Abstract
Purpose
The present study aims to investigate whether the combination treatment of cordycepin (an extracted pure compound from Cordyceps sinensis) and cisplatin (a platinum-based chemotherapy drug) has better apoptotic effect in head and neck squamous cell carcinoma (HNSCC).
Methods
The apoptotic influences of cordycepin and/or cisplatin treatments to human OC3, OEC-M1, and FaDu HNSCC cells were investigated by morphological observations, viability assay, flow cytometry assay, and Western blotting methods.
Results
Data showed that the cell death phenomenon increased as the dosage of cordycepin or cisplatin increased, and it appeared more in cordycepin plus cisplatin cotreatment among three cell lines. Cell survival rates significantly decreased as the dosage of cordycepin or cisplatin increased, and the better apoptotic effects were observed in cotreatment. Cell cycle analysis further demonstrated that percentages of subG1 cells in cordycepin or cisplatin treatments significantly increased, suggesting that cells underwent apoptosis, and cordycepin plus cisplatin induced many more subG1 cells. Furthermore, cordycepin or cisplatin induced caspase-8, caspase-9, caspase-3, and poly adenosine diphosphate-ribose polymerase protein cleavages, and stimulated c-Jun NH2-terminal kinase, extracellular signal-regulated kinase, and p38 protein phosphorylations. Moreover, cordycepin plus cisplatin cotreatment significantly activated those proteins with much better effects among three cell lines.
Conclusion
Cordycepin plus cisplatin have better apoptotic effect by activating caspase activation with possible MAPK pathway involvement in HNSCC cells.
Introduction
Betel quid-related oral cavity cancer is a unique type of head and neck squamous cell carcinoma (HNSCC) that occurs with an areca nut chewing habit, which is endemic in many areas around the world.Citation1 In Taiwan, there are over 2,000 deaths in oral cavity cancer yearly, and it is still increasing.Citation2 Surgery and radiation are often used to treat local advanced HNSCC,Citation3 but these treatments would damage a patient’s face and affect his or her salivary secretion and taste functions. For late-staged patients, chemotherapy is often used in combination with surgery and/or radiotherapy in order to improve the poor survival rate.Citation4 The addition of platinum-based chemotherapy, such as cisplatin (cis-DDP) or carboplatin (CBDCA), is the major agent in HNSCC treatment.Citation5 Cisplatin is the most efficient agent used to treat HNSCC; however, the development of cisplatin-resistance is the major limitation of treatment.Citation6 Studies have shown the possible mechanisms involved in cisplatin resistance, including the reduction of intracellular accumulation of the chemotherapy drug, the down-regulation of proapoptotic proteins, the increase of glutathione, and the upregulation of antiapoptotic proteins.Citation7
Cordycepin, a pure extracted compound of Cordyceps sinensis, has been shown to have antitumor properties as it activates cysteine aspartic-specific protease (caspase) pathways.Citation8,Citation9 It is reported that cordycepin could inhibit the formation of polyadenylate polymerase or inactivate messenger ribonucleic acid (RNA) polyadenylation to induce tumor cell apoptosis,Citation10 which is characterized by cellular rounding-up, cytoplasmic contraction, plasma membrane blebbing, chromatin condensation, and deoxyribonucleic acid (DNA) fragmentation.Citation11 During the course of apoptosis, the activation of caspases is commonly thought to be one of the earliest points in the no-return pathway of apoptosis.Citation12 In general, caspase can be divided into two groups: initiator caspases (including caspase-8, caspase-9, and caspase-10) and effector caspases (including caspase-3, caspase-6, and caspase-7). Initiator caspases are responsible for cleaving and activating effector caspases.Citation13 The cleavage of caspases, such as caspase-7 and caspase-3, could be activated, which will further cleave poly adenosine diphosphate-ribose polymerase (PARP), which is responsible for DNA repair,Citation12 and result in the execution of cell death.Citation14
Besides caspase cascades, mitogen-activated protein kinases (MAPKs) are also involved in apoptosis regulation.Citation15 MAPKs consist of three family membranes: extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p38 proteins.Citation16 Studies have been reported that stress signals can activate the stress-activated protein kinases/JNK protein kinases, which mediate cellular steps in the apoptosis of some cell types.Citation17,Citation18 It has been shown that ERK is response to growth stimuli is the important signal for anti-apoptosis;Citation16 however, the involvement of p38 in apoptosis is diverse. Phosphorylation of p38 can be initiated by MKK3 and MKK6 at the threonine and tyrosine regions, which control many transcriptional factors and kinases to enhance cell survival or prompt apoptosis.Citation16 Accordingly, caspase and MAPKs pathways may play important roles in the apoptosis of tumor cells activated by chemotherapy agents.
Cordycepin and cisplatin both have antitumor effects.Citation6,Citation8,Citation9,Citation19 Thus, the attempt to clarify the combined effect of cisplatin plus cordycepin on HNSCC cell death in addition to an investigation of the underlying mechanisms is being conducted in the present study. Three cell lines, OC3, OEC-M1, and FaDu cells, were used in the investigation. It should be noted that better effects in OC3, OEC-M1, and FaDu cells on apoptosis by cordycepin plus cisplatin were observed. These findings could encourage the development of more effective chemotherapy agents with different concomitant administration against betel nut-induced oral cancers.
