Abstract
Objective
Human epididymis protein 4 (HE4) has been approved for diagnosing ovarian cancer. The goal of this meta-analysis was to evaluate the clinical value of the serum HE4 in the diagnosis of ovarian cancer.
Methods
The PubMed and Embase databases were searched to identify suitable studies. The sensitivity (SEN), specificity (SPE), and positive and negative likelihood ratios (PLR and NLR, respectively) of HE4 for the diagnosis of ovarian cancer were commonly used as bivariates. Summary receiver operating characteristic curves were used to summarize overall test performances. Meta-Disc 1.4 software was used to analyze the data.
Results
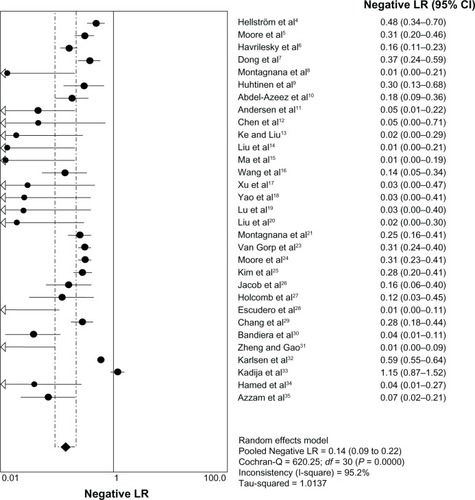
A total of 6,269 patients from 31 trials were subjected to meta-analysis. The summary estimates of HE4 for ovarian cancer diagnosis were as follows: SEN 0.73 (95% confidence interval [CI] 0.71–0.75); SPE 0.89 (95% CI 0.88–0.90); PLR 7.30 (95% CI 5.42–9.84); and NLR 0.15 (95% CI 0.10–0.23). SEN 0.74 (95% CI 0.72–0.76); SPE 0.89 (95% CI 0.88–0.90); PLR 7.35 (95% CI 5.55–9.73); NLR 0.14 (95% CI 0.09–0.21).
Conclusion
Our study demonstrates that the sensitivity and specificity of HE4 was higher than that of cancer antigen 125. The results indicated that HE4 could be a useful tumor marker for ovarian cancer diagnosis. However, the results of this meta-analysis should be interpreted with caution, due to the heterogeneity among study designs. Further study should pay more attention to the possibility that HE4 can be a marker for monitoring recurrence of ovarian cancer.
Keywords:
Background
Ovarian malignant tumor ranks third in female reproductive tract tumors. Only 25% of patients can be identified at stage I. Compared with stage I, 5-year survival of ovarian cancer patients of stage III and IV has decreased from 95% to 20%–25%.Citation1–Citation3 With this high mortality, early diagnosis of ovarian malignant tumor is of great importance. Serum tumor markers are widely used in screening, diagnosis, recurrence detection, and efficacy judgment. Serum cancer antigen (CA)-125 can elevate in 85% of ovarian cancer patients, with clinical value in preoperative diagnosis and progress monitoring of ovarian cancer. Nevertheless, CA125 levels do not elevate in the early stage of some ovarian cancer patients. It is difficult to establish an early diagnosis of ovarian cancer based on CA125 levels, and they can elevate in some benign gynecological diseases, such as endometriosis, benign ovarian tumors, pelvic inflammatory disease, the menstrual phase, early pregnancy, and laparotomy. Therefore, it is a hot issue for study to search and separate new markers for early diagnosis and disease surveillance of ovarian cancer.
Human epidermis protein 4 (HE4) is an ovarian cancer marker suggested recently. A study revealed that HE4 is highly expressed in ovarian cancer tissues, whereas it is expressed comparatively lower in precancerous, benign, and normal tissues. Detection of HE4 might have a certain value for diagnosis and monitoring of ovarian cancer. Now, HE4 is studied mostly by individual research centers; the role of the HE4 assay for diagnosing ovarian cancer has not been well-established. Application of evidence-based medicine provides a reliable research method that can integrate the results from different research centers and provide reliable scientific conclusions to guide clinical practice. Therefore, we conducted a meta-analysis based on relevant and available trials to assess the value of serum HE4 for the diagnosis of ovarian cancer to guide clinical treatment.
