Abstract
Purpose
The majority of Egyptian patients with lung cancer present at a late stage of the disease. Bevacizumab/carboplatin/paclitaxel, as well as cisplatin plus pemetrexed, are both standard regimens for advanced non-squamous bronchogenic cancer. This study compares both regimens, in terms of efficacy and toxicity profile, in Egyptian patients.
Patients and methods
This is a randomized Phase II study comparing toxicity profile and survival in 41 chemotherapy-naïve patients with stage IIIB or IV non-squamous NSCLC, with an ECOG performance status of 0 to 2. The epidermal growth factor receptor (EGFR) mutation detection was performed prior to treatment of all patients. Patients in the first group received: bevacizumab 7.5 mg/m2 on Day 1 and Day 15; carboplatin area under the curve-5 on Day 1; and paclitaxel 60 mg/m2 on Day 1, Day 8, and Day 15 every 4 weeks. In the second group, patients received cisplatin 75 mg/m2 and pemetrexed 500 mg/m2 every 3 weeks.
Results
The combination of bevacizumab/carboplatin/paclitaxel demonstrated higher Grade III–IV toxicity than cisplatin/pemetrexed regarding sensory/motor neuropathy (P = 0.06), DVT (P = 0.23), proteinuria (P = 0.23), and hypertension (P = 0.11), as well as Grade II alopecia (P = 0.001); however, no significant difference in toxicities between both arms was recorded regarding nausea and vomiting (P = 0.66), hematological toxicity, febrile neutropenia (P = 1) and fatigue (P = 0.66). Progression-free survival was similar for both treatment arms with a median of 6 months (P = 0.978). Overall median survival was comparable in both arms, 16.07 months versus 16.01 months (P = 0.89).
Conclusion
Bevacizumab/carboplatin/paclitaxel and cisplatin/pemetrexed provided meaningful and comparable efficacy in advanced non-squamous bronchogenic carcinoma not harboring EGFR mutation. No significant difference in toxicity was observed between both treatment arms, apart from bevacizumab/carboplatin/paclitaxel-related risks as DVT, hypertension, proteinuria, sensory/motor neuropathy, and alopecia.
Introduction
Primary lung cancers are the most common malignancies after nonmelanocytic skin cancer and the leading cause of human cancer deaths worldwide.Citation1 Non-small cell lung cancer (NSCLC) accounts for more than 85% of all lung cancers.Citation2 In advanced-stage (stage IIIB or IV) NSCLC, doublet combinations of platinum compounds (cisplatin or carboplatin) with gemcitabine, vinorelbine, or taxanes (paclitaxel or docetaxel) are reference regimensCitation3 when compared head-to-head in Phase III studies, these doublets have shown comparable efficacy, with variable differences in toxicity profiles.Citation4–Citation8
Pemetrexed is a potent inhibitor of thymidylate synthaseCitation9,Citation10 and other folate-dependent enzymes, including dihydrofolate reductase and glycinamide ribonucleotide formyltransferase.Citation11 Pemetrexed/cisplatin is currently approved in combination for first-line treatment of malignant pleural mesotheliomaCitation12 and in first-line treatment of non-squamous advanced NSCLC.Citation13
Different studies showed more favorable overall survival (OS) for adenocarcinoma related to low thymidine synthetase expression in non-squamous histology.Citation14,Citation15 Besides this, another study in chemotherapy-naïve patients with squamous and adenocarcinoma of the lung demonstrated that baseline expression of the thymidylate synthetase gene and protein were significantly higher in squamous cell carcinoma compared with adenocarcinoma;Citation16 however, molecular and other mechanisms that would explain the survival advantage for adenocarcinoma remain unclear. Further molecular-marker studies will help in better stratification of patients to different active regimens.
The addition of bevacizumab, a monoclonal antibody against vascular endothelial growth factor to paclitaxel and carboplatin, led to significant survival benefit. However, this efficacy benefit was seen with an increased risk of treatment-related morbidity and deaths.Citation17
The recombinant, humanized monoclonal antibody bevacizumab in combination with paclitaxel and carboplatin is approved by the US Food and Drug Administration for first-line treatment of patients with unresectable, locally advanced, recurrent, or metastatic non-squamous NSCLC.Citation17–Citation20 Bevacizumab binds to a vascular endothelial growth factor, which is an essential endothelial cell mitogen, a survival factor, and a key factor in tumor-associated angiogenesis.
