Abstract
Most cases of superior vena cava (SVC) syndrome resulting from neoplasm, especially from lung cancer, remain a serious challenge to treat. Here, for the first time as far as we are aware, we report the case of a non-small-cell lung cancer patient with a massive SVC malignant thrombosis who was treated with thoracic irradiation and erlotinib. The treatment regimen consisted of erlotinib 150 mg/day and a total dose of 66 Gy/33 fractions delivered to the tumor, malignant thrombosis, and metastasis mediastinal lymph nodes. The malignant thrombosis responded dramatically and the combined regimen was well tolerated. After discharge, the erlotinib was prescribed as maintenance therapy. The patient was followed closely for the next 3 years. During this time, positron emission tomography/computed tomography scans and serum tumor marker screens were undertaken. By 6 months, the primary tumor showed complete response and by 9 months, the SVC thrombosis had disappeared. No sign of relapse has been found to date.
Introduction
The majority of cases of superior vena cava (SVC) syndrome result from neoplasm, particularly lung cancer.Citation1 Here, for the first time as far as we are aware, we report the case of a non-small-cell lung cancer (NSCLC) patient with massive SVC malignant thrombosis who was treated with thoracic irradiation and erlotinib, subsequently achieving complete remission of the cancer and long-term disease-free survival over 3 years.
Case report
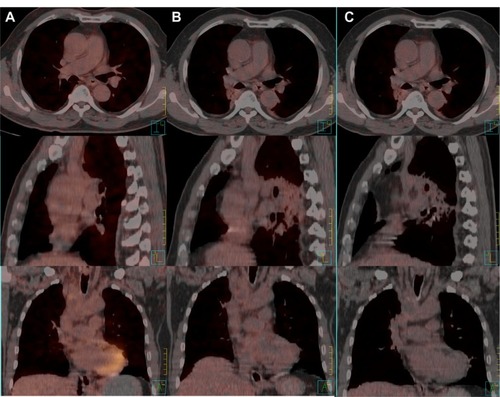
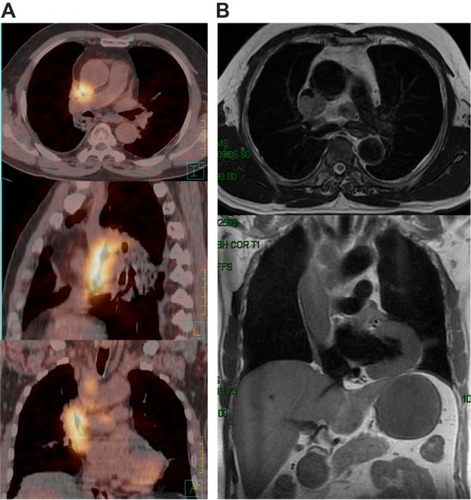
A 61-year-old man presented to the hospital with a 3-month history of progressive edema of the head, neck, arms, and upper chest, followed by shortness of breath and distention of the jugular and chest veins. The medical history of the patient revealed 30 years of tobacco use. The whole-body positron emission tomography/computed tomography (PET/CT) scan revealed a mass (1.5 × 1.0 cm) in the upper lobe of the right lung, with a maximal standard uptake value (SUVmax) of 4.2; enlarged lymph nodes in levels 1, 2R, and 4R; and a huge 7.4 cm long mass with a SUVmax of 8.9 () inside the SVC, which was confirmed by magnetic resonance imaging (). Core needle biopsy samples of the enlarged lymph nodes yielded NSCLC (possibly adenocarcinoma) with epidermal growth factor receptor (EGFR) protein expression. Owing to inadequate tumor sample volume, the EGFR gene was not tested for somatic mutation. No evidence of distant metastasis was found and the disease was diagnosed as primary adenocarcinoma of the lung (cT1N2M0), with malignant thrombosis of the SVC.
Figure 1 Malignant thrombosis of the superior vena cava before treatment: (A) whole-body positron emission tomography/computed tomography scan; (B) magnetic resonance image.