Materials and methods
Literature search and inclusion criteria
Literature searches of the PubMed and Embase databases (January 2000 to May 2013) were performed. Index words included HE4, WFDC2, human epididymis protein 4, human epididymis-specific protein 4, OC, ovarian cancer, ovarian carcinoma, and ovarian neoplasm.
English-language restriction was imposed. In addition, the reference lists of identified studies were manually checked to include other potentially eligible trials. This process was performed iteratively until no additional articles could be identified.
Inclusion criteria
All papers with pathological diagnosis of ovarian cancer and serum HE4 value were included. All papers were published from January 2000 to May 2013. There was a clear threshold reference value.
Exclusion criteria
Papers published before January 2000 or after May 2013 were excluded, as were case reports and reviews and papers without a serum HE4 test value of ovarian cancer, or where there was no clear threshold reference value.
Quality assessment
The methodological quality of each trial was evaluated by the quality-assessment tool for diagnostic accuracy studies (QUADAS). We requested the relevant information from authors if data were unreported.
The quality and bias of independent research was assessed based on the following aspects: whether the experimental design was scientific, whether inclusion criteria and basic structural characteristics of research objects were clear, whether factors and methods of treatment were accurate, whether statistical methods were appropriate, and whether biases in the study were discussed. Research that met one of these conditions scored 1 point. Quality was reliable when the total score was ≥3 points.
Statistical analyses
This meta-analysis was performed using Meta-Disc 1.4 software provided by the Cochrane Collaboration (Oxford, UK). Analysis of heterogeneity between studies was done using the χ2 test. When there was no significant heterogeneity between studies (P > 0.1, I2 ≤ 50%), we used fixed-effect meta-analysis and analyzed bias to obtain sensitivity, specificity, positive predictive, and negative predictive values. If there was statistical heterogeneity between studies, the meta-analysis was performed using the random-effects model (P ≤ 0.1, I2 > 50%).
Results
Basic information and quality evaluation of document retrieval
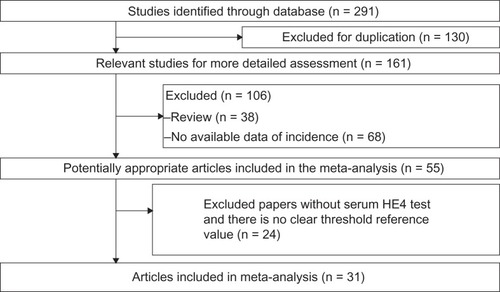
Basic information on studies included is shown in . A total of 291 potentially relevant papers were identified based on the search terms. Finally, 31 papers were scrutinized in full text as appropriate. The selection process for articles included in the meta-analysis is shown in . These studies were published between 2010 and 2013. The size of the articles ranged from 49 to 1,061 (total 6,269) words.
Table 1 Basic information about included papers
Quality evaluation of literature
The quality and bias of 31 papers were evaluated based on the evaluation criteria. Risk of bias and applicability concerns were used to evaluate the quality of the literature. Most studies were regarded as being at high risk of bias, and patient selection was unclear (description of QUADAS is shown in ).
Table 2 Quality-assessment tool for diagnostic accuracy studies (QUADAS) measure for included papers
Meta-analysis
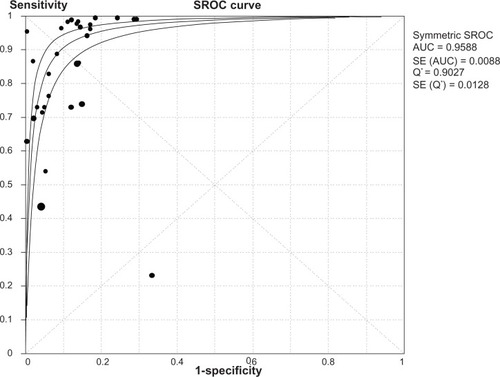
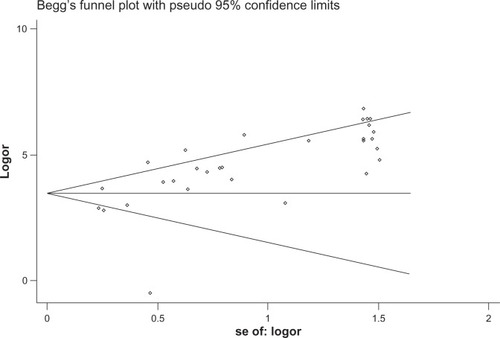
The heterogeneity analysis revealed a less homogeneous (P < 0.00001, I2 = 96.0%). The meta-analysis was performed using the random-effects model. The results showed sensitivity and specificity of serum HE4 in the diagnosis of ovarian cancer was 0.74 (95% CI 0.72–0.76), and 0.89 (95% CI, 0.88–0.90), respectively ( and ). The positive and negative predictive values of serum HE4 in the diagnosis of ovarian cancer were 7.28 (95% CI 5.48–9.68), and 0.14 (95% CI 0.09–0.22), respectively ( and ). The area under the summary receiver operating-characteristic curve was 0.96 (0.95–0.98) (). The asymmetry of the funnel plots using Egger’s and Begg’s tests showed that there was publication bias among the included studies (Egger’s test, P = 0.010; Begg’s test, P = 0.009) ().