Materials and methods
Chemicals
Cordycepin, cisplatin, penicillin-streptomycin, methylthiazol tetrazolium (MTT), dimethyltetrazolium bromide (DMSO), ribonuclease A, and propidium iodine (PI) were purchased from Sigma-Aldrich (St Louis, MO, USA). Fetal bovine serum, Dulbecco’s Modified Eagle’s Medium (DMEM), and Keratinocyte-SFM medium were purchased from Gibco® (Life Technologies, Carlsbad, CA, USA). Sodium hydroxide was purchased from Merck KGaA (Darmstadt, Germany). In addition, (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES) was purchased from Mallinckrodt Baker, Inc, (Phillipsburg, NJ, USA). Sodium bicarbonate, sodium carbonate, and sodium chloride were purchased from Riedel der Haen (Seelze, Germany). Fetal bovine serum, Roswell Park Memorial Institute 1640 medium, and lyophilized trypsin-ethylenediaminetetraacetic acid were purchased from Gibco® (Life Technologies). Tween 20, sodium hydroxide, and hydrochloric acid were purchased from Merck KGaA. Sodium dodecyl sulfate (SDS) and acrylamine, as well as Tris (base) were purchased from JT Baker® (Avantor™ Performance Materials, Phillipsburg, NJ, USA). Antibodies against cleaved caspase-8, caspase-9, caspase-3, and β-actin, as well as antibodies against phosphorylated-JNK, phosphorylated-ERK, and phosphorylated-p38 were purchased from Cell Signaling Technology, Inc, (Beverly, MA, USA). Anti-PARP antibody was purchased from Oncogene Research Products (San Diego, CA, USA).
Cell lines and cell culture
Three HNSCC cell lines – OC3 (established from an oral squamous cell carcinoma [OSCC] in a long-term areca [betel] chewer who does not smoke), OEC-M1 (derived from gingival epidermal carcinoma), and FaDu (a human pharyngeal squamous cell carcinoma) – were used in the experiments. It should be noted that OC3 and OEC-M1 cells are indigenous oral cavity cancer cell lines in Taiwan.Citation20 OC3 cells were maintained in DMEM in addition to a twofold volume of keratinocyte-SFM mixed medium supplemented with 24 mM of NaHCO3, 25 mM of HEPES, 100 ppm of penicillin, 100 ppm of streptomycin, and 10% v/v heat-inactivated fetal bovine serum; pH7.4. OEC-M1 cells were maintained in Roswell Park Memorial Institute 1640 medium supplemented with 24 mM of NaHCO3, 25 mM of HEPES, 10,000 U of penicillin, 10,000 U of streptomycin, and 10% heat-inactivated fetal bovine serum; and pH 7.4Citation21 FaDu cells were cultured in high-glucose DMEM supplemented with 10% fetal bovine serum and 0.1% penicillin/streptomycin. Cells were incubated in a humidified atmosphere containing 95% air and 5% CO2 at 37°C.Citation20,Citation21
MTT cell viability test
An MTT assay was employed to determine cell viability with the treatment of cordycepin and/or cisplatin. OC3 and OEC-M1 cells were seeded in a 96-well plate (Techno Plastic Products AG, Trasadingen, Switzerland) with 1 × 104 cells in 100 μL of serum medium among each well, and FaDu cells were seeded in a 96-well plate with 8 × 103 cells in 100 μL of serum medium among each well. After reaching 70%–80% confluence, cells were treated without or with cordycepin, cisplatin, or both agents in various combinations of concentrations (10 μM, 100 μM, or 1 mM of cordycepin alone; 30 μM, 300 μM, or 600 μM of cisplatin alone; and 300 μM or 600 μM of cisplatin combined with 100 μM of cordycepin, respectively, for 24 hours). MTT was added with a final concentration of 0.5 mg/mL, and then incubated for 4 hours at 37°C. The medium was removed and DMSO (50 μL) was added into each well to dissolve the crystals by gently shaking the plate for 20 minutes in the dark. The absorbance (optical density) values in each treatment were then determined at λ = 590 nm by an enzyme-linked immunosorbent assay microplate reader (VersaMax, Nordion, Ottawa, ON, Canada).
Morphological study
OC3 and OEC-M1 cells were seeded at a concentration of 6 × 105 cells and FaDu cells were seeded at a concentration of 4.5 × 105 cells in a 6 cm Petri dish (Techno Plastic Products AG) supplemented with 2 mL of serum medium. After reaching 70%–80% confluence, cells were treated without or with 100 μM cordycepin, 300 μM cisplatin, 600 μM cisplatin, or 100 μM cordycepin combined with 300 μM or 600 μM cisplatin for 24 hours, respectively. Cell morphology was then observed and recorded under light microscopy (Olympus CK40; Olympus Corporation, Tokyo, Japan).
Flow cytometry analysis
In order to investigate whether cordycepin and/or cisplatin could induce cell apoptosis, flow cytometric analysis was used with PI stain to determine both DNA fragmentation and the redistribution of the cell cycle. OC3 and OEC-M1 cells were seeded in a 6 cm Petri dish with 2 mL of serum medium, which contained 6 × 105 cells, while FaDu cells were seeded at a concentration of 4.5 × 105 cells. After reaching 70%–80% confluence, cells were treated with or without 100 μM of cordycepin only; 300 μM or 600 μM of cisplatin only; or 100 μM of cordycepin combined with 300 μM or 600 μM of cisplatin for 24 hours, respectively. The treated cells were harvested with trypsin, washed with phosphate buffered saline (PBS), and fixed in 75% ethanol for at least 2 hours at −20°C. After fixation, cells were washed in cold PBS and then collected by centrifugation and stained with PI solution (40 μg/mL of PI and 100 μg/mL of ribonuclease in PBS). The stained cells were analyzed using a fluorescence activated cell sorter (FACScan™; BD Biosciences, San Jose, CA, USA) at λ = 488 nm and analyzed by CellQuest™ software (BD Biosciences). The DNA content distribution of normal growing cells is characterized by two peaks phenomenon; G1/G0 and G2/M phases. The G1/G0 phase indicates that cells are arrested at the resting state of the cell cycle with the most diploid DNA content, while cell DNA content in the G2/M phase increases as a consequence of progressing in cell cycle. Cells in the subG1 phase have the least amount of DNA content in the cell cycle distribution, which is called hypodiploid. The hypodiploid DNA content represents the fragmentation of DNA, indicating cell apoptosis.Citation17
Immunoblotting analysis
Cells were lysed and protein extraction was performed. Protein concentration of the cell lysates was determined by the Lowry et al method.Citation22 Cell proteins (30 μg) were separated in 12% of SDS-polyacrylamide gel, which performed at 100 V for 2 hours using a standard running buffer (24 mM Tris-HCl, 0.19 M glycine, 0.5% SDS, pH8.3), and the proteins were electrophoretically transferred to a polyvinylidene difluoride membrane at 400 mA for 2 hours in transfer buffer (20 mM Tris-HCl, 150 mM glycine, 10% methanol, and 0.01% SDS). The membranes were blocked with 4% nonfat milk, washed and subsequently incubated with a specific antibody. After washing, the membrane was incubated with horseradish peroxidase-conjugated goat antimouse antibody or goat antirabbit antibody, and then visualized by an enhanced chemiluminescence detection kit (Amersham-Pharmacia International PLC, Amersham, UK). The optical density of each protein band was quantitated using a computer-assisted image analysis system (Quantity One, Huntington Station, NY, USA).Citation23 The amount of β-actin (43 kDa) in each lane was also detected as a control.
