Abstract
Purpose
Anthracycline-based chemotherapies for breast cancer are well known to have adverse effects and can also negatively affect host immune function. There is therefore a necessity for an adjuvant that maintains the quality of life (QOL) and immune function of cancer patients receiving anthracycline-based chemotherapies.
Patients and methods
The present study investigated the effectiveness of the concomitant use of Lentinula edodes mycelia extract (LEM), an oral immunomodulator, with FEC75 (5-fluorouracil + epirubicin + cyclophosphamide) therapy on host QOL and immune function in breast cancer patients with nodal metastases. Ten breast cancer patients with nodal metastases receiving surgery were enrolled in this study. Treatment with 5-fluorouracil (500 mg/m2), epirubicin (75 mg/m2), and cyclophosphamide (500 mg/m2) was performed every 21 days for two courses, and LEM (1800 mg/day by mouth) was administered during the second course.
Results
In the first course, hematological toxicity was observed and host QOL and immune function were exacerbated. In the second course, however, the number of white blood cells and lymphocytes did not decrease and host QOL was maintained. Furthermore, the cytotoxic activities of natural killer (NK) and lymphokine-activated killer cells and the proportion of activated NK and NK T-cells in lymphocytes were maintained in the second course.
Conclusion
It has been suggested that the concomitant use of LEM with FEC75 therapy can maintain host QOL and immune function, and offer important implications for an application of LEM as a useful oral adjuvant to anthracycline-based chemotherapies.
Introduction
In breast cancer patients with nodal metastases following radical surgery, anthracycline-based chemotherapies are efficacious,Citation1,Citation2 and widely used, but are well known to have adverse effects, including hematotoxicity, nausea and vomiting, and hair loss,Citation3,Citation4 which are potentially associated with an impaired quality of life (QOL).Citation5,Citation6 Furthermore, host immune function of breast cancer patients receiving anticancer chemotherapy has been reported to influence the relapse rate,Citation7 and anthracycline-based chemotherapies can negatively affect immune function.Citation8 A widely applicable treatment that maintains the host QOL and immune system of patients treated with anthracycline-based chemotherapies would therefore be of great benefit.
Lentinula edodes mycelia extract (LEM) is a dried powder of a hot water extract of the mycelia of L. edodes.Citation9 Oral administration of LEM has been shown to result in antitumor and immunomodulatory actions both in vitro and in vivo.Citation9–Citation14 LEM administered orally to patients with gastrointestinal cancers who were receiving chemotherapy was reported to reduce the adverse effects of chemotherapy, improve QOL, and enhance immunity.Citation15,Citation16 Moreover, a study of patients receiving postoperative chemotherapy for breast cancer indicated improvement in QOL after the use of LEM.Citation15 Thus, LEM has the potential to be a useful oral adjuvant for anthracycline-based chemotherapies. In the above mentioned studies, however, the baseline characteristics of the patients were not uniform,Citation15,Citation16 so further investigation under appropriately controlled conditions is essential to verify the effectiveness of LEM in patients receiving anthracycline-based chemotherapies. For this reason, we performed this study to verify the effectiveness of oral LEM intake for the QOL and immunity of patients receiving postoperative breast cancer chemotherapy with the 5-fluorouracil + epirubicin + cyclophosphamide (FEC75) regimen.
Patients and methods
Subjects comprised ten breast cancer patients positive for lymph node metastasis who underwent surgical resection in the Department of Surgery II at Yamaguchi University, Ube City, Yamaguchi, Japan between 2003 and 2004. All were women, aged between 37 years and 63 years (mean, 53.0 years), and all had a performance status of 0. Performance status was determined in conformity with the Eastern Cooperative Oncology Group performance status given in the Common Toxicity Criteria,Citation17 Version 2.0. Tumor stage was IIA in four patients, IIB in four patients, and IIIA in two patients. The trial protocol was approved by the institutional review board of Yamaguchi University Graduate School of Medicine, and the trial was conducted based on the ethical principles established in the Helsinki Agreement. All subjects received full information on the aims and methods of the trial before participation. Written consent for participation in the trial was obtained from all subjects.
Drug
The LEM used was manufactured as previously reported,Citation9 and supplied by Kobayashi Pharmaceutical Co, Ltd (Osaka, Japan). In brief, L. edodes mycelia were first inoculated onto solid medium consisting mainly of bagasse of sugar-cane and defatted rice polishings, and cultured until the mycelia spread. The culture was then extracted by hot water. The dissolved component was dehydrated to obtain a dry powder. This dry powder was used as the LEM in this trial.
