), bevacizumab in combination with chemotherapy (docetaxel and carboplatin chemotherapy + bevacizumab [bevacizumab group]), erlotinib in combination with chemotherapy (docetaxel and carboplatin chemotherapy + erlotinib [erlotinib group]), or bevacizumab in combination with erlotinib and chemotherapy (docetaxel and carboplatin chemotherapy + bevacizumab + erlotinib [combination group]). Randomization of this prospective four-arm study was performed with an allocation rate of 1:1:1:1 (). It was an open-label study, without placebo, bevacizumab, or erlotinib used alone.
All patients initially received two cycles of chemotherapy with docetaxel 100 mg/mCitation2 and carboplatin at a dose of area under the concentration-time curve of 5.5 every 28 days,Citation14,Citation15 and after laboratory assessment, were randomized into four groups. The first group (controls) received a further four cycles of docetaxel-carboplatin and continued with observation until disease progression. The second group (erlotinib) received four cycles of docetaxel-carboplatin plus erlotinib administered orally at 150 mg/dL per day beginning on the first day of the third cycle and continued with erlotinib monotherapy thereafter until progression. The third group (bevacizumab) received four cycles of docetaxel-carboplatin plus bevacizumab 7.5 mg/kg by intravenous infusion every 28 days and continued with bevacizumab every 21 days until disease progression. The fourth group (combination therapy) received four cycles of chemotherapy plus bevacizumab 7.5 mg/kg every 28 days and erlotinib 150 mg/dL, and continued with bevacizumab every 21 days and erlotinib until disease progression.
After the first two cycles of chemotherapy, patients who experienced progression of the disease to greater than 20%–25% of the initial dimensions of the tumor were not randomized. Patients who had progression to less than 20%–25% in the initial assessment were randomized and re-evaluated clinically and with a chest X-ray in the fourth cycle. If further deterioration of the disease was suspected and confirmed by computed tomography, patients were discontinued (three in the control group, four in the erlotinib group, none in the bevacizumab group, and one in the combination group), but they were included in the final statistical analysis for overall survival.
The second group received erlotinib with chemotherapy, although this combination has not demonstrated survival benefit compared with chemotherapy given alone,Citation8 because when the study was designed, monotherapy with erlotinib had approval only as second-line therapy. The primary endpoint was overall survival. Secondary endpoints included improvement in time to progression, objective response rate, initial response rate to chemotherapy in chemonaïve patients, safety, and assessment of associations between efficacy endpoints and expression of EGFR and VEGF in tissue and plasma. All patients provided their informed consent before starting chemotherapy. The study protocol was approved by the ethical committee at our hospital and the scientific medical council of the Aristotle University of Thessaloniki.
Laboratory correlates
Plasma VEGF and EGFR levels were measured at baseline and before the third and sixth cycle of treatment in all patients using an enzyme-linked immunosorbent assay. EGFR and VEGF protein expression in lung cancer tissue was determined by immunohistochemical staining analysis of unstained slides, if available (using the DakoCytomation PharmDx test kit, DakoCytomation, Carpinteria, CA). Immunoreactivity was graded as positive if more than 10% of carcinoma cells were stained and negative if less than 10% were stained.Citation16
Tumor response
Tumor response was determined by RECIST (Response Evaluation Criteria in Solid Tumors) version 1.0 criteria.Citation17 Tumor assessment by computed tomography was performed at baseline and on day 28 of chemotherapy cycles 2 and 6, and every 12 weeks following completion of chemotherapy. Chest X-ray, hematologic, renal, and hepatic function, and urine analysis were performed on day 28 of each cycle.
Adverse events
Adverse events were graded according to the US National Cancer Institute Common Toxicity Criteria for Adverse Events version 3.0. Patients were assessed for all grades of adverse events, serious adverse events, and adverse events requiring interruption or discontinuation of the study drug. For grade 1 or 2 toxic effects (diarrhea and rash occurred more frequently in the bevacizumab and combination groups), symptomatic treatment was recommended without reduction of the dose of erlotinib. For grade 3 toxic effects, a dose reduction (erlotinib 100 mg) or temporary interruption of therapy was needed. There was also special concern about pulmonary hemorrhage or any serious bleeding event (grade 3 or higher), especially in patients on bevacizumab and on the combination therapy.
Statistical analysis
Data are expressed as the median ± standard error (95% confidence interval [CI]). The null hypothesis was rejected for an á level < 0.05. Survival rates were calculated using the Kaplan-Meier method and survival curves were compared using the log-rank test. All calculations involved two-sided P values with an á = 0.05 and 80% power (r = 0.3 medium effect size) and a 100-day survival benefit, according to the G*Power 3 test.Citation18 We used stratified Cox proportional hazard models to estimate the hazard ratio (HR) and 95% CI for overall survival and time to progression.
