Abstract
Background
Bromelain is a pineapple stem extract with a variety of therapeutic benefits arising from interaction with a number of different biological processes. Several preclinical studies and anecdotal clinical observations have reported the anticancer properties of bromelain. In the present study, we investigated the cytotoxic effects of bromelain in four human cancer cell lines of gastrointestinal origin and the mechanisms involved.
Methods
The gastric carcinoma cell lines (KATO-III and MKN45) and two chemoresistant subpopulations of the HT29 colon adenocarcinoma cell line (HT29-5M21 and HT29-5F12) were treated with a range of concentrations of bromelain, as well as with cisplatin as a positive control. The effect of bromelain on the growth and proliferation of cancer cells was determined using a sulforhodamine B assay after 72 hours of treatment. Expression of apoptosis-associated proteins in MKN45 cells treated with bromelain was analyzed by Western blotting.
Results
Data from our sulforhodamine B assay showed that bromelain inhibited proliferation of HT29-5F12, HT29-5M21, MKN45, and KATO-III cells, with respective half maximal inhibitory concentration values of 29, 34, 94, and 142 μg/mL. Analyzing the expression of proapoptotic and antiapoptotic proteins in bromelain-treated MKN45 cells, we observed activation of the caspase system, cleavage of PARP and p53, overexpression of cytochrome C, attenuation of phospho-Akt and Bcl2, and removal of MUC1. Apart from the caspase-dependent apoptosis observed, emergence of cleaved p53 supports a direct, extranuclear apoptotic function of p53. Moreover, interrupted Akt signaling and attenuation of Bcl2 and MUC1 oncoproteins suggest impaired survival of cancer cells.
Conclusion
Our findings collectively indicate that bromelain exerts cytotoxic effects in a panel of human gastric and colon carcinoma cells. Our study of MKN45 cells implicated different mechanisms in bromelain-induced cell death. While promoting apoptosis with involvement of the caspase system and extranuclear p53, bromelain also appears to impair cancer cell survival by blocking the Akt pathway and attenuating Bcl2 and MUC1 oncoproteins.
Introduction
Pineapple, botanically named Ananas comosus, has been used for centuries as a folk medicine by the indigenous inhabitants of Central and South America to treat a range of ailments. The medicinal qualities of the plant are attributed to bromelain, a pineapple stem extract, which has been available as a pharmaceutical product since 1956.Citation1 The beneficial effects of bromelain are attributable to its multiple constituents. Although primarily comprised of sulfhydryl-containing proteolytic enzymes, bromelain also contains escharase (a nonproteolytic component with debriding effects),Citation2 peroxidase, acid phosphatase, glucosidases, cellulases, several protease inhibitors, glycoproteins, carbohydrates, and organically bound calcium.Citation3,Citation4 Bromelain has shown an ability to interact with a variety of effectors and pathways involved in physiological processes, including inflammation, the immune response, and coagulation. Thus, the therapeutic potential of bromelain, alone or in combination with other agents, has been tested in preclinical and clinical settings, suggesting a number of clinical indications.Citation5 However, as an anticancer agent, bromelain has been the subject of limited preclinical and clinical observations.Citation6
Gastrointestinal cancers account for more than one third of all deaths from malignant neoplasms worldwide,Citation7 among which colorectal and stomach cancers are the most common.Citation8 In the present study, as an initial attempt towards implementing a novel approach to the management of advanced gastrointestinal carcinoma, the anticancer efficacy of bromelain was investigated in a range of gastrointestinal carcinoma cells. To explore the efficacy of bromelain in a more comprehensive way, gastrointestinal cancer cells of different origins and various sensitivities to cytotoxic agents were employed. These included poorly differentiated human gastric cancer cell lines (MKN45 and KATO-III) and chemoresistant subpopulations of the HT29 human colon cancer cell line (HT29-5F12 and HT29-5M21).