Statistics
Each data point of the bar in the figures represents the mean ± standard error of the mean of three separate experiments. Statistically significant differences between treatments and controls were determined by one-way analysis of variance, and then Tukey’s test was used for post hoc testing. Statistical significance was set at P<0.05.
Results
Effect of cordycepin and/or cisplatin on morphological change in HNSCC cell lines
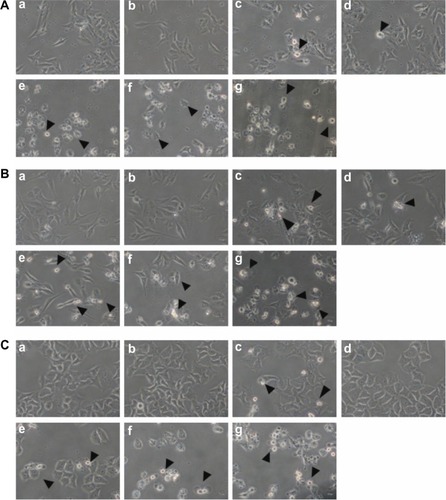
OC3, OEC-M1, and FaDu cells were treated with 100 μM of cordycepin only; 300 μM or 600 μM of cisplatin only; or 100 μM of cordycepin combined with 300 μM or 600 μM of cisplatin for 24 hours, respectively, and morphological changes related to cell death were examined under light microscopy. Among the three cell lines, cells in the control and 0.5% DMSO treatments showed a polygonal shape with a healthy appearance, which is a normal cell growth phenomenon (, , , , , and ). Twenty-four hours after treatment with 100 μM of cordycepin and 300 μM of cisplatin, cells appeared to be rounded-up, but they still adhered to the ground matrix (, , , , , and ). After 24 hours of treatment with 600 μM of cisplatin, many cells rounded up, with some floating in medium (, , and ). A combination of 100 μM of cordycepin plus 300 μM or 600 μM of cisplatin treatments for 24 hours resulted in a greater loss of cell attachment to ground matrix, more appearance of membrane blebbings, and more floating cells (, , , , , and ). These phenomena suggested that a combination of cordycepin plus cisplatin treatments induced apoptotic cell death in all three cell lines, which was more effective than cordycepin or cisplatin alone.
Figure 1 Effect of cordycepin and/or cisplatin on morphological change in HNSCC cell lines.
Abbreviations: HNSCC, head and neck squamous cell carcinoma; DMSO, dimethyltetrazolium bromide.
Effects of cordycepin and/or cisplatin on cell viability in HNSCC cell lines
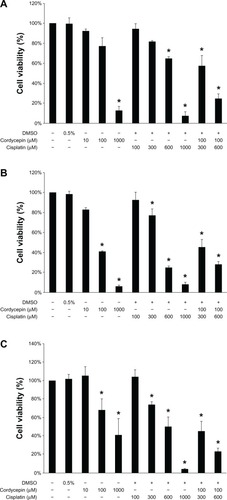
HNSCC cell morphological changes suggested that cordycepin and/or cisplatin might be involved in cell death. The MTT viability test was used to further examine cell viability. In the OC3 cell line, the cell survival rate with a treatment of 100 μM of cordycepin alone was 77%; 300 μM or 600 μM of cisplatin alone showed cell survival rates of 82% and 65%, respectively; and cordycepin (100 μM) plus cisplatin (300 μM or 600 μM) showed cell survival rates of 52% and 58%, respectively, which demonstrated that the cordycepin plus cisplatin cotreatment could significantly reduce the cell survival rate () (P<0.05). In fact, the cordycepin (100 μM) plus cisplatin (600 μM) treatment illustrated a synergistic cell death phenomenon (). In the OEC-M1 cell line, the cell survival rate with a treatment of 100 μM of cordycepin alone was 59%; the cell survival rates with 300 μM or 600 μM of cisplatin alone were 77% and 25%, respectively; and cell survival rates with cordycepin (100 μM) plus cisplatin (300 μM or 600 μM) were 45% and 28%, respectively, which demonstrated that the combination of cordycepin (100 μM) plus cisplatin (300 μM) could significantly reduce the cell survival rate () (P<0.05). In the FaDu cell line, the cell survival rate with the 100 μM of cordycepin treatment alone was 68%; the cell survival rates with 300 μM or 600 μM of cisplatin alone were 74% and 50%, respectively; and the cell survival rates with the cordycepin (100 μM) plus cisplatin (300 μM or 600 μM) treatments were 45% and 23%, respectively, which demonstrated that the combination of cisplatin plus cordycepin could significantly reduce the cell survival rate () (P<0.05).
