Abstract
Purpose
Primary small cell carcinoma of the urinary bladder is a rare malignant disease. It accounts for less than 1% of all urinary bladder carcinomas. The purpose of this study is to review the clinical features, the treatment modalities, and the overall survival of these patients. We also compare the clinical outcomes between patients of bladder small cell carcinoma (SCC) and bladder urothelial carcinoma (UC).
Materials and methods
We reviewed the charts of patients with bladder tumors from January 1995 to December 2012 in the Chang Gung Memorial Hospital. A total of 2421 malignant bladder tumor patients were reviewed and there were 18 patients who were diagnosed with primary bladder SCC. The patients’ characteristics, including age, gender, smoking history, presented symptoms, tumor size, locations, clinical stages, treatment modalities, pathology appearance, recurrence conditions, and survival conditions were all recorded. We also compared the clinical outcomes and the overall survival rates between patients with bladder SCC and those with UC.
Results
Bladder SCC accounted for about 0.74% of all bladder malignancies in our institution. The mean age at diagnosis was 70.67 years, and the male-to-female ratio was 2.6:1. Thirteen patients had a history of cigarette smoking. All patients presented with symptoms of gross hematuria, and three of them had bladder tamponade requiring blood clot evacuation by cystoscopy. Only one patient had T1 disease, ten patients had stage III disease, and seven patients had lymph node or distant metastasis (stage IV disease). The mean tumor size was 4.29 cm in diameter. For the majority (61.11%) of patients, SCC coexisted with UC components. The average survival time was 10.92 months. Patients with bladder SCC had worse overall survival rates than those of stage III and stage IV bladder UC. Performing radical cystectomy does not significantly improve their overall survival rates. None of the clinicopathologic parameters, including the presence of coexisting nonsmall cell carcinoma component (P = 0.831), receiving radical cystectomy (P = 0.194), distant metastasis (P = 0.062), and gender (P = 0.564), were significantly associated with survival.
Conclusion
SCC of the urinary bladder is a rare condition, and standard treatment outlines have not been well established. Most of the presented cases have a very poor prognosis. Prospective, multi-institutional, randomized studies are required to assess better treatment modalities. To the best of our knowledge, this is the largest reported case analysis of primary bladder SCC in a Taiwanese population.
Introduction
Primary small cell carcinoma of the urinary bladder (SCC) is a rare malignant disease. It accounts for less than 1% of all urinary bladder carcinomas.Citation1,Citation2 The first reported case was published in 1981 by Cramer et al.Citation3 Since that time, approximately 250 cases have been reported in the literature.Citation4 This disease affects mostly elderly male patients who present with gross hematuria, and the prognosis is very poor.Citation5,Citation6 However, most of the presented cases were reported in western countries and focused predominantly on the Caucasian population; not many studies focus on Asian populations. We reviewed the charts of Chang Gung Memorial Hospital in Taiwan from January 1995 to December 2012, where a total of 18 cases of primary bladder SCC were recorded. The purpose of this study is to review the clinical features, the treatment modalities, and the overall survival of the patients. We also compare the clinical outcomes between patients with bladder SCC to those with bladder UC. To the best of our knowledge, this is the first reported series of primar y bladder SCC in Taiwanese patients.
Materials and methods
We reviewed the charts of patients with bladder tumors from January 1995 to December 2012 in the Chang Gung Memorial Hospital. A total of 2421 malignant bladder tumor patients were reviewed. After excluding tumors of other sites with metastasis to the bladder, there were 18 patients who were diagnosed with primary bladder SCC. The tumors fulfilled the criteria established for small cell carcinoma according to the World Health Organization (WHO) classification system.Citation7 The patients’ characteristics including age, gender, smoking history, presented symptoms, tumor size, locations, clinical stages, treatment modalities, pathology appearance, recurrence conditions, and survival conditions were all recorded. The staging definition for bladder cancer was based on the American Joint Committee on Cancer (AJCC) staging 7th edition.Citation8 A Cox proportional hazards model was used to analyze survival outcomes with covariates. We also compared the clinical outcomes and the overall survival rates between patients with bladder SCC and those with bladder UC. Kaplan-Meier survival curves and Log-rank tests were used to analyze the survival outcomes among the different groups. SPSS 15.0 for windows (IBM Corporation, Armonk, NY, USA) was used for statistical analysis, and P-value s ≤ 0.05 were considered statistically significant.
Results
A total of 2421 malignant bladder tumor patients were reviewed, and there were 18 patients who were diagnosed with primary bladder SCC, which accounted for about 0.74% of all bladder malignancies in our institution. The 18 patients’ characteristics and clinical findings are summarized in . The mean age at diagnosis was 70.67 years (range 59–83 years), and the male-to-female ratio was 2.6:1. Thirteen (72.22%) patients gave a history of cigarette smoking. All patients presented with symptoms of gross hematuria, and three of them had bladder tamponade and required blood clot evacuation by cystoscopy. Acute renal failure occurred in two patients who received temporary hemodialysis. None of the 18 patients had paraneoplastic syndrome.
