Abstract
We herein report a patient with advanced hepatitis B virus-related hepatocellular carcinoma (HCC) beyond the Milan criteria. He underwent orthotopic liver transplantation after successful HCC downstaging that satisfied the University of California, San Francisco criteria, using concurrent chemoradiation therapy with a combination of repeated hepatic arterial infusion chemotherapy (HAIC) and sorafenib. A 52-year-old male was diagnosed with advanced hepatitis B virus-related HCC beyond the Milan criteria. He underwent concurrent chemoradiation therapy (50 Gy with 20 fractions over 5 weeks with HAIC using 5-fluorouracil at a dose of 500 mg/day, which was administered during the first and fifth weeks of radiation therapy) as an initial treatment modality. This was followed by the combined use of HAIC using 5-fluorouracil (500 mg/m2 for 5 hours on days 1–3) and cisplatin (60 mg/m2 for 2 hours on day 2) every 4 weeks (twelve cycles) and sorafenib (from the third to the twelfth cycle of HAIC) to treat the remaining HCC. Because a remarkable decrease in the tumor burden that satisfied the University of California, San Francisco criteria was observed after these combination treatments, the patient underwent orthotopic liver transplantation with curative aim and survived for 11 months without evidence of HCC recurrence.
Introduction
Orthotopic liver transplantation (OLT) has long been the most appropriate treatment modality for patients with HCC combined with advanced cirrhosis, not only because the tumor can be removed, but because the cirrhotic liver, which is at risk for development of new lesions, can be replaced. However, OLT has been reserved for a specific population with early HCC, and the Milan criteriaCitation1 (single tumor up to 5 cm or up to three tumors, each no larger than 3 cm, without macrovascular invasion or extrahepatic spread) have been its selection criteria. These criteria predicted almost identical outcomes when compared with OLT performed in subjects without HCC.
However, recent studies have tried to expand the indication for OLT to overcome the donor shortage.Citation2–Citation5 First, some investigators have proposed more extended selection criteria, such as the University of California, San Francisco (UCSF) expanded criteria (solitary tumor of 6.5 cm in diameter, or three nodules with the largest diameter of 4.5 cm and a total tumor diameter of 8 cm)Citation2 and the up-to-7 criteria (HCC with 7 as the sum of the size [in cm] of the largest tumor and the number of tumors).Citation3 Furthermore, downstaging of HCC by decreasing the tumor size with locoregional therapies with or without systemic therapies to meet the acceptable criteria for OLT has also been attempted in patients with HCC above the Milan criteria.Citation4 Indeed, several meta-analyses have reported that the post-transplant 5-year survival rates for patients with downstaged tumors were slightly below or even identical to those achieved in patients with HCC initially meeting the Milan criteria.Citation5
We herein report a patient who initially had advanced hepatitis B virus (HBV)-related HCC beyond the Milan criteria. He underwent OLT after successful downstaging of the HCC that satisfied the UCSF criteria using concurrent chemoradiation therapy (CCRT) with the combined use of twelve cycles of hepatic arterial infusion chemotherapy (HAIC) and sorafenib.
Case report
A 52-year-old male presented to our hospital in November 2009 with a history of abdominal pain and weight loss that had been present for a few months. He had been first diagnosed as an HBV carrier 20 years previously. His baseline laboratory test results were as follows: platelet count, 220,000/μL; international normalized ratio, 0.95; serum albumin, 4.6 g/dL; aspartate aminotransferase, 61 IU/L; alanine aminotransferase, 44 IU/L; total bilirubin, 0.37 mg/dL; and alkaline phosphatase, 119 IU/L. Hepatitis B e antigen was positive, and the serum HBV DNA level was 1.60 × 106 IU/mL. The α-fetoprotein (AFP) level was 3690.65 IU/mL.
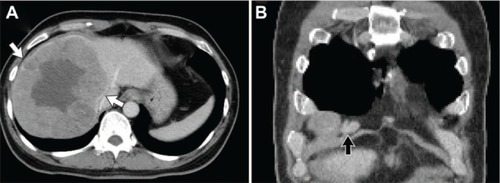
A computed tomography (CT) scan demonstrated findings typical of liver cirrhosis, an approximately 14.5 cm hypervascular mass in the right lobe of the liver without invasion of the middle hepatic vein, and an enlarged lymph node in the right anterior cardiophrenic space suggestive of metastasis in the lymph node ( and ). A huge liver mass with inner necrosis and intense peripheral fluorodeoxyglucose (FDG) uptake suggestive of high-grade HCC and borderline-size right cardiophrenic area also showed similar FDG uptake on positron emission tomography (PET) and CT. Finally, HCC was confirmed based on these typical radiological findings with elevated tumor markers.Citation6
Figure 1 Computed tomography demonstrated a cirrhotic liver with a 14.5 cm hepatocellular carcinoma (white arrow) in the right lobe (A) and an enlarged lymph node in the right anterior cardiophrenic area (black arrow, B).