After consulting with the oncologists, the patient was treated with thoracic intensity-modulated radiation therapy and erlotinib at a dosage of 150 mg/day, in order to avoid chemotherapy which may result in nausea and vomiting, could cause the drop of thrombosis. A loop diuretic (hydrochlorothiazide 50 mg) was also used to relieve the SVC syndrome for the first week. Thoracic intensity-modulated radiation therapy was delivered to the planning target volume at a total dose of 66 Gy at 2 Gy per fraction (five times per week). The planning target volume was created by a 5 mm isotropic expansion of the clinical target volume, which encompassed the gross tumor volume and the subcarinal nodes, ipsilateral mediastinum, and ipsilateral hilum. The gross tumor volume was contoured according to the PET/CT images, which included a primary lesion in the right upper lung, metastatic mediastinal lymph nodes, and malignant thrombosis of the SVC (). During weekly physical examinations of the patient, the distention of the jugular and chest veins was found to have resolved completely following radiotherapy delivered at 22 Gy, while significant tumor remission was observed after radiation treatment at 40 Gy ().
Figure 2 Dose distribution in the primary intensity-modulated radiation therapy. The red, olive, and grey lines represent dose distributions of 66, 30, and 20 Gy, respectively. The red, blue, and green areas represent malignant thrombosis in the superior vena cava, metastatic lymph nodes, and the planning target volume, respectively.
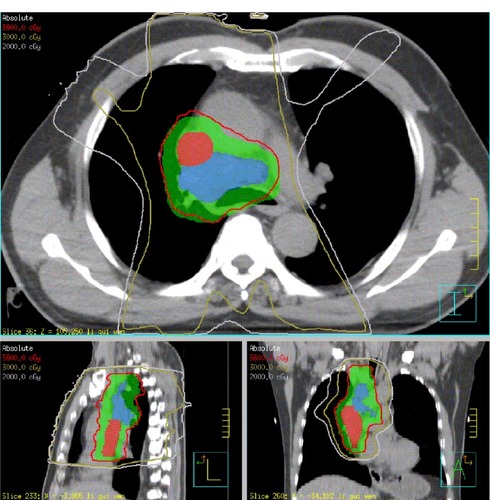
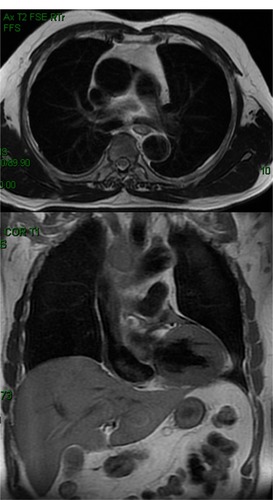
Figure 3 T1-weighted images showing residual malignant thrombosis of the superior vena cava at 40 Gy.
On discharge, the patient was prescribed erlotinib (150 mg/day) as maintenance therapy and monitored closely for the following 45 months with PET/CT scans and serum tumor marker (STM) screens every 3 months. At 6 months after treatment, the primary tumor was found to have completely responded and at 9 months post-treatment, the SVC thrombosis had disappeared. In addition, no signs of pulmonary interstitial abnormality were observed on PET/CT. All the STMs were controlled and the lymph nodes that had been enlarged before treatment were found to have shrunk significantly without abnormal SUV elevation by the last follow-up ().
Discussion
For patients with SVC syndrome resulting from intravascular thrombus by neoplasm, the prognosis is quite poor, and the condition carries a median life expectancy of 6 months and a 2-year survival rate of 5%, although estimates vary widely according to the underlying malignant conditions.Citation2–Citation6 However, the patient in this study continues to enjoy a disease-free survival time beyond 3 years. To our knowledge, this is the first report of conventional radiotherapy combined with erlotinib inducing complete remission and long-term disease-free survival time in NSCLC with malignant thrombosis of the SVC.
Erlotinib has been used to treat NSCLC patients with SVC syndrome in the past,Citation7,Citation8 and the tumors in these cases completely responded to the erlotinib. In one of these cases,Citation7 erlotinib was used concurrently with stereotactic body radiation therapy. However, regardless of the therapeutic effect, the patient developed radiation pneumonitis 3 months after treatment, with suspected interstitial lung disease. The most serious toxicity of tyrosine kinase inhibitors is interstitial lung disease. A previous study has implied that prior tissue injury from radiation therapy could lead to cells having altered responses to drugs administered subsequently.Citation9 Thus, it is possible for erlotinib to induce an altered response in normal tissue, such as in the lung. However, our patient showed good tolerance of the radiotherapy and erlotinib without symptomatic or radiographic radiation pneumonitis. It could be supposed that the difference in fractionated dose between conventionally fractionated radiotherapy and stereotactic body radiation therapy has a unique impact on radiation pneumonitis. In relation to acute radiation pneumonitis, continuous hyperfractionated accelerated radiotherapy is better tolerated than conventionally fractionated radiotherapy.10 Thus, conventionally fractionated individual doses of 2 Gy might have fewer side effects on the pulmonary system. The total dose and mean lung dose should be also considered.