Materials and methods
Cell culture
The human gastric carcinoma cell lines (MKN45 and KATO-III) were obtained from the Cancer Research Campaign Laboratories (University of Nottingham, NG7 2RD, UK) and the American Type Culture Collection (Manassas, VA, USA), respectively. The HT29-5F12 and HT29-5M21 cells were a kind gift from Dr Thécla Lesuffleur (Université Pierre et Marie Curie, Paris, France). All cell lines were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37°C in their respective media as follows: MKN45 in RPMI-1640, KATO-III cells in Iscove’s Dulbecco’s modified Eagle’s medium, and HT29-5F12 and HT29-5M21 cells in Dulbecco’s modified Eagle’s medium (all from Invitrogen, Carlsbad, CA, USA). All of the culture media used were supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) penicillin-streptomycin (Invitrogen), with the exception of Iscove’s Dulbecco’s modified Eagle’s medium, for which 20% fetal bovine serum supplementation was used.
Drug preparation
Bromelain and cisplatin were purchased from Sigma-Aldrich (St Louis, MO, USA). Bromelain and cisplatin stock solutions were prepared at concentrations of 1000 μg/mL and 15,000 μg/mL, respectively. For each experiment, bromelain stock solution was freshly made, filtered, and diluted with the appropriate culture medium to achieve the final treating concentrations.
Proliferation assay
The effect of bromelain on growth and proliferation of MKN45, KATO-III, HT29-5F12, and HT29-5M21 cells was determined using the sulforhodamine B assay. In brief, the cells were seeded into 96-well plates and cultured in complete culture medium at densities of 1500–5000 cells per well. At the desired confluence, the cells were treated for 72 hours with a range of concentrations of bromelain (5–1000 μg/mL) in serum-free medium, as well as with cisplatin as the positive control. At the end of the treatment period, the cells were fixed by 30 minutes of incubation with 10% (w/v) trichloroacetic acid at 4°C. This was followed by five washes with slow-running tap water. The plates were then stained with 0.4% (w/v) sulforhodamine B (Sigma-Aldrich) dissolved in 1% acetic acid. Unbound dye was removed by rinsing the plates with 1% acetic acid, and the plates were then allowed to dry out at room temperature. Bound sulforhodamine B was solubilized with 10 mM Tris base (Sigma-Aldrich) and absorbance was read using a microplate reader (PowerWaveX, Bio-Tek Instruments Inc, Winooski, VT, USA) with a working wavelength of 570 nm.
Western blotting
The efficacy of bromelain in activating apoptotic processes and modulating expression of apoptosis-associated proteins was explored in MKN45 cells using Western blotting. Briefly, the cells were homogenized in protein lysis buffer (RIPA) containing 10% protease inhibitor (Sigma-Aldrich), and the protein concentrations were then quantified using a BioRad protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). The following primary antibodies were then applied to the membranes according to the manufacturers’ protocols: rabbit polyclonal anticaspase 3, anti-Bcl2, anti-p53 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anticaspase 8 (R&D Systems, Minneapolis, MN, USA), anticaspase 9, anti-PARP, anticytochrome C, anti-Akt, rabbit monoclonal antiphospho-Akt, and mouse monoclonal anti-MUC1 (Cell Signaling Technology Inc, Danvers, MA, USA). The membranes were washed and treated with the appropriate horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology Inc). A similar process was carried out for β-actin as a loading control using mouse monoclonal anti-β-actin antibody (Santa Cruz Biotechnology). The antigen-antibody reaction was visualized using ImageQuant LAS 4000 Biomolecular imager and ImageQuant software (GE Healthcare, Chalfont, UK). Band densitometry was quantified and the data were normalized against the values of β-actin protein expression.
Statistical analysis
All data presented are representative of three independent experiments. Statistical analyses were performed using GraphPad InStat (GraphPad Prism 5, San Diego, CA, USA). The Student’s t-test was applied for unpaired samples and P values < 0.05 were considered to be statistically significant.