Figure 2 Forest plot of analyses for sensitivity.
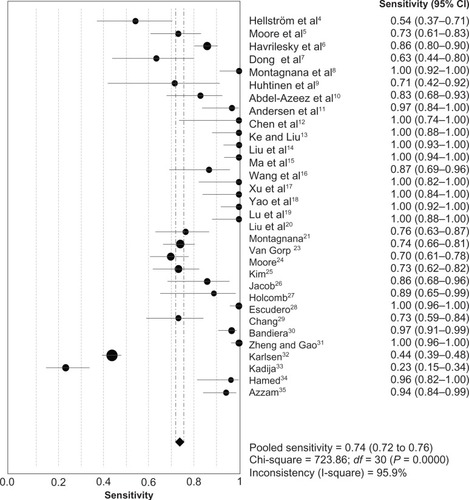
Figure 3 Forest plot of analyses for specificity.
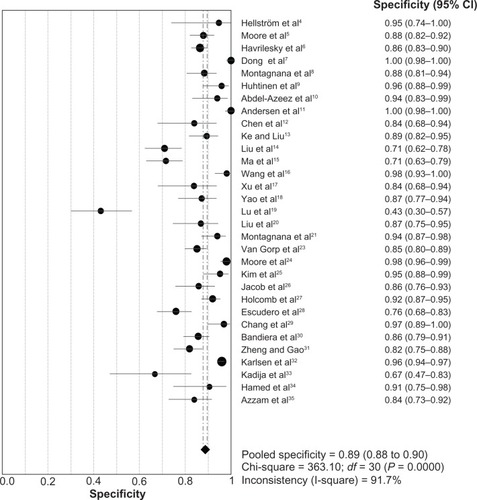
Figure 4 Forest plot of analyses for positive LR.
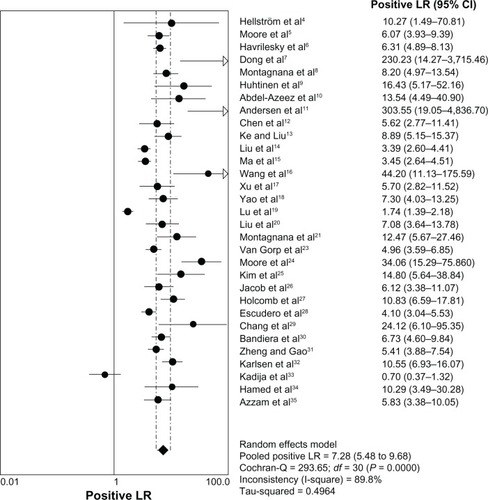
Figure 5 Forest plot of analyses for negative LR.
Discussion
Ovarian cancer is the most lethal gynecological malignancy. Unfortunately, the majority of patients remain clinically undetected until they have developed late-stage disease, and only a mere 25% of cancers are detected as stage I disease, due to the fact that there are no typical clinical symptoms. The tumor marker is of great significance for early cancer detection and disease surveillance. CA125 is the most studied and widely used in the clinical setting. However, it has been reportedCitation4 that about 40%–50% of patients with early stage epithelium ovarian cancer did not have abnormal CA125. Therefore, it is necessary to find a new tumor marker to supplement CA125.