Because the initial tumor stage of the patient was T2N1M0 (stage IVa) according to the modified American Joint Committee on Cancer/Union for International Cancer Control staging system in 2002,Citation7 the HCC was not resectable. Thus, the patient received CCRT from October 2009 (50 Gy with 20 fractions over 5 weeks with HAIC using 5-fuorouracil at a dose of 500 mg/day, which was administered during the first and fifth weeks of radiation therapy) via an implanted chemoport ().Citation8 One month after CCRT, the HCC decreased from 14.5 to 9.0 cm, and the metastatic lymph node in the right cardiophrenic area disappeared (partial response according to the modified Response Evaluation Criteria in Solid Tumors [RECIST]).Citation9 In addition, the AFP level markedly dropped from 3690.65 to 31.55 IU/mL.
Figure 2 Scheme of therapy protocol. After CCRT with subsequent combined use of HAIC and sorafenib, the patient underwent OLT.
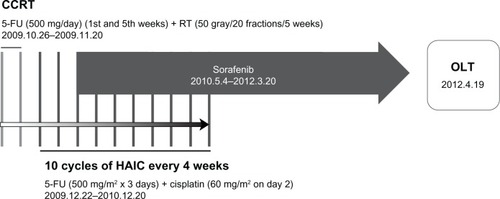
Because an intrahepatic tumor was still viable, HAIC using 5-fuorouracil (500 mg/m2 for 5 hours on days 1–3) and cisplatin (60 mg/m2 for 2 hours on day 2) were additionally administered and repeated every 4 weeks. After two cycles of HAIC following CCRT, a new 1.6 cm HCC lesion appeared. To prevent locoregional cancer progression and distant metastasis due to the potential resistance of HCC to HAIC, sorafenib (Nexavar; Bayer, Leverkusen, Germany) was additionally initiated in May 2010 (400 mg orally, twice daily), just after the completion of the second cycle of HAIC. The dose of sorafenib was reduced to 400 mg once daily because of grade 1 hand–foot syndrome (NCI CTCAE v3.0) after 9 weeks of sorafenib use.Citation10 A total of twelve cycles of HAIC following CCRT were completed by November 2010. However, sorafenib was maintained for an additional 2 years until the end of March 2012 ().
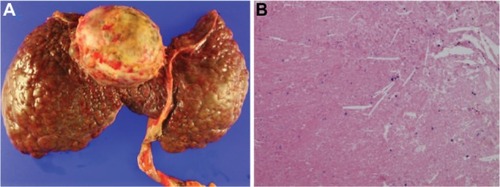
After the completion of 2 years of sorafenib monotherapy in March 2012, the size of the primary tumor had further decreased to 6.4 cm, and a suspicious HCC lesion that had developed after two cycles of HAIC had also disappeared (). In addition, a remarkable decrease in FDG uptake in the primary tumor was identified without visible distant metastasis on PET-CT (). The serum AFP level was maintained as normal, at 1.7 IU/mL. Although the treatment response was favorable, liver function was decompensated with a large amount of ascites and peripheral edema. At that time, the Model for End Stage Liver Disease score was 36.56, and the patient’s expected survival was less than 3 months.Citation11 Thus, the patient underwent OLT from an unrelated living donor in April 2012 (31 months from initial anticancer treatment) to cure the remaining HCC meeting the UCSF criteria and decompensated liver cirrhosis. Grossly, the extracted liver showed mixed macro- and micronodular cirrhotic changes (). Microscopic examination showed total necrosis of the HCC and fibrous capsule formation (). The non-tumorous liver revealed sinusoidal obstruction syndrome related to preoperative chemoradiation therapy and diffusely dysplastic change due to chronic HBV. There were no complications in the postoperative course, and the patient was discharged on postoperative day 29. As of March 2013, he had survived for more than 11 months after OLT without evidence of HCC recurrence.
Figure 3 Computed tomography scan before orthotopic liver transplantation demonstrated a decrease of the primary tumor to 6.4 cm with a large amount of ascites (A). A remarkable decrease in fluorodeoxyglucose uptake in the primary tumor was identified without visible distant metastasis on positron emission tomography and computed tomography (B).