These two combinations bevacizumab/carboplatin/paclitaxel and cisplatin/pemetrexed achieved better and almost similar OS in different trials, and are standard regimen for advanced non-squamous NSCLC.
The primary aim of the present study is to analyze a toxicity profile as well as the efficacy of both regimens in term of progression-free survival (PFS), with OS as the secondary endpoint.
Patients and methods
During the period from September 2008 to May 2010, 65 chemotherapy-naïve patients presented to Dar Al Fouad Hospital with advanced non-squamous bronchogenic cancer. Sixteen patients were excluded, being epidermal growth factor receptor (EGFR)-mutant. Five patients were excluded because of the presence of brain metastasis. Three others were excluded as well because of an initial presentation with hemoptysis. The remaining 41 patients fulfilled the selection criteria and were assigned to this randomized study.
All patients were eligible if: they had histologically or cytologically confirmed NSCLC, classified as stage IIIB not amenable to curative treatment or stage IV, with at least one unidimensionally measurable lesion, according to the Response Evaluation Criteria in Solid Tumors (RECIST);Citation21 an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2;Citation22 and were at least 18 years of age. EGFR mutation detection was performed in all non-squamous NSCLC prior to study entry, using an EGFR mutation detection kit (EntroGen Inc, Tarzana, CA, USA). Patients had adequate bone marrow reserve and organ function, including calculated creatinine clearance ≥45 mL/minutes, based on the standard Cockcroft–Gault formula.Citation23
Exclusion criteria included: peripheral neuropathy ≥ Grade 1, according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 3.0; progressive brain metastases; or uncontrolled third-space fluid retention before study entry. Patients were also excluded on the basis on: harboring the sensitizing mutation to the EGFR gene; a recent history of bleeding or thrombotic events and ongoing therapeutic anticoagulation; uncontrolled hypertension; unable to interrupt aspirin and other nonsteroidal anti-inflammatory drugs; or if they were unable or unwilling to take folic acid, vitamin B12, or corticosteroids.
Non-squamous EGFR wild types fulfilling the selection criteria were randomly allocated into the treatment groups on a ratio of 1:1, using a computer system with a closed envelope to one of these two treatment arms:
Baseline and treatment assessments
Prior to treatment, all patients underwent a medical history and physical examination, and tumor measurements were taken both for palpable lesions as well as lesions assessed by imaging techniques. Positron emission tomography and ultrasound scans were not permitted. The baseline assessment method was repeated every other cycle and then every 8 weeks after treatment discontinuation until disease progression. Disease status was assessed in solid tumors according to RECIST.Citation21
Twenty patients were assigned to arm 1 (bevacizumab/carboplatin/paclitaxel), and 21 patients were assigned to arm 2 (cisplatin/pemetrexed). The primary endpoints of the study were PFS and toxicity profile and secondary endpoint was OS.
Statistical analysis
Comparison of toxicity was done using Fisher’s exact test. Survival was estimated using Kaplan–Meier and log rank for comparing curves. P-value is always two tailed; and significance was at the 0.05 level.
Results
In the period from September 2008 to May 2010, a total of 41 patients were randomly assigned (20 patients to bevacizumab/carboplatin/paclitaxel and 21 patients to cisplatin/pemetrexed). The baseline patient and disease characteristics are shown in .
Table 1 Baseline characteristics for randomly assigned patients
Efficacy
In terms of response rate, partial response was witnessed in (12/20 patients) in arm 1 and (10/21 patients) in arm 2, while stable disease was seen in (6/20 patients) in arm 1 and (9/21 patients) in arm 2 (P = 0.81).
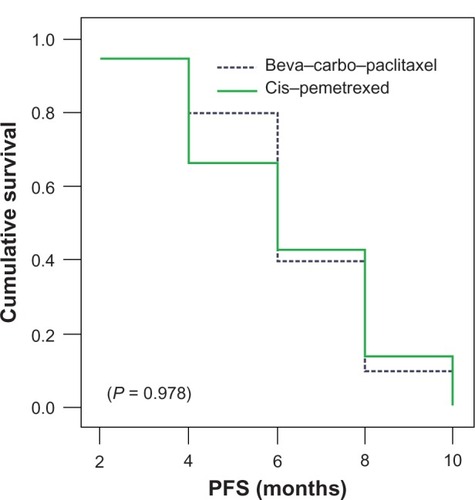
shows the progression-free survival estimate (95% confidence interval), which was similar for both treatment arms with a median for arm 1 of 6 months (5 to 7 months) versus (vs) 6 months (4 to 8 months) for arm 2 as well (P = 0.978).