HE4 is also known as WAP four-disulfide core domain protein 2, the coding gene of which was separated from the distal epithelium epidermis in 1991. The encoded protein is mainly composed of WAP four-disulfide core. Hellström et alCitation4 was the first to subject the HE4 protein to a double-blind test by enzyme-linked immunosorbent assay (ELISA), and found the sensitivity of HE4 in the detection of ovarian cancer was equal to CA125 and the specificity even higher than CA125.
This is a comprehensive systematic review and meta-analysis of 31 articles to evaluate the clinical value of the serum HE4 assay in the diagnosis of ovarian cancer. We reached a contradictory conclusion. Wu et alCitation38 and Yu et alCitation36 suggested that HE4 was better than CA125. However Li et alCitation37 showed that HE4 was not better than CA125, for either epithelial ovarian cancer or ovarian cancer prediction. The main finding of our meta-analysis seems to strengthen the conclusions of Wu et alCitation38 and Yu et al.Citation36 However, the results of this meta-analysis should be interpreted with caution, due to the heterogeneity among study designs. In detail, the previous meta-analyses included nine, twelve, and eleven articles, respectively, including twelve studies described here. Meanwhile, articles published in 2012 and 2013 were also included. Two previous meta-analysesCitation36,Citation38 and our meta-analysis showed that the sensitivity and specificity of HE4 was higher than that of CA125. These results are not conclusive, as they are not adequately powered for unified cutoff, control group (healthy women or women with benign disease), and different methods to evaluate HE4 in serum (ELISA, chemiluminescent microparticle immunoassay, bead-based array system).
In 2008, Moore et alCitation5 reported that as a single ovarian tumor marker, the sensitivity of serum HE4 in diagnosis of ovarian cancer was 72.9% When HE4 combined with CA125, sensitivity and specificity were 76.4% and 95%, respectively, but when HE4 combined with other tumor markers, the increase in sensitivity was very low. HE4 may be the best marker used alone for stage I disease, because the sensitivity of combination with CA125 or other ovarian cancer markers cannot increase. Montagnana et alCitation8 assayed the preoperative serum concentration of both HE4 and CA125 in patients with different forms of benign and malignant pelvic masses. The results showed that the median CA125 and HE4 serum levels were significantly higher among ovarian cancer patients compared with healthy subjects and with those with benign mass. The receiver operating characteristic-curve analysis on healthy controls and patients with ovarian cancers revealed that HE4 had a significantly higher area under the curve when compared with CA125 (0.99 vs 0.91), with sensitivity and specificity of 98% and 100%, respectively. The sensitivity and specificity of HE4 are higher than CA125. Thus, HE4 is considered a promising ovarian cancer marker. In this study, we conducted a meta-analysis to explore further the value of HE4 in the diagnosis of ovarian cancer. The specificity was 95% (95% CI 0.88–0.90). The positive predictive value was 7.35 (95% CI, 5.55–9.73). The negative predictive value was 0.14 (95% CI, 0.09–0.21). The results and reports revealed that the sensitivity of HE4 in detection ovarian cancer is the same as CA125, but specificity is higher than CA125. It suggested that HE4 is a promising marker of ovarian cancer for clinical application. In addition, this study shows the funnel plot is asymmetric. An unfilled corner at the bottom of the graph indicated that there was bias in the research. It is necessary to collect more data to improve the quality of research. In addition, there are still many papers in which HE4 serum was tested but concentration of HE4 in serum was not classified as positive or negative. These only mentioned the median value or range of ovarian cancers, benign diseases, and the control group.
One potential limitation of the present meta-analysis is that the cutoff values of HE4 used among the studies were various. There is no gold standard for the cutoff value and method to evaluate HE4. The heterogeneity with regard to the cutoff value of HE4, assay method, menopausal status, and small samples may also account for the lack of clear evidence to support HE4 as a potential tumor marker for ovarian cancer diagnosis. The second limitation is that we were unable to assess the impact of unpublished papers on publication bias. The third limitation is that the stage of ovarian cancer has insufficient statistical power. Although the evidence of diagnostic effectiveness in detecting early stage tumors is of pivotal relevance, there are currently not enough studies for estimating HE4 performance in this clinical scenario. Additional studies or data are warranted, particularly to evaluate HE4 capability to identify ovarian cancer at an early stage.