Study design
This trial was an open-label trial with a single group. Subjects were treated with two courses of FEC75 chemotherapy (5-fluorouracil [5-FU], 500 mg/m2; cyclophosphamide, 500 mg/m2; epirubicin, 75 mg/m2) for 3 weeks as one course. The first course comprised FEC75 chemotherapy alone, whereas the second course used LEM in combination with FEC. In the second course, subjects took 1800 mg/day of LEM every day for the 3 weeks.
Measurement
In all tests, measurements were made five times: at week 0 (before administration of FEC in the first course); week 1 (after administration of FEC for 1 week in the first course); week 3 (after administration of FEC for 3 weeks in the first course and before administration of FEC in the second course); week 4 (after administration of FEC for 1 week in the second course); and week 6 (after administration of FEC for 3 weeks in the second course). A survey of quality of life (QOL) was measured by the QOL Questionnaire for Cancer Patients Treated with Anticancer Drugs (QOL-ACD),Citation18 and was evaluated from scores on the questionnaire. Among immune indices, immunosuppressive acidic protein in serum was measured by using enzyme-linked immunosorbent assay. The proportion of interleukin (IL)-4-positive and interferon-γ-positive cells among cluster of differentiation (CD)4-positive lymphocytes and the proportion of perforin-secreting cells among CD161- and CD8-positive lymphocytes were measured with flow cytometry as peripheral blood lymphocyte subsets. Anti-CD4 PC-5-labeled antibody, anti-CD161 fluorescein isothiocyanate-labeled antibody, and anti-CD8 allophycocyanin-labeled antibody (BD Japan, Tokyo, Japan) were used as cell surface antigen markers. In addition, anti-IL-4 phycoerythrin-labeled antibody, anti-interferon-γ fluorescein isothiocyanate-labeled antibody, and antiperforin phycoerythrin Cy5-labeled antibody (BD Japan) were used as intracellular protein markers. The activity of natural killer (NK) cells was measured using a 51Cr-release assay. Peripheral blood mononuclear cells (PBMCs) were isolated by using Ficoll solution® (GE Healthcare, Tokyo, Japan). The isolated PBMCs were rinsed in RPMI1640 medium(Sigma-Aldrich, Tokyo, Japan) containing 10% fetal bovine serum, adjusted to 1 × 106 cells/mL, and used as effector cells. K562 cells (DS Pharma Biomedical Co, Ltd, Osaka, Japan), a human immortalized myelogenous leukemia cell line, were used in adjusting the target cells. One hundred microcuries of 51Cr was added to the K562 cells and cultured for 1 hour at 37°C. After rinsing twice with phosphate-buffered saline, adjustment was made to 1 × 106 cells/mL in RPMI1640 medium containing 10% fetal bovine serum, and these cells were taken as the target cells. Mixed culture of effector cells and target cells was done for 3.5 hours at an effector/target ratio of 20, and then 51Cr released from dead target cells was measured with a γ-scintillation counter. In the control, 1 N HCl was added in place of effector cells, and 51Cr released from dead target cells was measured with the γ-scintillation counter. The activity of NK-cells was calculated as the proportion of the volume of released 51Cr at the time of effector cell addition to the volume of released 51Cr in the control. In calculation of lymphokine-activated killer (LAK) cell activity, IL-2 was added to RPMI1640 medium to a final concentration of 25 U/mL and peripheral blood PBCMs were cultured for 72 hours, after which cells from washed cultures were taken as effector cells. The 51Cr-labeled Daudi cells were used as target cells. Mixed culture of effector cells and target cells was performed for 3.5 hours at an effector/target ratio of 20, 51Cr released from dead target cells was measured, and then LAK cell activity was calculated. For phytohemagglutinin (PHA) blastogenesis, PHA was added to peripheral blood PBMCs as a mitogen and cultured for 64 hours, after which 3H-thymidine was added and culture was continued for another 8 hours. After culturing, the amount of 3H-thymidine taken up by PBMCs was measured, and PHA blastogenesis was calculated. Both hematology and blood biochemistry were performed at each measurement point.
Statistical analysis
Measurements are shown as mean ± standard deviation. Changes between before and after administration of FEC75 in the first and second courses were analyzed with Student’s paired t-test (two-sided test) using SPSS version 13 software (IBM Corporation, Armonk, NY, USA).