Figure 1 PFS for both treatment arms.
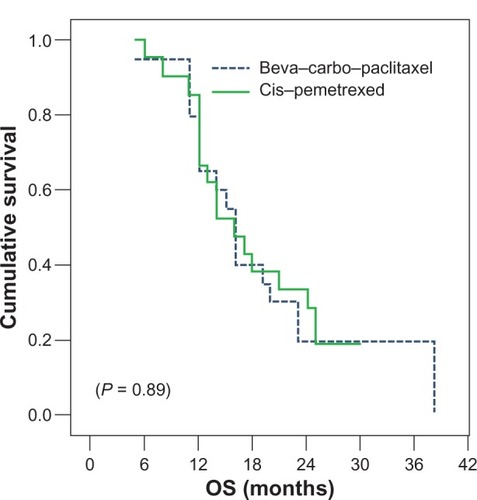
Overall survival estimate (95% confidence interval) for the patient randomly assigned to (bevacizumab/carboplatin/paclitaxel) was almost similar to that of (cisplatin/pemetrexed), 16.01 (11.47–20.55) months vs 16.07 (14.66–17.49) months (P = 0.89).
shows the Kaplan–Meier curve for overall survival. Survival at 12 months and 24 months was 80% and 20% for bevacizumab/carboplatin/paclitaxel, respectively, and 85.7% and 33% for cisplatin/premetrexed.
Figure 2 Overall survival for both treatment arms.
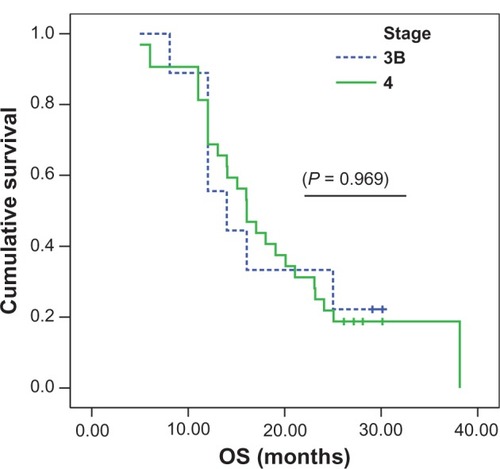
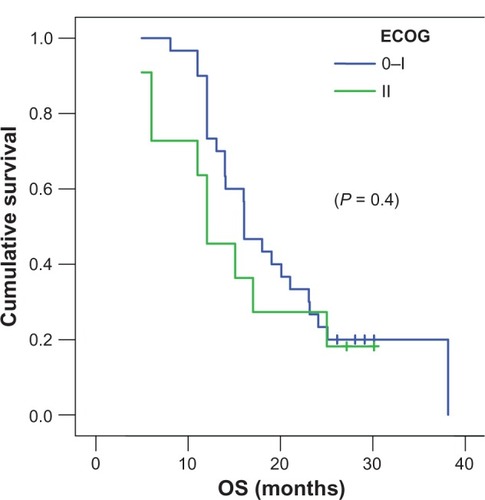
In subgroup analysis to which stage IIIB (nine of 41 patients) was compared to stage IV (32 of 41 patients), the overall survival was 13.99 months vs 16.07 months (), which didn’t reach a statistical significance (P = 0.9697). However, subgroup analysis to which ECOG 0 to1 (30 of 41 patients) was compared to EGOG II (eleven of 41 patients) showed that the patient with better performance 0 to 1 () tended to achieve better survival at 16.07 months vs 12.05 months (P = 0.4046). Finally, subgroup analysis to which positive smoking status (36 of 41 patients) was compared to never-smoker status (five of 41 patients) revealed an overall survival of 16.07 months vs 12.05 months, not reaching statistical significance (P = 0.6571).