Results
Between May 2007 and December 2010, 229 patients were randomly assigned at our medical center. The first patient was enrolled on May 1, 2007 and the last patient on December 20, 2010. Sixty-one patients were assigned to the control group, 52 to the docetaxel and carboplatin chemotherapy + erlotinib group, 56 were assigned to the docetaxel and carboplatin chemotherapy + bevacizumab group, and 60 were assigned to docetaxel and carboplatin chemotherapy + bevacizumab + erlotinib group. The median follow-up duration was 440 ± 51.20 days (95% CI 341–545). shows selected demographic and baseline characteristics for all patients randomized, and shows a consort diagram for the eligible patients.
Figure 2 Consort diagram. 248 patients were enrolled. The patients received firstly 2 cycles of chemotherapy (docetaxel plus carboplatin) and then they were randomly divided into four groups. In total 229 patients were eligible for data analysis.
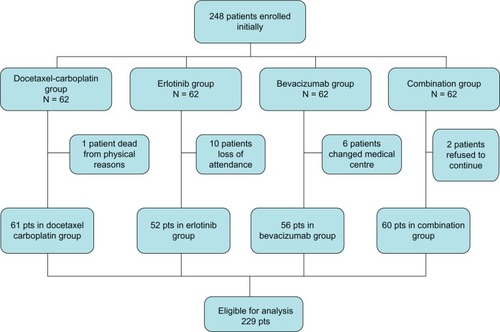
Table 1 Patient characteristics
Efficacy analysis
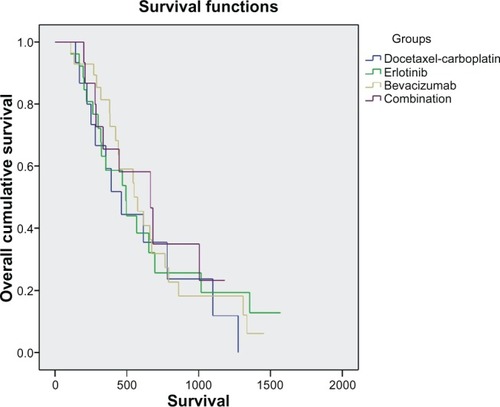
Overall survival did not differ between patients in the four groups (P = 0.381). Median duration of overall survival was longer in the combination group than in the other groups, although not statistically significant (). Pairwise comparisons did not reveal any statistically significant difference between the four study arms. Median overall survival was 460 days (95% CI 270–650) in the control group, 491 (95% CI 290–692) in the erlotinib group, 574 (95% CI 378–769) in the bevacizumab group, and 663 (95% CI 370–955) in the combination group ().
Table 2 Median overall survival in the four treatment groups
By Kaplan-Meier analysis, one-year survival was 16% in the control group, 27% in the erlotinib group, 39% in the bevacizumab group, and 18% in the combination group. Based on observation of the Kaplan-Meier curves, a statistical analysis in the first 450 days (15 months) of the study was performed and showed that the bevacizumab group had a survival benefit compared with the other groups, ie, 248 (190–305) days for the control group, 299 (229–368) days for the erlotinib group, 380 (317–442) for the bevacizumab group, and 284 (275–292) for the combination group (P = 0.023, ).
Time to progression did not differ significantly between the four groups at the end of the study, but time to progression of the disease was significantly longer in the combination group at the end of the first year of the study (P = 0.001, ).
Table 3 Time to progression among the four treatment groups
shows an analysis of objective response rate, which was greater in the groups receiving targeted therapies than in the control group. When the patients were divided into responders (those with a complete, partial, or minor response, or stable disease) and nonresponders (those with progressive disease) according to their response in the initial assessment, it was evident that patients who did not respond to the initial two cycles of cytotoxic chemotherapy do not respond at all to chemotherapy overall (). After two cycles of chemotherapy, nonresponders who received additional treatment with bevacizumab or combination therapy had a survival benefit [657 (349–970) days and 681 (315–912) days, respectively], which was statistically significant compared with the continuation of treatment with cytotoxic chemotherapy (P < 0.001). Moreover, this survival benefit in nonresponders with the addition of bevacizumab to cytotoxic docetaxel and carboplatin chemotherapy continued after six cycles of docetaxel and carboplatin chemotherapy and after 3 months of maintenance therapy with bevacizumab ().