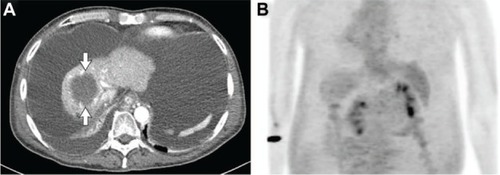
Figure 4 Macroscopic aspect and microscopic examination of liver specimen. Grossly, the liver showed macronodular cirrhotic change and a yellow necrotic mass (6.3 × 5.6 cm) at segment 4/8 (A). Microscopic examination showed complete necrosis of the hepatocellular carcinoma (B, hematoxylin and eosin, 100×).
Discussion
HCC is the fifth-commonest cancer globally and is diagnosed in more than 550,000 people worldwide each year.Citation12 In South Korea, the incidence of HCC is increasing.Citation13 Although active surveillance programs for early detection of HCC in patients with chronic liver disease have increased the proportion of early HCC patients,Citation14 a considerable number of patients remain at high risk of diagnosis with advanced HCC at first presentation. If HCC is detected in its early stage, surgical resection, liver transplantation, and ablative locoregional therapies can be performed with a curative aim.
Among these treatment options, OLT is an attractive therapeutic modality, because the tumor and diseased cirrhotic liver can be cured simultaneously. Mazzaferro et al first defined a subgroup of patients with unresectable HCC for whom liver transplantation was appropriate; these definitions are now referred to as the Milan criteria.Citation1 More recently, a considerable number of suggestions have been proposed in terms of expansion of traditional transplant criteria in several specialized centers; these expansions include the UCSF,Citation2 up-to-7,Citation3 Tokyo,Citation15 Kyoto,Citation16 and Kyushu University criteria.Citation17 Meanwhile, downstaging through neoadjuvant locoregional therapy including transarterial chemoembolization (TACE), radiofrequency ablation (RFA), and selective radioembolization with yttrium-90 labeled microspheres have also been recently attempted in patients who were initially beyond the Milan criteria.Citation18 Chapman et al reported that selected patients with stages III/IV HCC could be downstaged to the Milan criteria with TACE in about one-fourth of cases with favorable midterm disease-free and overall survival rates, similar to stage II HCC.Citation19 In addition, the combined approach of TACE plus either RFA or radioembolization has been known to successfully downstage HCC in ~60% of cases, which is higher than with either monotherapy.Citation20,Citation21 More recently, sorafenib has also been suggested for downstaging as a bridging or neoadjuvant therapy in selected patients with advanced HCC by slowing or halting tumor progression, especially when combined with locoregional therapies.Citation22
Similar to previous studies of TACE, RFA, or yttrium-90 as locoregional treatment modalities to downstage HCC,Citation19,Citation23 we also tried to downstage HCC in our case using CCRT followed by the combined use of sorafenib and HAIC. CCRT and HAIC are used frequently in Asian countries, including South Korea and Japan.Citation24 In a recent pilot study, CCRT showed excellent tumor response, with a median survival of 13.1 months and a 3-year overall survival rate of 24.1% in locally advanced tumors.Citation8 In other studies conducted in South Korea and Japan, the efficacy and safety of repeated HAIC have also been reported with a median time to disease progression and overall survival of 4.1 to 7.0 months and 12.0 to 15.9 months, respectively.Citation25,Citation26 We also added sorafenib when a new HCC lesion appeared during the repeated HAIC to overcome the development of potential resistance to repeated HAIC and to prevent distant metastasis. To date, several reports have supported the rationale of combining sorafenib with locoregional treatments, especially when the patient is refractory to TACE or develops TACE failure.Citation27,Citation28 In addition, in a case report, sorafenib combined with HAIC showed significant tumor regression in patients with advanced HCC and portal vein thrombosis.Citation29
Although we cannot clearly distinguish all therapeutic effects of CCRT, repeated HAIC, and sorafenib, the present case is the first report of OLT being performed via successful down-staging through a combination of locoregional treatment modalities and a systemic target agent in a patient who was initially beyond the Milan or other extended criteria. In this case, there were no significant complications, with the exception of a mild degree of hand–foot syndrome related to sorafenib, during the entire treatment period. This suggests that the combination of sorafenib and locoregional treatment is safe and effective in terms of down-staging of HCC in select patients with advanced HCC. However, the appropriateness of this combined anti-cancer treatment strategy should be further investigated in a randomized large-scale study.
Acknowledgment
The authors are grateful to Dong-Su Jang, (medical illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, South Korea) for his help with the figures.