Safety
According to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 3.0, the grade III–IV drug-related toxicity incidents recorded were neutropenia, anemia, and thrombocytopenia. There was no difference between both arms (neutropenia: 3 of 20 patients in arm 1 vs 4 of 21 patients in arm 2 [P = 1], anemia: 2 of 20 patients in arm 1 vs 3 of 21 patients in arm 2 [P = 1]; and thrombocytopenia: 1 in 20 patients in arm 1 vs 4 of 21 patients in arm 2 [P = 0.43]). Febrile neutropenia requiring hospital admission was noted in 1 in 20 patients in arm 1 and 2 in 21 patients in arm 2 (P = 1). Nausea and vomiting was in 2 of 20 patients in arm 1 and higher in arm 2 (4 in 21 patients) (P = 0.66). Fatigue was noted in 3 in 20 patients in arm 1 and 2 in 21 patients in arm 2 (P = 0.66).
Hypertension was also higher in arm 1 (3 of 20 patients) vs no hypertension recorded in arm 2 (P = 0.11). Proteinuria also was recorded in arm 1 (2 of 20 patients) and was not recorded in arm 2 (P = 0.23). Deep venous thrombosis (DVT) was higher in the bevacizumab-containing arm (4 in 20 patients) but not significantly different compared to arm 2 (2 of 21 patients) (P = 0.41). Sensory/motor neuropathy was more evident in arm 1 (8 of 20 patients) vs arm 2 (3 of 21 patients), although not reaching significance (P = 0.06). Alopecia (Grade II alopecia) was significantly higher in arm 1 (18 of 20 patients) vs arm 2 (1 of 21 patients) (P = 0.001). The toxicity profile difference between both arms is demonstrated in .
Table 2 Randomly assigned treated patients common toxicity criteria (Worst Grade 3–Grade 4)
Discussion
It has been reported that the addition of bevacizumab to carboplatin/paclitaxel in previously untreated patients with advanced non-squamous NSCLC was associated with improved OS.Citation17 On the other hand, the combination of cisplatin/pemetrexed improved OS, when compared to the doublet combination of platinum compound with gemcitabine.Citation13 Both of these two combinations achieved better and almost similar OS in different trials, and are standard regimen for advanced non-squamous NSCLC. However, direct comparison of efficacy across different randomized clinical studies could lead to some biased conclusions due to different patient populations.
The issue of bevacizumab dosing in advanced nonsquamous NSCLC, whether 7.5 mg/kg or 15 mg/kg, remains discussable and depends on the chemotherapy backbone. In the AVAstin in Lung (AVAiL) study,Citation24 15 mg/kg every 3 weeks with paclitaxel/carboplatin in (ECOG 4599) and 7.5 mg/kg every 3 weeks with gemcitabine/cisplatin were studied; yet, in our study, we assumed that 7.5 mg/kg every 2 weeks would be a compromise between toxicity and efficacy, which is probably why we didn’t record any life-threatening hemorrhagic side effects or treatment-related mortality.
This study compared both regimens in terms of toxicity and efficacy (response rates, PFS, and OS rates) in a small sample of the Egyptian population. A partial response was observed in 60% in arm 1 and 47.6% in arm 2, while stable disease was seen in 30% in arm 1 and 42% in arm 2. This response rate was meaningful and denoted the comparable activity of both regimens in non-squamous EGFR nonmutant bronchogenic cancer.
The median time to progression in both arms was 6 months, which was also comparable to that achieved in ECOG of 6.2 months and that of the study conducted by Scagliotti et al of 5.3 months.Citation13
The combination of bevacizumab/carboplatin/paclitaxel demonstrated higher Grade 3–4 toxicity than cisplatin/pemetrexed, in terms of the well-known bevacizumab/carboplatin/paclitaxel-related hazards, such as sensory/motor neuropathy, DVT, proteinuria, and hypertension, as well as alopecia Grade 2. However, no significant difference in toxicity was observed regarding nausea and vomiting, hematological toxicity, and febrile neutropenia between both arms.
The median survival for both arms was equivalent at 16.07 months and 16.01 months; this is longer than both the median previously reported in ECOG 4599Citation17 of 12.3 months and the 12.6 months reported in the study conducted by Scagliotti et al.Citation13 Despite the small size of our study, this better overall survival can be attributed to eventual population-related biological factors. The achieved median survival in both arms reflects the positive effect of both regimens on survival.
Surprisingly, stage IV patients (32 of 41 patients) and smokers (36 of 41 patients) tended to have a better OS than stage IIIB patients (9 of 41 patients) and nonsmokers (5 of 41 patients), which might be attributed to the smaller number of stage IIIB patients and nonsmokers in comparison to Stage IV patients and smokers.