Table 4 Analysis of objective response rate
Table 5 Patients with progressive disease (nonresponders)
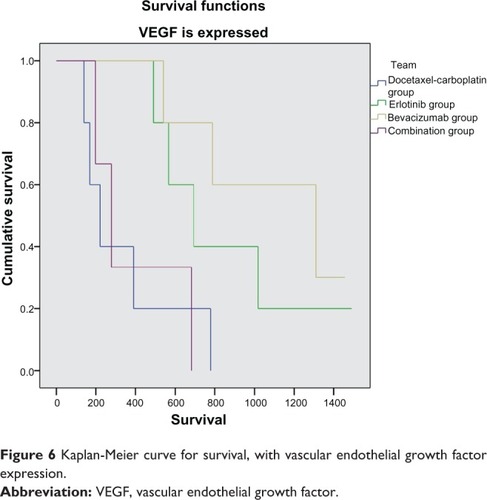
One hundred and thirty-two (57%) of the 229 patients enrolled had tumor tissue available for EGFR and VEGF immunohistochemistry. Further, 183 patients had results for serum EGFR and VEGF levels. VEGF and EGFR expression in tumor tissue in general had no correlation with survival (P = 0.18 and P = 0.19, respectively). Nevertheless, if we compare patients according to their treatment allocation, a statistically significant survival benefit is observed in patients who received erlotinib or bevacizumab and expressed VEGF (P = 0.002 and P = 0.013, respectively), while patients receiving cytotoxic chemotherapy had a survival benefit compared with the other groups (P = 0.034) when VEGF and EGFR were not expressed in tumor tissue (, and ). Serum VEGF and EGFR levels before and after treatment did not correlate with overall survival rate (P = 0.15).
Figure 5 Kaplan-Meier curve for survival without vascular endothelial growth factor expression.
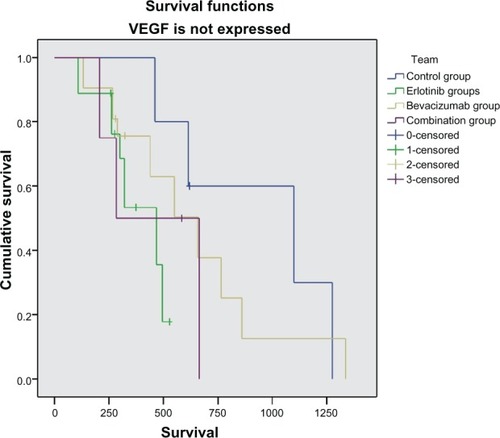
Figure 6 Kaplan-Meier curve for survival, with vascular endothelial growth factor expression.
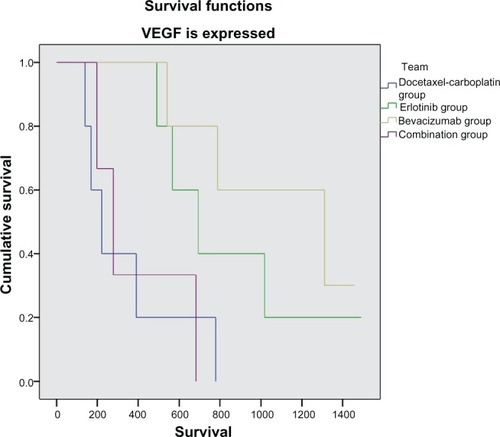
Table 6 VEGF and EGFR in tissue and survival in days
EGFR mutations and prognosis in NSCLC
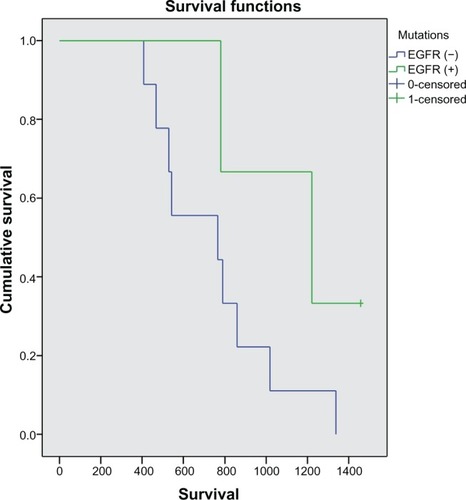
A subgroup analysis of patients with longer survival (>1.5 years) was performed. In 24 patients for whom tumor tissue was available for determination of EFGR mutation status, we investigated the sequence of the gene encoding a tyrosine kinase region (exons 18–21). These mutations were detected using high resolution melting analysis and identified by direct determination of the DNA sequence (sequencing using ABI Prism 3130 sequencer) in the exons. Of 24 patients, six had EGFR-mutated tumors. Although subgroup analysis of overall survival seemed to favor patients with EGFR-mutated tumors compared with wild-type EGFR tumors, the difference did not reach statistical significance (P = 0.134, ).