Figure 2 Effects of cordycepin and/or cisplatin on cell viability in HNSCC cell lines.
Abbreviations: DMSO, dimethyltetrazolium bromide; HNSCC, head and neck squamous cell carcinoma; MTT, methylthiazoletetrazolium.
Effects of cordycepin and/or cisplatin on cell cycle in HNSCC cell lines
To further investigate whether cordycepin and/or cisplatin could induce apoptosis, OC3, OEC-M1, and FaDu cells were examined by flow cytometry analysis to determine whether DNA fragmentation occurred, and whether there was any change in cell cycle progression. Distribution of the subG1, G1, and G2/M phase cells among the OC3, OEC-M1, and FaDu cells with different treatments were illustrated in –, respectively. The results showed that cordycepin (100 μM) plus cisplatin (300 μM or 600 μM) could notably induce more subG1 phase cells among the three cell lines (–). In order to elucidate the changes of the subG1, G1, and G2/M phase cells between different treatments, the cell number percentage among the OC3, OEC-M1, and FaDu cells from – were statistically analyzed and illustrated in –, respectively.
Figure 3 The analysis of cell cycle under cordycepin and/or cisplatin influence in HNSCC cell lines.
Abbreviations: DMSO, dimethyltetrazolium bromide; HNSCC, head and neck squamous cell carcinoma; DNA, deoxyribonucleic acid.
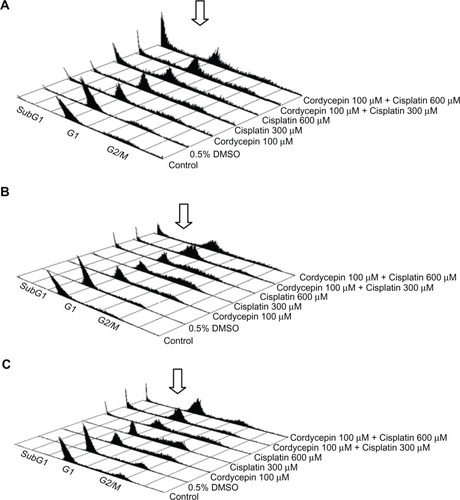
Figure 4 Quantification and analysis in percentage among subG1, G1, and G2/M phase cell number in HNSCC cell lines.
Abbreviations: HNSCC, head and neck squamous cell carcinoma; DMSO, dimethyltetrazolium bromide.
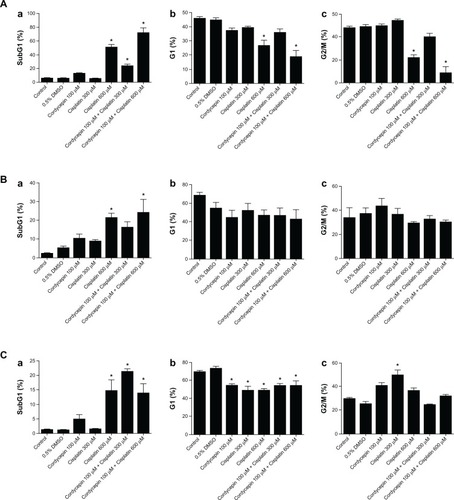
The percentage of subG1 phase cells in the control groups was 5% in the OC3 cells (), 2% in the OEC-M1 cells (), and 1% in the FaDu cells (), respectively. Cordycepin (100 μM) induced subG1 phase cells to 12% in the OC3 cells (), 11% in the OEC-M1 cells (), and 5% in the FaDu cells (), respectively. In treatment with cisplatin alone, 300 μM of cisplatin induced the subG1 phase cells to 5% in the OC3 cells (), 10% in the OEC-M1 cells (), and 2% in the FaDu cells (), respectively. However, 600 μM of cisplatin induced the subG1 phase cells to 52% in the OC3 cells (), 23% in the OEC-M1 cells (), and 15% in the FaDu cells (), respectively. Interestingly, cordycepin (100 μM) plus cisplatin (300 μM) or cordycepin (100 μM) plus cisplatin (600 μM) cotreatments induced the subG1 phase cells to 27% and 73% in the OC3 cells (), 17% and 24% in the OEC-M1 cells (), and 23% and 14% in the FaDu cells (), respectively. These data illustrated that cordycepin plus cisplatin cotreatments had a better apoptotic effect in the subG1 phase among the three cell lines. It should be noted that cordycepin plus cisplatin (300 μM or 600 μM, respectively) in the OC3 cells and cordycepin plus cisplatin (300 μM) in the FaDu cells did demonstrate a much better apoptotic effect in the subG1 phase.
Effects of cordycepin and/or cisplatin on caspase pathway in HNSCC cell lines
Previous results illustrated that cordycepin and/or cisplatin would cause cell apoptosis among OC3, OEC-M1, and FaDu cells. Thus, the expressions of cleavage caspase and PARP proteins were investigated.