Several articles have reported on HE4 in the early diagnosis of ovarian cancer, and found its sensitivity (76.9%–82.7%) to be higher than CA125 (45.9%).Citation4,Citation7 HE4 is also reported in some literature for ovarian cancer disease surveillance. Allard et alCitation22 reported that 83.8% of patients’ CA125 or HE4 levels matched the clinical state. Meanwhile, more and more research has focused on the clinical performance of the risk of ovarian malignancy algorithm (ROMA) and on CA125 and HE4. Sandri et alCitation39 demonstrated that the ROMA index combined the advantage of each single marker and reached a sensitivity of 89.3% and a specificity of 81.7%. However, the results should be interpreted with caution due to heterogeneity. Such heterogeneity means there cannot be a uniform standard in ROMA projects, at least in the beginning. Further indicated that HE4 can be a marker to monitor recurrence of ovarian cancer, which is consistent with the CA125.
Conclusion
The studies described indicate that HE4 is valuable as an ovarian tumor marker in diagnosis and monitoring the disease state. The value of serum HE4 in diagnosis and disease monitoring of ovarian cancer still needs large-scale and multicenter randomized study.
Acknowledgments
This work was supported by a grant from the Provincial Research Project Funding of Guangxi, People’s Republic of China (2010GXNSFD013053 and 2011Z2011217).
Disclosure
The authors report no conflicts of interest in this work.
References
- BastRCJrStatus of tumor markers in ovarian cancer screeningJ Clin Oncol200321200s205s12743135
- PiverMSBakerTRDriscollDLLack of substantial five year disease-free survival by primary aggressive surgery and cisplatin-based chemotherapy or by salvage intraperitoneal cisplatin-based chemotherapyEur J Gynaecol Oncol1990112432502245807
- OzolsRRubinSThomasGRobboySJEpithelial ovarian cancerHoskinsWJPerezCAYoungRCBarakatRMarkmanMRandallMPrinciples and Practice of Gynecologic Oncology4th edPhiladelphiaLippincott Williams & Wilkins2005895989
- HellströmIRayeraftJHayden-LedbetterMThe HE4 (WFDC2) protein is a biomarker for ovarian carcinomaCancer Res2003633695370012839961
- MooreRGBrownAKMillerMCThe use of multiple novel tumor biomarkers for the detection of ovarian carcinoma patients with a pelvic massGynecol Oncol200810840240818061248
- HavrileskyLJWhiteheadCMRubattJMEvaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrenceGynecol Oncol200811037438218584856
- DongLChangXHYeXThe values of serum human epidermis secretory protein 4 and CA125 assay in the diagnosis of ovarian malignancyZhonghua Fu Chan Ke Za Zhi200843931936 Chinese19134334
- MontagnanaMLippiGRuzzenenteOThe utility of serum human epididymis protein 4 (HE4) in patients with a pelvic massJ Clin Lab Anal20092333133519774626
- HuhtinenKSuvitiePHiissaJSerum HE4 concentration differentiates malignant ovarian tumors from ovarian endometriotic cystsBr J Cancer20091001315131919337252
- Abdel-AzeezHALabibHASharafSMRefaiANHE4 and mesothelin: novel biomarkers of ovarian carcinoma in patients with pelvic massesAsian Pac J Cancer Prev20101111111620593939
- AndersenMRGoffBALoweKAUse of a symptom index, CA125, and HE4 to predict ovarian cancerGynecol Oncol201011637838319945742
- ChenGDLiZGQianDYThe value of human epidermis secretory protein in the diagnosis of pelvic massesJ Pract Med20102627742776
- KeLLiuFHThe value of serum HE4 and CA125 in the diagnosis of ovarian cancerMod Med (Chic)2010108586
- LiuGRWangALLiuQCombined detection of serum CA125 and HE4 for ovarian cancer diagnosis and differential diagnosisChin J Clin Lab Sci201028119121
- MaLYanCLPiaoWHThe clinical application of serum human epidermis protein 4 in the aided diagnosis of ovarian cancerNingxia Med J20103210381039
- WangKYLengJHDengHThe value of combined detection of serum HE4 and CA125 in the diagnosis of ovarian cancerChin J Health Lab20102011391140