Results
Bromelain inhibited proliferation of human gastrointestinal carcinoma cells
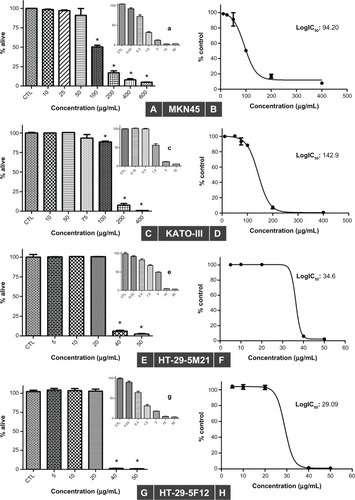
Using the sulforhodamine B assay, we investigated the potential of bromelain to inhibit growth of gastrointestinal cancer cells. As shown in , bromelain significantly inhibited cell proliferation in MKN45 (P = 0.0018, 0.0010, 0.0002, and <0.0001 using concentrations of 100, 200, 400, and 600 μg/mL, respectively), KATO-III (P < 0.0001 using concentrations of 100, 200, and 400 μg/mL, respectively), and 5F12 and 5M21 (P < 0.0001 using concentrations of 40 and 50 μg/mL, respectively). Fifty percent inhibitory concentration (IC50) values were calculated from concentration-response curves plotting growth percentage versus drug concentration using GraphPad Prism 5 (). Our data revealed IC50 values of 29, 34, 94, and 142 μg/mL for HT29-5F12, HT29-5M21, MKN45, and KATO-III cells, respectively.
Figure 1 Sulforhodamine B assay in MKN45 (A and B), KATO-III (C and D), HT29-5M21 (E and F), and HT29-5F12 (G and H) cells after 72 hours of treatment with bromelain concentrations ranging from 5 μg/mL to 600 μg/mL, and with different concentrations of cisplatin used as a positive control (shown as smaller graphs a, c, e and g).
Bromelain induced caspase-dependent apoptosis
To investigate the inhibitory effects of bromelain observed in the proliferation assay further, the implication of caspase-driven apoptotic events was evaluated by examining expression of cytochrome C, caspases 3, 8, and 9 in MKN45 cells after 72 hours of treatment with concentrations ranging from 25 to 200 μg/mL (). Along with overexpression of cytochrome C, the appearance of immunoreactive subunits of caspase 3 and caspase 8, as well as withering of procas-pase 9, was observed in a concentration-dependent manner. Moreover, the functionality of activated caspase 3 was confirmed by cleavage of PARP Expression of cleaved PARP was more prominent at a concentration of 200 μg/mL, where the precursor protein was no longer detectable.
Figure 2 Western blot imaging (A) and densitometric quantification (B–E) for a range of proteins involved in apoptotic death of MKN45 cells treated for 72 hours with bromelain concentrations of 25, 50, 100, and 200 μg/mL.
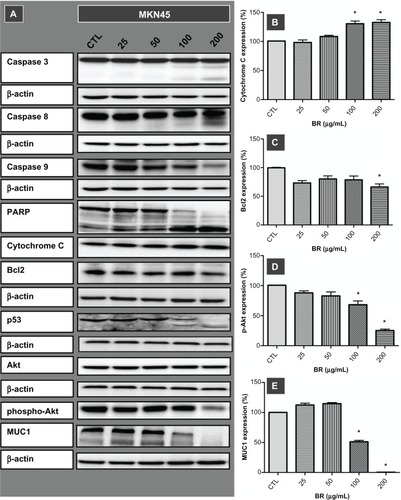
Bromelain caused cleavage of p53, removal of MUC1, and attenuation of phospho-Akt and Bcl2
Exploring the role of other mediators of apoptosis, our results showed cleavage of p53 protein and emergence of cleaved fragments at concentrations of 100 μg/mL and 200 μg/mL. Moreover, disappearance of MUC1, along with attenuation of phospho-Akt and Bcl2, occurred when the MKN45 cells were treated with a bromelain concentration of 200 μg/mL ().