Results
QOL
shows the QOL of patients. One week following the first course of FEC75 therapy (5-FU [500 mg/m2], epirubicin [75 mg/m2], and cyclophosphamide [500 mg/m2]) without LEM, the QOL total score and the physical subscale score decreased significantly (). However, 1 week following the second course of FEC75 therapy with the concomitant use of LEM, QOL was maintained compared with that observed after the first course without LEM.
Figure 1 QOL score.
Abbreviations: QOL, quality of life; SE, standard error.
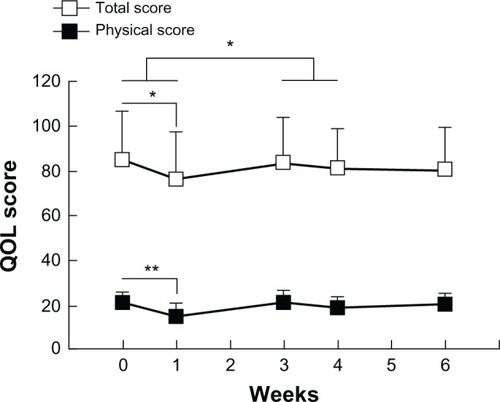
Table 1 QOL score
Hematotoxicity
shows the parameters of hematotoxicity. All hematological parameters exhibited a decrease 1 week following the first course of FEC75 therapy without LEM. However, decreases in white blood cells, neutrophils, and hemoglobin were suppressed 1 week following the second course of FEC75 therapy with concomitant use of LEM. In addition, 3 weeks following the first course of FEC75 therapy, the lymphocytes, hemoglobin, and hematocrit were insufficiently recovered, whereas both parameters were recovered 3 weeks following the second course.
Table 2 Hematotoxicity
Immune parameters
shows the immune parameters. One week following the first course of FEC75 therapy without LEM, LAK-cell and NK-cell activity as well as the percentages of NK (CD3negativeCD161positive) and activated NK (CD3negativeCD161positive perforinpositive) cells were decreased among lymphocyte subsets (). On the other hand, all of these immune parameters were maintained after the second course with concomitant use of LEM. The percentage of CD8+ T-cells was increased 3 weeks following both the first and second courses of FEC75 therapy.
Figure 2 Immunological parameters.
Abbreviations: LAK, lymphokine-activated killer; NK, natural killer; SE, standard error.
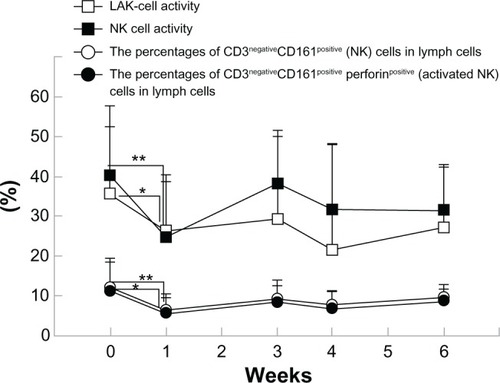
Table 3 Immunological parameters
Other parameters
There were no differences in changes in blood biochemistry parameters between the first and second courses (data not shown).
Follow-up at 5 years
shows the status of patients at a 5-year follow-up. All patients were treated with four more courses of FEC75 therapy regimen following the completion of the present study. Of the ten patients, nine patients could be followed up at 5 years. Of these, six patients were confirmed alive with no relapses, and two patients were alive with relapses. One patient was confirmed to have died of liver metastases.
Table 4 Five-year follow-up
Discussion
In breast cancer patients with positive nodes following radical surgery, anthracycline-based chemotherapies such as FEC therapy are widely performed.Citation1,Citation2 Such chemotherapies are often given as outpatient treatment in 21-day cycles, but clinically involve adverse effects such as hematotoxicity, nausea, and vomiting, which can impose substantial burden on the patients.Citation3,Citation4 Similarly, in the present study, hematotoxicity was observed 1 week following the first course of chemotherapy without LEM. Furthermore, 3 weeks after treatment, there was insufficient recovery of lymphocytes and hemoglobin values. On the other hand, 1 week following the second course of chemotherapy with concomitant use of LEM, no decreases were observed in white blood cells, neutrophils, or hemoglobin; and subsequently all hematological parameters showed recovery at 3 weeks following treatment. QOL, assessed by the QOL-ACD questionnaire, showed decreases in the total score and the subscale score for physical condition including nausea and vomiting after the first course of chemotherapy without LEM, but showed no decreases in these scores following the second course of chemotherapy with concomitant use of LEM. These findings indicate that LEM can reduce the averse effects of FEC75 therapy, including hematotoxicity as well as nausea and vomiting, and help maintain the QOL of the patients.