It is unfortunate that we did not obtain information about the presence or lack of somatic mutation in the EGFR gene of the tumor, just as in a previously reported case,Citation8 as this has been associated with a higher responsiveness to erlotinib.Citation11 We were limited by the biopsy sample volume and by the need to treat the SVC obstruction, as EGFR gene sequencing usually takes 1 to 2 weeks. Nevertheless, we recommend testing for the EGFR gene mutation to guide the tyrosine kinase inhibitor treatment, as less than 30% of unselected patients have been found to carry the mutation.Citation12,Citation13
Owing to the rarity of SVC syndrome caused by malignant thrombosis, such cases represent a serious challenge to both internists and oncologists. There are no formal professional guidelines addressing the management of SVC obstruction. Most data regarding management of SVC syndrome are from case series; randomized trials are scarce. Besides best supportive care and treatment to relieve symptoms, such as diuretics and corticosteroids, certain emergent treatments can be considered.Citation14–Citation16 Percutaneous placement of an intravascular stent to bypass the obstruction of the SVC is a possible intervention. Because the stent can be placed before a tissue diagnosis is available, it is a useful procedure for patients with severe symptoms, such as respiratory distress that require urgent intervention. Complications of stent placement have been reported in 3%–5% of patients with SVC syndrome, including infection, pulmonary embolus, stent migration, hematoma at the insertion site, bleeding, and, very rarely, perforation.Citation6,Citation17,Citation18 Another, less common, treatment is surgical bypass grafting when no original malignancies are found or when they are not sensitive to chemotherapy or radiotherapy. Bypass grafting that involves a subcutaneous jugular–femoral graft, for example,Citation19 can be performed with relatively few complications. A more common approach is sternotomy or thoracotomy with extensive resection and reconstruction of the SVC. Case series indicate an operative mortality of approximately 5%.Citation16,Citation20,Citation21
Further treatment of the original malignancy should not be compromised after symptom remission and is usually guided by the histology of the tumor. For example, in patients with lymphoma, small-cell lung cancer, or germ-cell tumors, the clinical response to systemic chemotherapy alone is typically rapid. For patients with NSCLC, chemotherapy (Stage IV disease) or chemotherapy combined with radiotherapy (Stage III disease) should be considered. Stent placement should also be strongly considered for patients with mesothelioma, which tends not to respond well to chemotherapy or radiation. In addition, surgery is often appropriate when SVC syndrome has been caused by a thymoma, which is relatively resistant to chemotherapy and radiation compared with lymphomas.
The other main clinical reason for an SVC is when patients have been at risk of a thrombotic complication because of cancer. Patients with cancer who develop thrombosis have a poor prognosis, and their risk of dying after an acute thrombotic event is four- to eightfold higher than patients without cancer.Citation22 However, the benefit of either short- or long-term anticoagulation therapy for this syndrome is unclear.
Conclusion
Based on the general recommendation of the American College of Chest PhysiciansCitation23 and the National Comprehensive Cancer NetworkCitation24 for radiotherapy, we suggest that radiotherapy combined with erlotinib be considered for selected Stage III NSCLC patients with malignant thrombosis of the SVC, rather than chemotherapy, which may cause nausea and vomiting and carries the risk of cardiovascular embolism. However, the safety and efficiency of this combined regimen should be further explored in clinical trials.
Disclosure
The authors declare no relationships with industry and organizations or potential conflicts of interest between the authors and other persons in relation to this work. All authors read and approved the final manuscript for publication.