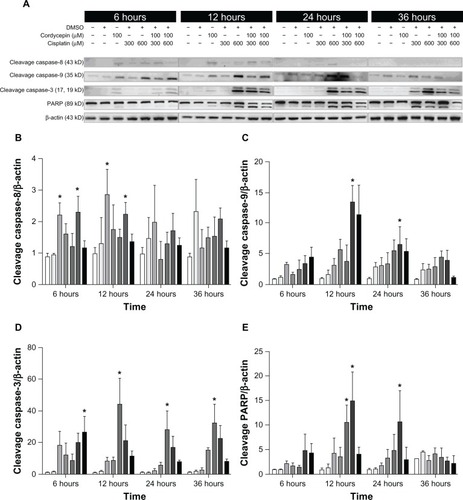
The expression of caspase-8 cleavage in the three cell lines under control and DMSO treatments was very low, and was slightly increased by treatment with cordycepin or cisplatin alone (300 μM or 600 μM, respectively) from 6 hours to 36 hours. However, cordycepin plus cisplatin cotreatment significantly induced more caspase-8 cleavage between 6 hours to 24 hours among OC3, OEC-M1, and FaDu cells (, , , , and ) (P<0.05). The expression of cleavage caspase-9 in the three cell lines under the control and DMSO treatments was low, and it was slightly increased by treatment with cordycepin or cisplatin alone (300 μM or 600 μM, respectively) from 6 hours to 24 hours. However, the cordycepin plus cisplatin cotreatment significantly induced more caspase-9 cleavage between 6 hours to 24 hours among OC3, OEC-M1, and FaDu cells (, , , , and ) (P<0.05). In addition, the expression of cleavage caspase-3 in these three cell lines under control and DMSO treatments was very low, and it was slightly increased by treatment with cordycepin or cisplatin alone (300 μM or 600 μM, respectively) from 6 hours to 36 hours. However, the cordycepin plus cisplatin cotreatment significantly induced more caspase-3 cleavage between 6 hours to 12 hours in OC3 and FaDu cells (, , , , and ) (P<0.05). Moreover, there was no cleavage of PARP among the three cell lines under control and DMSO treatments, and PARP cleavage slightly increased by treatment with cordycepin or cisplatin alone (300 μM or 600 μM, respectively) from 6 hours to 36 hours. However, the cordycepin plus cisplatin cotreatment significantly induced more PARP cleavage between 6 hours to 24 hours among OC3, OEC-M1, and FaDu cells (, , , , and ) (P<0.05). It should be noted that there were different levels of sensitivity among the caspase pathway activated by cordycepin and/or cisplatin between the different cell lines.
Figure 5 Effects of cordycepin and/or cisplatin on caspase-8, caspase-9, caspase-3, and PARP protein expressions in OC3 cells.
Abbreviations: DMSO, dimethyltetrazolium bromide; PARP, poly adenosine diphosphate-ribose polymerase.
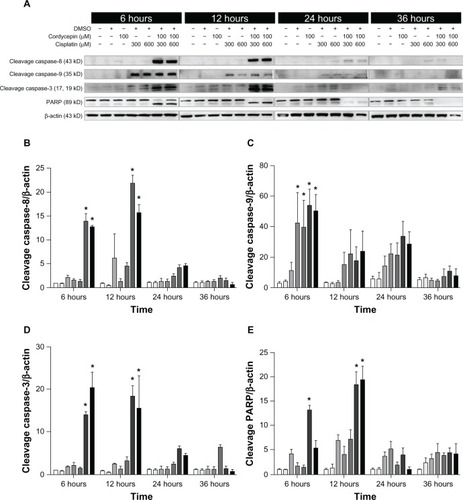
Figure 6 Effects of cordycepin and/or cisplatin on caspase-8, caspase-9, caspase-3, and PARP protein expressions in OEC-M1 cells.
Abbreviations: DMSO, dimethyltetrazolium bromide; PARP, poly adenosine diphosphate-ribose polymerase.
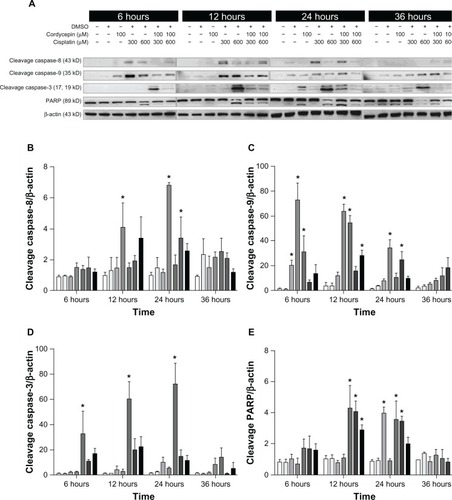
Figure 7 Effects of cordycepin and/or cisplatin on caspase-8, caspase-9, caspase-3, and PARP protein expressions in FaDu cells.
Abbreviations: DMSO, dimethyltetrazolium bromide; PARP, poly adenosine diphosphate-ribose polymerase.
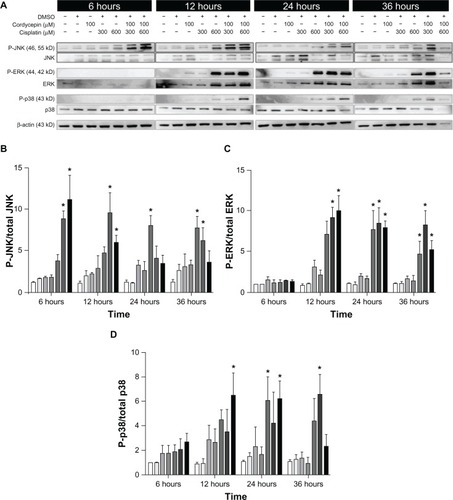
Effects of cisplatin and/or cordycepin on the regulation of MAPK pathway in HNSCC cell lines
Studies have shown that the phosphorylation of the MAPK pathway could either positively or negatively regulate cell mitosis, proliferation, and apoptosis.Citation16,Citation24 To determine whether cordycepin- and/or cisplatin-induced HNSCC cell apoptosis would be mediated by the MAPK pathway, the phosphorylation of JNK, ERK1/2, and p38 among the OC3, OEC-M1, and FaDu cells were analyzed by Western blotting.