Host immune function of breast cancer patients taken with anticancer chemotherapy can influence the relapse rate.Citation7 In the present study, NK- and LAK-cell activity as well as the percentages of NK-cells (CD3negativeCD161positive) and activated NK-cells (CD3negativeCD161positive perforinpositive) decreased 1 week after the first course of FEC therapy without LEM, indicating that FEC therapy leads to decreased cytotoxic activity of lymphocytes. Conversely, no decreases were observed in these parameters after the second course of FEC therapy with concomitant use of LEM, and, in comparison with the first course, NK T cells (CD3positiveCD161positive) were increased 1 week after the second course, thereby indicating that the oral ingestion of LEM can reduce immune depression associated with FEC75 therapy. Although the mechanism by which LEM reduces immune depression by chemotherapy is not known, LEM has been reported to mitigate regulatory T-cell (Treg)-mediated immunosuppression in the tumor host.Citation13,Citation14 In cancer patients, Treg was shown to release soluble IL-2 receptors (sIL-2R),Citation19 which are involved in immunosuppression, particularly in decreasing NK cytotoxicity.Citation20 In addition, Archili et alCitation21 reported that FEC therapy increased the ratio of sIL-2R/lymphocytes, suggesting that, in the present study, FEC therapy might cause a decrease in the activity of NK-cells by increasing the ratio of sIL-2R/lymphocytes. Subsequently, LEM might decrease the ratio of sIL-2R/lymphocytes and contribute to the conservation of NK-cell activity or other immune functions during the second course of treatment. The present study also demonstrated an increased percentage of CD8+ T-cells among other lymphocyte subsets at 3 weeks following the treatment of chemotherapy. It has been reported that B-cells decrease to a greater extent than T-cells in anthracycline-based chemotherapies,Citation22 suggesting that, in the present study, B-cells may have been largely decreased whereas the percentage of CD8+ T-cells was relatively increased.
Among nine subjects who could be followed in the present study, the 5-year postoperative survival rate was 88.9%, and the 5-year relapse-free survival rate was 66.7%. These survival rates are comparable to the treatment effect for positive nodes after surgery for breast cancer of FEC100 therapy (a regimen in which the dose of epirubicin is increased from 75 mg/m2 to 100 mg/m2 compared with FEC75; the regimen consists of 5-FU [500 mg/m2], epirubicin [100 mg/m2], and cyclophosphamide [500 mg/m2]), and a higher effect may be expected than with FEC75.Citation2 However, because the period of LEM intake was limited to 3 weeks in this study, the effect of LEM on the 5-year relapse-free survival rate is unknown. To verify the effect of LEM in FEC therapy on the 5-year relapse-free survival rate, it will be necessary to conduct a large-scale, controlled trial with longer-term LEM intake.
Although advances in chemotherapy are being made today for many types of malignant disease other than breast cancer, a therapeutic approach that also emphasizes maintenance of patient QOL during the course of chemotherapy is generally sought. In postoperative adjuvant chemotherapy especially, the maintenance of QOL is a matter that should be given priority. Attempts are being made to maintain QOL in various types of cancer, including pancreatic cancer,Citation23 colon cancer,Citation24 and lung cancer,Citation25 but as yet there is no reliable method. Further investigation of the possibility that LEM is an adjuvant that will improve QOL and immune function in postoperative adjuvant chemotherapy for these cancers is therefore warranted.
In conclusion, FEC therapy in breast cancer patients with lymph node metastases after surgery can exacerbate QOL and immunity of the patients. Concomitant use of oral LEM is expected to reduce FEC therapy-related impairment of QOL and immune depression. LEM may therefore be a useful oral adjuvant to anthracycline-based chemotherapies. In addition, a larger-scale controlled study is required.