Adverse events
The adverse event rate was similar in the four treatment groups. Twenty-eight (47%) of the 61 patients in the control group experienced adverse hematological events, ten (17%) of which were grade 3 or 4, compared with seven (13%) of the 52 patients in the erlotinib group, ten (18%) of the 56 patients in the bevacizumab group, and 18 (28%) of the 60 patients in the combination group (). The incidence of grade 3 arterial thromboembolic events (pulmonary embolism) was higher in the bevacizumab group than in the other groups, but was much lower than the rates previously reported in patients with advanced NSCLC and treated with bevacizumab.Citation4 Two patients (4%) in the bevacizumab group and two (3%) in the combination group had grade 3 or 4 hypertension ().
Table 7 Adverse events
Three patients (5%) in the control group discontinued treatment because of adverse events compared with four (8%) in the erlotinib group, two (4%) in the bevacizumab group, and six (9%) in the combination group. After discontinuation of treatment, patients received palliative therapy, second/third-line chemotherapy, or radiotherapy, as necessary.
Discussion
Dual inhibition reduces tumor endothelial proliferation compared with VEGF or EGFR blockade alone.Citation19 However, our trial did not find a statistically significant advantage in favor of the combination of bevacizumab and erlotinib, as did the recent BeTa trial,Citation13 although the combination group had a survival benefit of 200 days (6.5 months) compared with the control group, albeit not statistically significant.
Moreover, the combination of bevacizumab and erlotinib delayed disease progression until the end of the first year of treatment. A synergistic role of inhibition of angiogenesis and tumor growth might account for the delay in disease progression, with development of resistance by tumor cells rendering the targeted agents inactive after a period of time.Citation20
The response to the second cycle of chemotherapy can predict overall response in patients with NSCLC.Citation18 Dividing patients into responders and nonresponders according to their response at initial assessment, nonresponders to initial cytotoxic chemotherapy did not respond to the next cycles of the same initial regimens.Citation21
Another issue in patients with NSCLC is the optimal treatment duration. Large studies, including AVAIL (Avastin in Lung) and ATLAS (A Study Comparing Bevacizumab Therapy With or Without Erlotinib for First-Line Treatment of Non-Small Cell Lung Cancer),Citation5,Citation22 confirmed the efficacy of bevacizumab as maintenance therapy and demonstrated that the benefit is further improved by addition of erlotinib. During the first year of treatment, bevacizumab combined with chemotherapy seemed to confer a significant survival benefit compared with the other treatment groups.
In our study, patients with progressive disease who did not respond to initial chemotherapy survived for longer when they received bevacizumab, not only as initial treatment, but also as maintenance therapy for more than six cycles. Further, better response rates were achieved in the groups receiving targeted therapies (0% for the control group, 22% for the erlotinib group, 27% for the bevacizumab group, and 30% for the combination group, after 3 months of cytotoxic chemotherapy and continuation with targeted agents).
The targeted therapies had an acceptable safety profile, despite being combined with cytotoxic chemotherapy, probably because of the low dose of bevacizumab used (7.5 mg/kg). The importance of identifying molecular prognostic factors has been emphasized with the development of targeted treatment, but for NSCLC the field remains open as a result of the large volume of conflicting data, especially for bevacizumab.Citation23 In our study, expression of VEGF and EGFR in tumor tissue in general had no correlation with survival. Nevertheless, patients who expressed VEGF and received erlotinib or bevacizumab had a statistically significant survival benefit compared with the control group, perhaps because of blockade of VEGF.
Expression, overexpression, and mutation of EGFR have been implicated in the pathogenesis of NSCLC,Citation24 suggesting that patients with EGFR mutations might derive increased benefit from EGFR-targeted therapies.Citation25 In our study, although subgroup analysis suggested that overall survival was better in patients with EGFR-mutated tumors compared with wild-type EGFR tumors, the difference did not achieve statistical significance. However, this result should be interpreted with caution because examination of EGFR mutation in cancer tissue was performed retrospectively in patients with prolonged survival, and the patient population in our study was quite small.
The main limitation of our study is that, when the protocol was designed, erlotinib was not approved for first-line therapy as a single agent, and this is why we administered erlotinib in combination with chemotherapy. Another limitation of the study was the lack of data for VEGF and EGFR expression in lung cancer tissue in all patients enrolled in the study, some of whom were diagnosed cytologically. Further, for financial reasons, determination of EGFR mutation was performed retrospectively and not in all patients. It should be noted that the survival benefit was statistically significant in the bevacizumab group at the 450-day survival analysis, and this was an ad hoc result based on initial observation of the Kaplan-Meier curve. Another limitation is the need for prolonged (more than 2 years) follow-up, especially in patients with long-term survival and positive expression of VEGF. It would be interesting in the future to compare other targeting agents administered alone or in combination with traditional chemotherapy in NSCLC patients who are nonresponders.