The expression of the phosphor-JNK protein in three cell lines under the control and DMSO treatments was very low, and slightly increased by treatment with cordycepin or cisplatin alone (300 μM or 600 μM, respectively) from 6 hours to 36 hours. However, the cordycepin plus cisplatin cotreatment significantly induced more phosphor-JNK protein between 6 hours to 36 hours among the OC3, OEC-M1, and FaDu cells (, , , , and ) (P<0.05). In addition, the expression of the phosphor-ERK protein in the three cell lines under the control and DMSO treatments was very low, and slightly increased by treatment with cordycepin or cisplatin alone (300 μM or 600 μM, respectively) from 6 hours to 36 hours. However, the cordycepin plus cisplatin cotreatment significantly induced more phosphor-ERK protein between 6 hours to 36 hours among the OC3, OEC-M1, and FaDu cells (, , , , and ) (P<0.05). Furthermore, the expression of the phosphor-p38 protein in the three cell lines under the control and DMSO treatments was very low, and slightly increased by treatment with cordycepin or cisplatin alone (300 μM or 600 μM, respectively) from 6 hours to 36 hours. However, the cordycepin plus cisplatin cotreatment significantly induced more phosphor-p38 protein between 12 hours to 36 hours among the OC3, OEC-M1, and FaDu cells (, , , , and ) (P<0.05). It should be recognized that there were different levels of sensitivity among the MAPK pathway, which were activated by cordycepin and/or cisplatin between the different cell lines.
Figure 8 Effects of cordycepin and/or cisplatin on the protein expression of the MAPK pathway in OC3 cells.
Abbreviations: DMSO, dimethyltetrazolium bromide; P, phosphorylated; JNK, Jun NH2-terminal kinase; ERK, signal-regulated kinase; MAPK, mitogen-activated protein kinases.
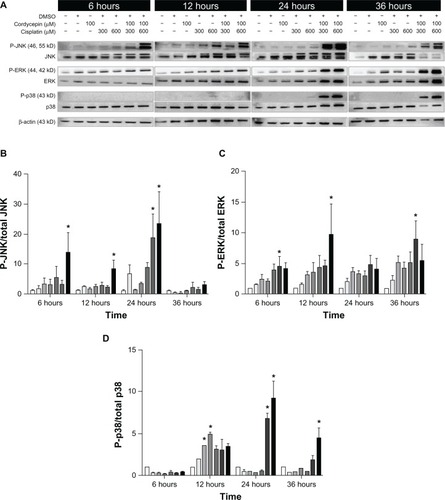
Figure 9 Effects of cordycepin and/or cisplatin on the protein expression of the MAPK pathway in OEC-M1 cells.
Abbreviations: DMSO, dimethyltetrazolium bromide; P, phosphorylated; JNK, Jun NH2-terminal kinase; ERK, signal-regulated kinase; MAPK, mitogen-activated protein kinases.
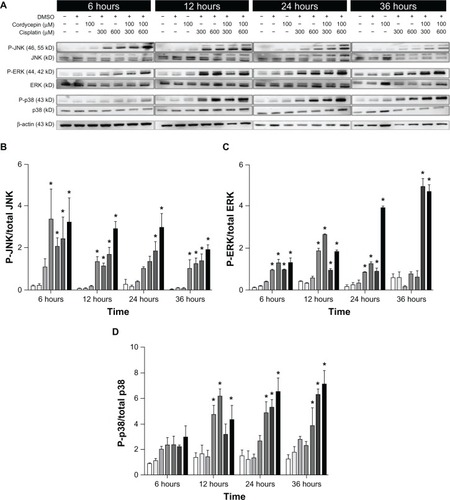
Figure 10 Effects of cordycepin and/or cisplatin on the protein expression of MAPK pathway in FaDu cells.
Abbreviations: DMSO, dimethyltetrazolium bromide; P, phosphorylated; JNK, Jun NH2-terminal kinase; ERK, signal-regulated kinase; MAPK, mitogen-activated protein kinases.
Discussion
Betel quid chewing is a popular oral habit with potential links to the occurrence of oral cancer.Citation25 In Taiwan, the incidence of oral cancer has increased in recent years.Citation2 Cisplatin is one of the most well-known potent antitumor agents, displaying clinical activity against a wide variety of solid tumors.Citation26 Studies have shown that cisplatin combined with other anticancer drugs can enhance more cell death.Citation6 It has been reported that cordycepin could effectively inhibit tumor cell growth concomitant with the induction of apoptotic and/or autophagic cell death in breast cancer cells, Leydig tumor cells, and oral cavity cancer cells;Citation9,Citation19,Citation27 thus, the combination of different chemicals might be more effective in treating cancer cells.Citation28 In fact, we have just reported that treatment with cordycepin plus cisplatin combined could cause a better effect of cell death in OC3 cells through a very preliminary mechanism investigation.Citation29 In the present study, we used three oral cavity cancer cells lines (OC3, OEC-M1, and FaDu) to further investigate the combined apoptotic effect of cordycepin plus cisplatin with a detailed examination of the cellular mechanisms.