References
- WilsonLDDetterbeckFCYahalomJClinical practice. Superior vena cava syndrome with malignant causesN Engl J Med2007356181862186917476012
- SchraufnagelDEHillRLeechJAPareJASuperior vena caval obstruction. Is it a medical emergency?Am J Med1981706116911747234887
- YellinARosenAReichertNLiebermanYSuperior vena cava syndrome. The myth – the factsAm Rev Respir Dis19901415 Pt 1111411182339833
- TanigawaNSawadaSMishimaKClinical outcome of stenting in superior vena cava syndrome associated with malignant tumors. Comparison with conventional treatmentActa Radiol19983966696749817039
- MarcyPYMagneNBentolilaFDrouillardJBrunetonJNDescampsBSuperior Vena cava obstruction: is stenting necessary?Support Care Cancer20019210310711305067
- GreillierLBarlésiFDoddoliCVascular stenting for palliation of superior vena cava obstruction in non-small-cell lung cancer patients: a future ‘standard’ procedure?Respiration200471217818315031575
- HsiehCHChangHTLinSCToxic risk of stereotactic body radiotherapy and concurrent helical tomotherapy followed by erlotinib for non-small-cell lung cancer treatment – case reportBMC Cancer20101069667021194444
- SalmiRGaudenziPDi TodaroFMorandiPNielsenIManfrediniRMassive thrombosis of brachiocephalic veins and superior vena cava syndrome in a patient with non-small cell lung cancer treated with the epidermal growth factor receptor inhibitor erlotinibClin Drug Investig2007277499503
- KitaniHKosakaTFujiharaTLindquistKElkindMMThe “recall effect” in radiotherapy: is subeffective, reparable damage involved?Int J Radiat Oncol Biol Phys19901836896952318703
- JenkinsPD’AmicoKBensteadKElyanSRadiation pneumonitis following treatment of non-small-cell lung cancer with continuous hyperfractionated accelerated radiotherapy (CHART)Int J Radiat Oncol Biol Phys200356236036612738310
- TsaoMSSakuradaACutzJCErlotinib in lung cancer – molecular and clinical predictors of outcomeN Engl J Med2005353213314416014883
- ShepherdFARodrigues PereiraJCiuleanuTNational Cancer Institute of Canada Clinical Trials GroupErlotinib in previously treated non-small-cell lung cancerN Engl J Med2005353212313216014882
- DongQGHanBHHuangJSAnalysis of EGFR mutations in 176 cases of non-small cell lung cancerZhonghua Zhong Liu Za Zhi2006289686690 Chinese17274376
- YimCDSaneSSBjarnasonHSuperior vena cava stentingRadiol Clin North Am200038240942410765398
- ThonyFMoroDWitmeyerPEndovascular treatment of superior vena cava obstruction in patients with malignanciesEur Radiol19999596597110370001
- ShenKRMeyersBFLarnerJMJonesDRAmerican College of Chest PhysiciansSpecial Treatment Issues in Lung Cancer: ACCP Evidence-Based Clinical Practice Guidelines2nd edChest2007132Suppl 3290S305S17873175
- NicholsonAAEttlesDFArnoldAGreenstoneMDyetJFTreatment of malignant superior vena cava obstruction: metal stents or radiation therapyJ Vasc Interv Radiol1997857817889314368
- UrruticoecheaAMesíaRDomínguezJTreatment of malignant superior vena cava syndrome by endovascular stent insertion. Experience on 52 patients with lung cancerLung Cancer200443220921414739042
- DhaliwalRSDasDLuthraSSinghJMehtaSSinghHManagement of superior vena cava syndrome by internal jugular to femoral vein bypassAnn Thorac Surg200682131031216798237
- ChenKNXuSFGuZDSurgical treatment of complex malignant anterior mediastinal tumors invading the superior vena cavaWorld J Surg200630216217016425072
- InoueMMinamiMShionoHEfficient clinical application of percutaneous cardiopulmonary support for perioperative management of a huge anterior mediastinal tumorJ Thorac Cardiovasc Surg2006131375575616515944
- LevitanNDowlatiARemickScRates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims dataMedicine (Baltimore)199978528529110499070
- KvalePaSimoffMPrakashUbLung Cancer. Palliative CareChest2003123Suppl 1284S311S12527586
- National Comprehensive Cancer Network (NCCN)NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer V.I.2007Fort Washington, PANCCN2007 Available from: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdfAccessed April 9, 2007