Despite improvements in several efficacy endpoints, improving survival remains a challenge in the treatment of NSCLC. This randomized study suggests that bevacizumab enhances the activity of chemotherapy, mainly in patients who do not respond to initial cytotoxic chemotherapy. Taking into account the cost of biological agents, we could use initial response as a predictor of whether to add bevacizumab to standard chemotherapy for the treatment of NSCLC. Combination of erlotinib and bevacizumab did not prolong overall survival. Results from larger studies are eagerly awaited to help determine how these antiangiogenic agents may be best used either alone or in combination with traditional chemotherapy regimens. The advantages and disadvantages have to be presented along with the disadvantages of toxicity and cost effect.
Disclosure
The authors report no conflicts of interest in this work.
References
- JungYDMansfieldPFAkagiMEffects of combination antivascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse modelEur J Cancer20023881133114012008203
- KerbelRSTumor angiogenesisN Engl J Med2008358192039204918463380
- GridelliCMaionePRossiADe MarinisFThe role of bevacizumab in the treatment of non-small cell lung cancer: current indications and future developmentsOncologist200712101183119317962612
- SandlerAGrayRPerryMCPaclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancerN Engl J Med2006355242542255017167137
- ReckMvon PawelJZatloukalPPhase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAILJ Clin Oncol20092781227123419188680
- ArteagaCTargeting HER1/EGFR: a molecular approach to cancer therapySemin Oncol2003303 Suppl 7314
- ShepherdFARodrigues PereiraJCiuleanuTErlotinib in previously treated non-small-cell lung cancerN Engl J Med2005353212313216014882
- HerbstRSPragerDHermannRTRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancerJ Clin Oncol200523255892589916043829
- CappuzzoFCiuleanuTStelmakhLErlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 studyLancet Oncol201011652152920493771
- O’ReillyMSTherapeutic strategies using inhibitors of angiogenesisMethods Mol Biol200322359963412777753
- HerbstRSJohnsonDHMininbergEPhase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancerJ Clin Oncol200523112544255515753462
- HerbstRSO’NeillVJFehrenbacherLPhase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancerJ Clin Oncol200725304743475017909199
- HerbstRSAnsariRBustinFEfficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trialLancet201137797801846185421621716
- MillwardMJBoyerMJLehnertMDocetaxel and carboplatin is an active regimen in advanced non-small-cell lung cancer: a phase II study in Caucasian and Asian patientsAnn Oncol200314344945412598352
- ZarogoulidisKKontakiotisTHatziapostolouPA Phase II study of docetaxel and carboplatin in the treatment of non-small cell lung cancerLung Cancer200132328128711390009
- CascinuSStaccioliMPGaspariniGExpression of vascular endothelial growth factor can predict event-free survival in stage II colon cancerClin Cancer Res2000672803280710914727
- TherassePArbuckSGEisenhauerEANew guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of CanadaJ Natl Cancer Inst200092320521610655437
- FaulFErdfelderELangAGBuchnerAG*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciencesBehav Res Methods200739217519117695343
- NaumovGNNilssonMBCasconeTCombined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistanceClin Cancer Res200915103484349419447865
- SattlerMAbidoyeOSalgiaREGFR-targeted therapeutics: focus on SCCHN and NSCLCScientific World Journal2008890991918836658
- HannaNShepherdFAFossellaFVRandomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapyJ Clin Oncol20042291589159715117980
- MillerVAO’ConnorPSohCATLAS InvestigatorsA randomized, double-blind, placebo-controlled, phase IIIb trial (ATLAS) comparing bevacizumab (B) therapy with or without erlotinib (E) after completion of chemotherapy with B for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC)J Clin Oncol.200927Suppl 18Abstr LBA8002
- TimotheadouESkarlosDVSamantasEEvaluation of the prognostic role of a panel of biomarkers in stage IB-IIIA non-small cell lung cancer patientsAnticancer Res2007276C4481448918214064
- PaoWMillerVZakowskiMEGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to geftinib and erlotinibProc Natl Acad Sci USA200410136133061331115329413
- ReguartNCardonaAFRosellRRole of erlotinib in first-line and maintenance treatment of advanced non-small-cell lung cancerCancer Manag Res2010214315621188105