Our data showed that treatment with cordycepin or cisplatin alone induced the cell death phenomenon with the loss of cell attachment to the ground matrix, the appearance of membrane blebbings, and floating cells. Cordycepin plus cisplatin cotreatments induced more cell death, which showed a greater effect on three HNSCC cell lines. In fact, similar results have been illustrated on other oral cavity cancer cell lines, and the same appearance induced by cisplatin can also be found on other tumor cells.Citation7,Citation28 Indeed, other studies have shown that many factors could activate different cellular pathways and can respond to enhance cisplatin antitumor effects; for instance, luteolin enhanced p53 stabilization and accumulation,Citation30 and dexamethasone enhanced angiostatic activity and modulating cell cycle kinetics in different cell types.Citation31 Our study showed that cordycepin could induce a greater expression of caspase and the MAPK protein to enhance the antitumor effect of cisplatin in HNSCC cells. Hence, our observations are parallel to those studies. Moreover, cell viability results showed that the cordycepin plus cisplatin cotreatment could induce more cell death among the three HNSCC cell lines as compared to cordycepin or cisplatin treatment alone, and an enhanced effect could be observed in the OC3 cells. Interestingly, the amount of cell death among the OEC-M1 and FaDu cells affected by cordycepin or cisplatin was higher than in the OC3 cells, which demonstrated that there were different sensitivities among the three HNSCC cell lines to cordycepin and/or cisplatin. In fact, these phenomena are also found in other studies.Citation32,Citation33
In the cell cycle analysis, the percentage of subG1 phase cells increased to 11%–12% in the OC3 and OEC-M1 cells after treatment with cordycepin alone, which demonstrated greater efficiency than in the FaDu cells (only 5%). The percentage of subG1 phase cells significantly increased to 54% after treatment with 600 μM of cisplatin alone in the OC3 cells, and increased to 15%–20% in the OCE-M1 and FaDu cells, respectively. Exclusively, the percentage of subG1 phase cells in the cordycepin (100 μM) plus cisplatin (300 μM) cotreatment group significantly increased in all three cell lines. It should be noted that cordycepin plus cisplatin (300 μM and 600 μM, respectively) in the OC3 cells and cordycepin plus cisplatin (300 μM) in the FaDu cells did demonstrate a synergistically apoptotic effect in the subG1 phase. In fact, the synergistic/additive effect of cisplatin plus other drugs has been demonstrated in nasopharyngeal cancer (NPC) cell lines (NPC-TW01 and NPC-TW04), human HNSCC cell line SCC25, and human epidermoid carcinoma A431 cells.Citation34,Citation35 It has also been shown that a combination of different agents could induce more antitumor efficiency;Citation36 thus, our findings are consistent with those investigations.
In the present study, the cordycepin plus cisplatin cotreatment significantly induced greater expressions of cleavage caspase-3 and PARP compared to treatment with cordycepin or cisplatin alone. We further investigated whether the caspase-8 (extrinsic) and/or caspase-9 (intrinsic) pathways would be activated. Results showed that both extrinsic and intrinsic caspase pathways were activated by the cordycepin plus cisplatin cotreatment. Many studies have shown the synergistic effect on caspase and/or PARP protein cleavages to induce apoptosis among different tumor cell types.Citation37 Therefore, our results are not unprecedented. It should be noted that the better effects in caspase-8, caspase-3, and PARP cleavages by cordycepin plus cisplatin cotreatment could be observed in OC3, but not in the OEC-M1 and FaDu cells. Also, it should be recognized that there are different levels of sensitivity among the caspase pathway activated by cordycepin and/or cisplatin between the OC3, OEC-M1, and FaDu cells.
In our observations, the cordycepin alone treatment slightly increased the expression of the MAPK proteins. However, cordycepin plus cisplatin induced a greater expression of phosphorylated-JNK and phosphorylated-ERK among the OC3, OEC-M1, and FaDu cells. The phosphorylated-p38 could be hardly detected in the OC3 and FaDu cells, but could be observed in the OEC-M1 cells from 6 hours to 12 hours after combined treatment. A higher dosage of the combination showed a greater expression of phosphorylated-p38. These findings suggest that cordycepin and/or cisplatin could activate the phosphorylation of JNK and ERK proteins to induce HNSCC cell apoptosis; however, only the cordycepin plus cisplatin combinations could activate the phosphorylation of the p38 protein to induce HNSCC cell apoptosis. It should be noted that the greater effect of phosphorylated-JNK, phosphorylated-ERK, and phosphorylated-p38 protein expressions could be observed by cordycepin plus cisplatin (300 μM) among the OC-3, OEC-M1, and FaDu cells. Also, it should be noted that there were different levels of sensitivity among the MAPK pathway activated by cordycepin and/or cisplatin between the OC-3, OEC-M1, and FaDu cell lines.
Conclusion
In conclusion, cordycepin and cisplatin possess better apoptotic effects by activating the expression of extrinsic and intrinsic caspase and MAPK pathways in human oral cavity cancer cell lines, which highly suggests that the combination treatment of cordycepin and cisplatin might be a potential anticancer drug when compared to the single agent chemotherapy.
Acknowledgments
This work was supported by National Science Council Grants NSC101-2320-B-006-005-MY3 (BMH), Taiwan.
Disclosure
The authors report no conflicts of interest in this work.