Disclosure
The authors report no conflicts of interest in this work.
References
- MazzaferroVRegaliaEDociRLiver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosisN Engl J Med1996334116936998594428
- YaoFYFerrellLBassNMLiver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survivalHepatology20013361394140311391528
- MazzaferroVLlovetJMMiceliRPredicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysisLancet Oncol2009101354319058754
- Gordon-WeeksANSnaithAPetrinicTFriendPJBurlsASilvaMASystematic review of outcome of downstaging hepatocellular cancer before liver transplantation in patients outside the Milan criteriaBr J Surg20119891201120821618496
- LlovetJMSchwartzMMazzaferroVResection and liver transplantation for hepatocellular carcinomaSemin Liver Dis200525218120015918147
- TanwarSKhanSAGroverVPGwiltCSmithBBrownALiver transplantation for hepatocellular carcinomaWorld J Gastroenterol200915445511551619938188
- VarottiGRamacciatoGErcolaniGComparison between the fifth and sixth editions of the AJCC/UICC TNM staging systems for hepatocellular carcinoma: multicentric study on 393 cirrhotic resected patientsEur J Surg Oncol200531776076715975760
- HanKHSeongJKimJKAhnSHLeeDYChonCYPilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosisCancer20081135995100318615601
- LencioniRLlovetJMModified RECIST (mRECIST) assessment for hepatocellular carcinomaSemin Liver Dis2010301526020175033
- TrottiAColevasADSetserACTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatmentSemin Radiat Oncol200313317618112903007
- KamathPSWiesnerRHMalinchocMA model to predict survival in patients with end-stage liver diseaseHepatology200133246447011172350
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- HanKHKudoMYeSLAsian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in AsiaOncology201181Suppl 115816422212951
- BruixJShermanMManagement of hepatocellular carcinomaHepatology20054251208123616250051
- SugawaraYTamuraSMakuuchiMLiving donor liver transplantation for hepatocellular carcinoma: Tokyo University seriesDig Dis200725431031217960065
- TakadaYItoTUedaMLiving donor liver transplantation for patients with HCC exceeding the Milan criteria: a proposal of expanded criteriaDig Dis200725429930217960063
- TaketomiASanefujiKSoejimaYImpact of des-gamma-carboxy prothrombin and tumor size on the recurrence of hepatocellular carcinoma after living donor liver transplantationTransplantation200987453153719307789
- YuCYOuHYHuangTLHepatocellular carcinoma downstaging in liver transplantationTransplant Proc201244241241422410030
- ChapmanWCMajella DoyleMBStuartJEOutcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantationAnn Surg2008248461762518936575
- BharatABrownDBCrippinJSPre-liver transplantation locoregional adjuvant therapy for hepatocellular carcinoma as a strategy to improve longterm survivalJ Am Coll Surg2006203441142017000383
- ChanKMYuMCChouHSWuTJLeeCFLeeWCSignificance of tumor necrosis for outcome of patients with hepatocellular carcinoma receiving locoregional therapy prior to liver transplantationAnn Surg Oncol20111892638264621584831
- VagefiPAHiroseRSorafenib combined with locoregional therapy prior to liver transplantation for hepatocellular carcinoma: an update on a previous case reportJ Gastrointest Cancer201344224624722815137
- KulikLMAtassiBvan HolsbeeckLYttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantationJ Surg Oncol200694757258617048240
- AndoEYamashitaFTanakaMTanikawaKA novel chemotherapy for advanced hepatocellular carcinoma with tumor thrombosis of the main trunk of the portal veinCancer19977910189018969149014
- ParkJYAhnSHYoonYJRepetitive short-course hepatic arterial infusion chemotherapy with high-dose 5-fluorouracil and cisplatin in patients with advanced hepatocellular carcinomaCancer2007110112913717508408
- UeshimaKKudoMTakitaMHepatic arterial infusion chemotherapy using low-dose 5-fluorouracil and cisplatin for advanced hepatocellular carcinomaOncology201078Suppl 114815320616598
- KimHYParkJWJooJSeverity and timing of progression predict refractoriness to transarterial chemoembolization in hepatocellular carcinomaJ Gastroenterol Hepatol20122761051105622098152
- KudoMIzumiNKokudoNManagement of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated versionDig Dis201129333936421829027
- YangMYJeongSWKimDKTreatment of hepatocellular carcinoma with portal vein thrombosis by sorafenib combined with hepatic arterial infusion chemotherapyGut Liver20104342342720981227