Acknowledgments
This study received a research grant from the Osaka Cancer Research Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trialsLancet200536594721687171715894097
- French Adjuvant Study GroupBenefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French Adjuvant Study Group 05 randomized trialJ Clin Oncol200119360261111157009
- RiccardiABrugnatelliSGiordanoMMyeloprotective effect of early primary granulocyte-colony stimulating factor during six courses of intensified 5-fluorouracil, epirubicin and cyclophosphamide (120FEC) chemotherapy for advanced breast cancer. Cooperative Group of Study and Treatment of Breast CancerTumori19988455405469862513
- BaltaliEGunelNOnatDANeoadjuvant chemotherapy in locally advanced breast cancer: a preliminary report. Turkish Oncology Study GroupTumori199985648348710774570
- KornblithABLanLArcherLQuality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: a companion study to cancer and leukemia group B 49907J Clin Oncol20112981022102821300923
- MartinMLluchASeguiMAToxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimenAnn Oncol20061781205121216766587
- ApetohLGhiringhelliFTesniereAToll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapyNat Med20071391050105917704786
- MozaffariFLindemalmCChoudhuryANK-cell and T-cell functions in patients with breast cancer: effects of surgery and adjuvant chemo- and radiotherapyBr J Cancer200797110511117551492
- KawanishiTIkeda-DantsujiYNagayamaAEffects of two basidiomycete species on interleukin 1 and interleukin 2 production by macrophage and T cell linesImmunobiology2010215751652019913939
- SuganoNChojiYHibinoYAnticarcinogenic action of an alcohol-insoluble fraction (LAP1) from culture medium of Lentinus edodes myceliaCancer Lett1985271164039972
- SuganoNHibinoYChojiYMaedaHAnticarcinogenic actions of water-soluble and alcohol-insoluble fractions from culture medium of Lentinus edodes myceliaCancer Lett19821721091146891904
- LiuMLiJKongFLinJGaoYInduction of immunomodulating cytokines by a new polysaccharide-peptide complex from culture mycelia of Lentinus edodesImmunopharmacology19984031871989858062
- TanakaKMatsuiYIshikawaSKawanishiTHaradaMOral ingestion of Lentinula edodes mycelia extract can restore the antitumor T cell response of mice inoculated with colon-26 cells into the subserosal space of the cecumOncol Rep201227232533222086364
- TanakaKIshikawaSMatsuiYTamesadaMHarashimaNHaradaMOral ingestion of Lentinula edodes mycelia extract inhibits B16 melanoma growth via mitigation of regulatory T cell-mediated immunosuppressionCancer Sci2011102351652121261790
- YamaguchiYMiyaharaEHiharaJEfficacy and safety of orally administered Lentinula edodes mycelia extract for patients undergoing cancer chemotherapy: a pilot studyAm J Chin Med201139345145921598414
- OkunoKUnoKEfficacy of orally administered Lentinula edodes mycelia extract for advanced gastrointestinal cancer patients undergoing cancer chemotherapy: a pilot studyAsian Pac J Cancer Prev20111271671167422126542
- National Cancer Institute, U.S.:Common Terminology Criteria for Adverse Events V2.0 [Publish Date April 30, 1999]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf
- KuriharaMShimizuHTsuboiKDevelopment of quality of life questionnaire in Japan: quality of life assessment of cancer patients receiving chemotherapyPsychooncology19998435536310474853
- LindqvistCAChristianssonLHSimonssonBEnbladGOlsson-StrömbergULoskogAST regulatory cells control T-cell proliferation partly by the release of soluble CD25 in patients with B-cell malignanciesImmunology2010131337137620518821
- MocchegianiECiavattiniASantarelliLRole of zinc and alpha2 macroglobulin on thymic endocrine activity and on peripheral immune efficiency (natural killer activity and interleukin 2) in cervical carcinomaBr J Cancer19997922442509888464
- ArchiliCLissoniPCattaneoGLack of changes in soluble interleukin-2 receptor serum levels during chemotherapy-induced lymphocyte damageInt J Biol Markers19905143452172411
- MurtaEFde AndradeJMFalcãoR PBighettiSLymphocyte subpopulations in patients with advanced breast cancer submitted to neoadjuvant chemotherapyTumori200086540340711130570
- ToyamaYYoshidaSSaitoRSuccessful adjuvant bi-weekly gemcitabine chemotherapy for pancreatic cancer without impairing patients’ quality of lifeWorld J Surg Oncol201311323302293
- LinKYShunSCLaiYHLiangJTTsauoJYComparison of the effects of a supervised exercise program and usual care in patients with colorectal cancer undergoing chemotherapyCancer Nurs [Epub January 25, 2013]
- StigtJAUilSMvan RiesenSJA randomized controlled trial of postthoracotomy pulmonary rehabilitation in patients with resectable lung cancerJ Thorac Oncol20138221422123238118