References
- GuptaPCWarnakulasuriyaSGlobal epidemiology of areca nut usageAddict Biol200271778311900626
- HoPSKoYCYangYHShiehTYTsaiCCThe incidence of oropharyngeal cancer in Taiwan: an endemic betel quid chewing areaJ Oral Pathol Med200231421321912076324
- ForastiereAAIs there a new role for induction chemotherapy in the treatment of head and neck cancer?J Natl Cancer Inst200496221647164915547172
- ForastiereAAGoepfertHMaorMConcurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancerN Engl J Med2003349222091209814645636
- AdelsteinDJLiYAdamsGLAn intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancerJ Clin Oncol2003211929812506176
- BoulikasTVougioukaMRecent clinical trials using cisplatin, carboplatin and their combination chemotherapy drugs (review)Oncol Rep200411355959514767508
- LiRZangYLiCPatelNSGrandisJRJohnsonDEABT-737 synergizes with chemotherapy to kill head and neck squamous cell carcinoma cells via a Noxa-mediated pathwayMol Pharmacol20097551231123919246337
- JenCYLinCYHuangBMLeuSFCordycepin induced MA-10 mouse Leydig tumor cell apoptosis through caspase-9 pathwayEvid Based Complement Alternat Med2011201198453719131393
- WuWCHsiaoJRLianYYLinCYHuangBMThe apoptotic effect of cordycepin on human OEC-M1 oral cancer cell lineCancer Chemother Pharmacol200760110311117031645
- ThomadakiHTsiapalisCMScorilasAPolyadenylate polymerase modulations in human epithelioid cervix and breast cancer cell lines, treated with etoposide or cordycepin, follow cell cycle rather than apoptosis inductionBiol Chem2005386547148015927891
- LallasGCCourtisNHavredakiMK562 cell sensitization to 5-fluorouracil- or interferon-alpha-induced apoptosis via cordycepin (3′-deoxyadenosine): fine control of cell apoptosis via poly(A) polymerase upregulationInt J Biol Markers2004191586615077928
- GuptaSMolecular steps of death receptor and mitochondrial pathways of apoptosisLife Sci20016925–262957296411758823
- ThornberryNALazebnikYCaspases: enemies withinScience19982815381131213169721091
- YangXChangHYBaltimoreDAutoproteolytic activation of pro-caspases by oligomerizationMol Cell1998123193259659928
- WangLGLiuXMKreisWBudmanDRThe effect of antimicrotubule agents on signal transduction pathways of apoptosis: a reviewCancer Chemother Pharmacol199944535536110501907
- WadaTPenningerJMMitogen-activated protein kinases in apoptosis regulationOncogene200423162838284915077147
- KimGYMercerSEEwtonDZYanZJinKFriedmanEThe stress-activated protein kinases p38 alpha and JNK1stabilize p21(Cip1) by phosphorylationJ Biol Chem200227733297922980212058028
- TohWHSiddiqueMMBoominathanLLinKWSabapathyKC-Jun regulates the stability and activity of the p53 homologue, p73J Biol Chem200427943447134472215302867
- PanBSLinCYHuangBMThe effect of cordycepin on steroidogenesis and apoptosis in MA-10 mouse Leydig tumor cellsEvid Based Complement Alternat Med2011750468
- LinSCLiuCJChiuCPChangSMLuSYChenYJEstablishment of OC3 oral carcinoma cell line and identification of NF-kappa B activation responses to areca nut extractJ Oral Pathol Med2004332798614720193
- MengCLYangCYShenKLWongPYLeeHKInhibition of the synthesis of eicosanoid-like substances in a human oral cancer cell line by interferon-gamma and eicosapentaenoic acidArch Oral Biol199843129799869877329
- LowryOHRosenbroughNJFarrALRandallRJProtein measurement with the Folin phenol reagentJ Biol Chem1951193126527514907713
- ChenYCHuangYLHuangBMCordyceps sinensis mycelium activates PKA and PKC signal pathways to stimulate steroidogenesis in MA-10 mouse Leydig tumor cellsInt J Biochem Cell Biol200537121422315381163
- PearsonGRobinsonFBeers GibsonTMitogen-activated protein (MAP) kinase pathways: regulation and physiological functionsEndocr Rev200122215318311294822
- JengJHChangMCHahnLJRole of areca nut in betel quid-associated chemical carcinogenesis: current awareness and future perspectivesOral Oncol200137647749211435174
- SiddikZHCisplatin: mode of cytotoxic action and molecular basis of resistanceOncogene200322477265727914576837
- ChoiSLimMHKimKMJeonBHSongWOKimTWCordycepin-induced apoptosis and autophagy in breast cancer cells are independent of the estrogen receptorToxicol Appl Pharmacol2011257216517321933677
- YoonCYParkMJLeeJSAbdullajanovMThe histone deacety-lase inhibitor trichostatin A synergistically resensitizes a cisplatin resistant human bladder cancer cell lineJ Urol201118531102111121255805
- ChenYHHaoLJHungCPChenJWLeuSFHuangBMApoptotic effect of cisplatin and cordycepin on OC3 human oral cancer cellsChin J Integr Med Epub412013
- ShiRHuangQZhuXLuteolin sensitizes the anticancer effect of cisplatin via c-Jun NH2-terminal kinase-mediated p53 phosphorylation and stabilizationMol Cancer Ther2007641338134717431112
- ArafaHMAbdel-HamidMAEl-KhoulyAAElmazarMMOsmanAMEnhancement by dexamethasone of the therapeutic benefits of cisplatin via regulation of tumor angiogenesis and cell cycle kinetics in a murine tumor paradigmToxicology20062221–210311316567030
- KaczirekKSchindlMWeinhäuselACytotoxic activity of camptothecin and paclitaxel in newly established continuous human medullary thyroid carcinoma cell linesJ Clin Endocrinol Metab20048952397240115126569
- PushkarevVMStarenkiDVSaenkoVAMolecular mechanisms of the effects of low concentrations of taxol in anaplastic thyroid canacer cellsEndocrinology200414573143315215044368
- ChanLPChouTHDingHYApigenin induces apoptosis via tumor necrosis factor receptor- and Bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatinBiochim Biophys Acta2012182071081109122554915
- HungSHLeeFPSuCHTsengHEffect of all-trans retinoic acid on the growth of two nasopharyngeal cancer cell lines and its treatment potential in combination with cisplatinEur Arch Otorhinolaryngol2013270269570422673737
- van den BroekGBWildemanMRaschCRMolecular markers predict outcome in squamous cell carcinoma of the head and neck after concomitant cisplatin-based chemoradiationInt J Cancer2009124112643265019253368
- SungESParkKJChoiHJKimCHKimYSThe proteasome inhibitor MG132 potentiates TRAIL receptor agonist-induced apoptosis by stabilizing tBid and Bik in human head and neck squamous cell carcinoma cellsExp Cell Res2012318131564157622513214