Discussion
In this study, we observed the efficacy of bromelain in inhibiting growth and proliferation of four human gastrointestinal cancer cell lines in vitro. Clinical evaluation of the efficacy of bromelain as an anticancer agent, alone or in combination with other agents, has been confined to few anecdotal observations.Citation6 This may have stemmed from inadequate preclinical studies. Our literature search of PubMed yielded a limited number of investigations which had explored the anticancer effects of bromelain in preclinical settings, few of which had used cancer cells of human origin. Taussig et al reported bromelain-induced growth inhibition in three mouse tumor cell lines.Citation9 Byrnes et al indicated a role of bromelain in reversibly inhibiting the invasive properties of glioma cells.Citation10 In a study by Beuth et al, treatment with bromelain led to significant reduction of tumor growth in mice inoculated by murine sarcoma L-1 cells.Citation11 In vivo antitumoral and antimetastatic activity of bromelain against a panel of murine cancer cell lines has been shown by Baez et al.Citation12 Kalra et al reported reduced formation of mouse skin tumor with bromelain,Citation13 and Paroulek et al demonstrated the antitumor effects of bromelain against the GI101A human breast cancer cell line.Citation14 Bhui et al also demonstrated that pretreatment with bromelain reduced the number and volume of murine skin tumors,Citation15 and recently showed that bromelain had an antiproliferative effect against human A431 epidermoid carcinoma and A375 melanoma cells.Citation16
Our study of MKN45 cells demonstrates that bromelain induces caspase-dependent apoptotic cell death. Earlier studies have reported the proapoptotic effects of bromelain in a number of in vitro and in vivo cancer models. In their mouse models, Kalra et al and Bhui et al observed that treatment with bromelain resulted in upregulation of p53 and subsequent activation of caspase 3 and caspase 9.Citation13,Citation15 Using the M30 Apoptosense® assay (Peviva, Bromma, Sweden), Paroulek et al reported increased levels of the cytokeratin 18 protein, along with a large number of apoptotic cell bodies following bromelain treatment of GI-101A breast cancer cells.Citation14 They recently indicated a concentration-dependent increase in the activities of caspase 9 and caspase 3 coinciding with elevation of cytokeratin 18 levels.Citation17
An interesting feature of bromelain-induced apoptosis in our study was the cleavage of p53. Apart from the known anti-tumorigenic role of p53 as a sequence-specific transcription factor activating several proapoptotic genes, there is some evidence suggesting a direct, extranuclear apoptotic function of p53, activating so-called transcription-independent p53 apoptosis.Citation18,Citation19 Leu et al showed that p53 could interact with Bak, a proapoptotic mitochondrial membrane protein, resulting in release of cytochrome C from mitochondria.Citation20 Sayan et al demonstrated that p53 could be targeted and cleaved by caspases, generating two cytosolic fragments that translocate to the mitochondria and induce depolarization of the mitochondrial membrane.Citation21
In our study, bromelain also exhibited an intriguing aspect of its anticancer function by eliminating the MUC1 oncoprotein, which could be of particular therapeutic importance. Being expressed as a transmembrane mucin in normal epithelial cells of various organs, MUC1 is overexpressed and aberrantly glycosylated in most carcinomas.Citation22 Of the 1.4 million tumors diagnosed each year in the US, about 900,000 show overexpression of MUC1. MUC1 is exploited by malignant cells to induce transformation and tumorigenicity. It also plays an important role in cancer cell survival, tumor invasion, and metastasis, which is why MUC1 has emerged as a particularly attractive target for the development of anticancer agents.Citation23,Citation24
Diminished expression of phospho-Akt, but not total Akt, represents another molecular event of bromelain-induced apoptosis in the present study. This might be due to at least two mechanisms, ie, bromelain modulation of upstream kinase versus phosphatase activity or bromelain enhancement of protein phosphatase activity, and thus reduced phosphorylation status of phospho-Akt. Given that constitutive activation of Akt via crucial phosphorylation events promotes survival of cancer cells as well as resistance to treatment,Citation25 inhibition of phosphorylation may play an important role in the cytotoxicity of bromelain. In our study, bromelain appeared to impair survival of cancer cells by inhibiting Akt signaling and attenuating Bcl2 and MUC1.