- XuCLYangYHWangHLThe value of serum human epidermis protein 4 epithelial in the diagnosis of ovarian cancerChin J Gynecol Obstet201026684686
- YaoYLLiuQLiXYThe application value of joint inspections of serum HE4, TPS and CA125 in the diagnosis of ovarian cancerRadiat J Immunol201023409411
- LuMDChenDZZhaoJThe expression of joint detection of HE4 and CA125 in ovarian tumors and its clinical significanceChin Matern Child Health2010536233625
- LiuYNYeXChengHYMeasurement of serum human epidermis secretory protein 4 combined with the CA125 assay in the differential diagnosis of endometriosis cyst and ovarian benign and malignant tumorsChin J Obstet Gynecol201045363366
- MontagnanaMDaneseERuzzenenteOThe ROMA (risk of ovarian malignancy algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: is it really useful?Clin Chem Lab Med20114952152521288178
- AllardJSomersETheilRMooreRGUse of a novel biomarker HE4 for monitoring patients with epithelial ovarian cancerJ Clin Oncol2008265535
- Van GorpTCadronIDespierreEHE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the risk of ovarian malignancy algorithmBr J Cancer201110486387021304524
- MooreRGMillerMCDisilvestroPEvaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic massObstet Gynecol201111828028821775843
- KimYMWhangDHParkJEvaluation of the accuracy of serum human epididymis protein 4 in combination with CA125 for detecting ovarian cancer: a prospective case-control study in a Korean populationClin Chem Lab Med20114952753421320028
- JacobFMeierMCaduffRNo benefit from combining HE4 and CA125 as ovarian tumor markers in a clinical settingGynecol Oncol201112148749121420727
- HolcombKVuceticZMillerMCKnappRCHuman epididymis protein 4 offers superior specificity in the differentiation of benign and malignant adnexal masses in premenopausal womenAm J Obstet Gynecol2011205358. e1e621722869
- EscuderoJMAugeJMFilellaXTorneAPahisaJMolinaRComparison of serum human epididymis protein 4 with cancer antigen 125 as a tumor marker in patients with malignant and nonmalignant diseasesClin Chem2011571534154421933899
- ChangXYeXDongLHuman epididymis protein 4 (HE4) as a serum tumor biomarker in patients with ovarian carcinomaInt J Gynecol Cancer20112185285821633297
- BandieraERomaniCSpecchiaCSerum human epididymis protein 4 and risk for ovarian malignancy algorithm as new diagnostic and prognostic tools for epithelial ovarian cancer managementCancer Epidemiol Biomarkers Prev2011202496250622028406
- ZhengHGaoYSerum HE4 as a useful biomarker in discriminating ovarian cancer from benign pelvic diseaseInt J Gynecol Cancer2012221000100522426406
- KarlsenMASandhuNHøgdallCEvaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic massGynecol Oncol201212737938322835718
- KadijaSStefanovicAJeremicKThe utility of human epididymal protein 4, cancer antigen 125, and risk for malignancy algorithm in ovarian cancer and endometriosisInt J Gynecol Cancer20122223824422214964
- HamedEOAhmedHSedeekOBMohammedAMAbd-AllaAAAbdel GhaffarHMSignificance of HE4 estimation in comparison with CA125 in diagnosis of ovarian cancer and assessment of treatment responseDiagn Pathol201381123343214
- AzzamAZHashadDIKamelNAEvaluation of HE4 as an extrabio-marker to CA125 to improve detection of ovarian carcinoma: is it time for a step forward?Arch Gynecol Obstet Epub1302013
- YuSYangHJXieSQBaoYXDiagnostic value of HE4 for ovarian cancer: a meta-analysisClin Chem Lab Med2012501439144622868811
- LiFTieRChangKDoes risk for ovarian malignancy algorithm excel human epididymis protein 4 and ca125 in predicting epithelial ovarian cancer: a meta-analysisBMC Cancer20121225822712526
- WuLDaiZYQianYHShiYLiuFJYangCDiagnostic value of serum human epididymis protein 4 (HE4) in ovarian carcinoma: a systematic review and meta-analysisInt J Gynecol Cancer2012221106111222854652
- SandriMTBottariFFranchiDComparison of HE4, CA125 and ROMA algorithm in women with a pelvic mass: correlation with pathological outcomeGynecol Oncol201312823323823200911