In conclusion, cisplatin/pemetrexed and bevacizumab/carboplatin/paclitaxel provided meaningful and comparable efficacy in advanced non-squamous EGFR nonmutant bronchogenic carcinoma. There was no significant difference in toxicities, apart from in DVT, hypertension, sensory/motor neuropathy, proteinuria, and alopecia, which were more common in the bevacizumab-containing regimen. Their efficacy may allow these to become the preferred regimens, although with special attention to bevacizumab-related risks in certain patients.
These results warrant future prospective studies, specifically designed to evaluate biological markers which may guide the selection of patients most likely to benefit from either of these two regimens.
Acknowledgments
The authors would like to acknowledge the oncology team registrars and nurses at Dar Al Fouad Hospital, who made it possible to conduct this clinical trial. Last, but not least, we acknowledge support from Nelly Ali Eldin of the National Cancer Institute Egypt for her volunteer contribution for statistical analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
- Karim-KosHEde VriesESoerjomataramILemmensVSieslingSCoeberghJWRecent trends of cancer in Europe: a combined approach of incidence, survival, and mortality for 17 cancer sites since the 1990sEur J Cancer200844101345138918280139
- GovindanRPageNMorgenszternDChanging epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results databaseJ Clin Oncol200624284539454417008692
- PfisterDGJohnsonDHAzzoliCGAmerican Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003J Clin Oncol200422233035314691125
- SchillerJHHarringtonDBelaniCPComparison of four chemotherapy regimens for advanced non-small-cell lung cancerN Engl J Med20023462929811784875
- ScagliottiGVDe MarinisFRinaldiMPhase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancerJ Clin Oncol200220214285429112409326
- KellyKCrowleyJBunnPAJrRandomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: a Southwest Oncology Group trialJ Clin Oncol200119133210321811432888
- FossellaFPereiraJRvon PawelJRandomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study groupJ Clin Oncol200321163016302412837811
- Le ChevalierTScagliottiGNataleREfficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer: a meta-analysis of survival outcomesLung Cancer2005471698015603856
- TaylorECKuhntDShihCA dideazatetrahydrofolate analogue lacking a chiral center at C-6, N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic acid, is an inhibitor of thymidylate synthaseJ Med Chem19923523445044541447744
- SchultzRMPatelVFWorzallaJFShihCRole of thymidylate synthase in the antitumor activity of the multitargeted antifolate, LY231514Anticancer Res1999191A43744310226579
- ShihCHabeckLLMendelsohnLGChenVJSchultzRMMultiple folate enzyme inhibition: mechanism of a novel pyrrolopyrimidine-based antifolate LY231514 (MTA)Adv Enzyme Regul1998381351529762351
- VogelzangNJRusthovenJJSymanowskiJPhase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesotheliomaJ Clin Oncol200321142636264412860938
- ScagliottiGVParikhPvon PawelJPhase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancerJ Clin Oncol200826213543355118506025
- SigmondJBackusHHWoutersDTemminkOHJansenGPetersGJInduction of resistance to the multitargeted antifolate Pemetrexed (ALIMTA) in WiDr human colon cancer cells is associated with thymidylate synthase overexpressionBiochem Pharmacol200366343143812907242
- GiovannettiEMeyVNannizziSCellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cellsMol Pharmacol200568111011815795320
- CeppiPVolanteMSaviozziSSquamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthaseCancer200610771589159616955506
- SandlerAGrayRPerryMCPaclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancerN Engl J Med2006355242542255017167137
- Avastin® (bevacizumab) [package insert]South San Francisco, CA, USAGenentech Inc2007
- JohnsonDHFehrenbacherLNovotnyWFRandomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancerJ Clin Oncol200422112184219115169807
- SandlerABevacizumab in non-small cell lung cancerClin Cancer Res20071315 Pt 2s4613s461617671151
- TherassePArbuckSGEisenhauerEANew guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of CanadaJ Natl Cancer Inst200092320521610655437
- OkenMMCreechRHTormeyDCToxicology and response criteria of the Eastern Cooperative Oncology GroupAm J Clin Oncol1982566496557165009
- CockcroftDWGaultMHPrediction of creatinine clearance from serum creatinineNephron197616131411244564
- ReckMvon PawelJZatloukalPPhase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAiLJ Clin Oncol2009271227123419188680