Conclusion
Here, we report the anticancer effects of bromelain, a pineapple stem extract, in a panel of gastrointestinal cancer cell lines in vitro. Treatment with bromelain inhibited the growth and proliferation of cancer cells. Bromelain not only appeared to activate caspase-dependent apoptotic pathways and transcription-independent p53 apoptosis, but also induced concomitant inhibition of cell survival. The role of bromelain in removing MUC1 oncoprotein and attenuating phospho-Akt could be of particular therapeutic importance and warrants further investigation.
Disclosure
The authors report no conflicts of interest in this work.
References
- TaussigSJBatkinSBromelain, the enzyme complex of pineapple (Ananas comosus) and its clinical application. An updateJ Ethnopharmacol19882221912033287010
- HouckJCChangCMKleinGIsolation of an effective debriding agent from the stems of pineapple plantsInt J Tissue React1983521251346352539
- MaurerHRBromelain: biochemistry, pharmacology and medical useCell Mol Life Sci20015891234124511577981
- HaleLPGreerPKTrinhCTJamesCLProteinase activity and stability of natural bromelain preparationsInt Immunopharmacol20055478379315710346
- Bromelain. MonographAltern Med Rev201015436136821194252
- ChobotovaKVernallisABMajidFABromelain’s activity and potential as an anti-cancer agent: current evidence and perspectivesCancer Lett2010290214815619700238
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- TaussigSJSzekerczesJBatkinSInhibition of tumour growth in vitro by bromelain, an extract of the pineapple plant (Ananas comosus)Planta Med198551653853917345291
- TysnesBBMaurerHRPorwolTProbstBBjerkvigRHooverFBromelain reversibly inhibits invasive properties of glioma cellsNeoplasia20013646947911774029
- BeuthJBraunJMModulation of murine tumor growth and colonization by bromelaine, an extract of the pineapple plant (Ananas comosum L)In Vivo200519248348515796214
- BaezRLopesMTSalasCEHernandezMIn vivo antitumoral activity of stem pineapple (Ananas comosus) bromelainPlanta Med200773131377138317893836
- KalraNBhuiKRoyPRegulation of p53, nuclear factor kappaB and cyclooxygenase-2 expression by bromelain through targeting mitogen-activated protein kinase pathway in mouse skinToxicol Appl Pharmacol20082261303717889918
- ParoulekAFJaffeMRathinaveluAThe effects of the herbal enzyme bromelain against breast cancer cell line GI101AFASEB J200923LB18
- BhuiKPrasadSGeorgeJShuklaYBromelain inhibits COX-2 expression by blocking the activation of MAPK regulated NF-kappa B against skin tumor-initiation triggering mitochondrial death pathwayCancer Lett2009282216717619339108
- BhuiKTyagiSSrivastavaAKBromelain inhibits nuclear factor kappa-B translocation, driving human epidermoid carcinoma A431 and melanoma A375 cells through G(2)/M arrest to apoptosisMol Carcinog201251323124321432909
- DhandayuthapaniSPerezHDParoulekABromelain-induced apoptosis in GI-101A breast cancer cellsJ Med Food201215434434922191568
- ChipukJEGreenDRp53’s believe it or not: lessons on transcription-independent deathJ Clin Immunol200323535536114601643
- SpeidelDTranscription-independent p53 apoptosis: an alternative route to deathTrends Cell Biol2010201142419879762
- LeuJIDumontPHafeyMMurphyMEGeorgeDLMitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complexNat Cell Biol20046544345015077116
- SayanBSSayanAEKnightRAMelinoGCohenGMp53 is cleaved by caspases generating fragments localizing to mitochondriaJ Biol Chem200628119135661357316531411
- YonezawaSHigashiMYamadaNMucins in human neoplasms: clinical pathology, gene expression and diagnostic applicationPathol Int2011611269771622126377
- KufeDWMucins in cancer: function, prognosis and therapyNat Rev Cancer200991287488519935676
- KufeDWFunctional targeting of the MUC1 oncogene in human cancersCancer Biol Ther20098131197120319556858
- LoPiccoloJGranvilleCAGillsJJDennisPATargeting Akt in cancer therapyAnticancer Drugs200718886187417667591