Table 1 Patient characteristics (n = 18)
Except for one patient who had Tl disease, all patients presented with either locally advanced disease or metastatic disease when they were initially diagnosed. As shown in , ten patients (55.56%) had stage III disease, and seven patients (38.89%) had lymph node or distant metastasis (stage IV). Treatment varied greatly among patients for whom information was available, and this is presented in . There were eleven patients who received a major operation (either radical or partial cystectomy). However, very high margin positive rates were noted (6 of 11,54.55%). Among the patients who received chemotherapy, cisplatin plus etoposide combination and carboplatin/etoposide were the most common regimens.
Table 2 Treatment modalities of the 18 cases of primary bladder small cell carcinoma
The tumor characteristics are shown in . The mean tumor size was 4.29 cm (0.5–9.0 cm) in diameter. The intravesical distributions of the tumor were dispersed. The tumor did not occur in any specific location within the bladder. In the majority of patients (61.11%), SCC coexisted with UC components. Only seven patients (38.89%) had pure SCC of the bladder.
Table 3 Tumor characteristics
The mean follow-up time was 19.85 months (range 2–128 months). Among the eleven patients who were initially metastasis free (Tl to T3 disease), ten patients had tumor recurrence on the extravesical organs. The mean time to recurrence was 8.03 months (range 2.13–25.43 months). As shown in , a total of 25 recurrent tumors were found in these patients. The most common metastatic organs were the lung and the liver (44.4%), followed by the bone (27.78%).
Table 4 Locations of tumor recurrence (n = 25)
Among the 18 patients who were followed up in our study to date, only three of them survived, and the other 15 had died. These deceased patients survived an average of 10.92 (2–38) months since they were first diagnosed with cancer. The overall survival rates of the patients with bladder SCC and UC are demonstrated in . We can see that patients with bladder SCC had worse overall survival rates than those of stage III and stage IV bladder UC (Log-rank <0.0001), as well as patients of stage I and II disease (Log-Rank <0.0001). Since most of the patients with bladder SCC presented with stage III and stage IV disease, we further compared their overall survival rates with those of stage III and stage IV bladder UC, as shown in .
Figure 1 Kaplan-Meier analysis of the overall survival rates of the patients with bladder SCC and UC.
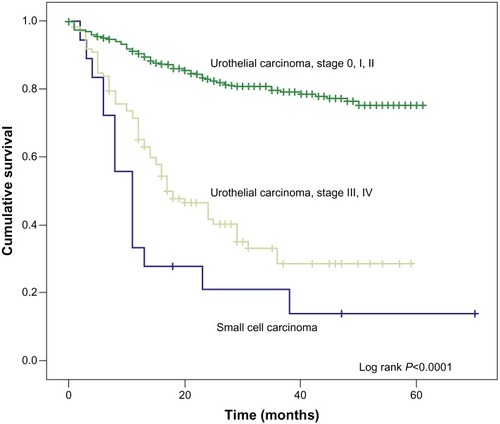
Figure 2 Kaplan-Meier analysis of the overall survival rates with those of stage III and stage IV bladder UC. Note that the Group I represented patients with bladder SCC who received radical cystectomy. Group 2 represented patients with bladder SCC who did not receive radical cystectomy. Group 3 represented patients with bladder UC who received radical cystectomy. Group 4 represented patients with bladder UC who did not receive radical cystectomy.
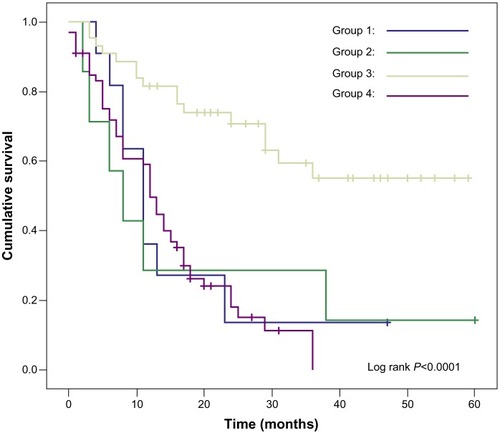
Group 1 represented patients with bladder SCC who received radical cystectomy. Group 2 represented patients with bladder SCC who did not receive radical cystectomy. Group 3 represented patients with bladder UC who received radical cystectomy, Group 4 represented patients with bladder UC who did not receive radical cystectomy. We can see that patients with bladder UC who received radical cystectomy had a better overall survival rate than those who did not (Log-Rank <0.0001). On the contrary, in the SCC groups, the overall survival rates were not significantly different whether they received radical cystectomy or not. In other words, performing radical cystectomy for patients with bladder SCC does not significantly improve their overall survival rates. shows the possible predictive value for the overall survival rate of bladder SCC patients. None of the clinicopathologic parameters, including the presence of coexisting nonsmall cell carcinoma component (P = 0.831), receiving radical cystectomy (P = 0.194), distant metastasis (P = 0.062), and gender (P = 0.564), were significantly associated with survival.
Table 5 Analysis of factors of possible predictive value for the overall survival rate
Discussion
Although primary small cell carcinoma of the urinary bladder is a rare malignant disease and accounts for only less than 1% of all urinary bladder cancers, a marked increase in the incidence of bladder SCC in the past 15 years was reported. According to Koay et al, there was a significant increase in incidence from 0.05 to 0.14 cases per 100,000 people, and an increase in the percentage of all bladder cancers from 1991 to 2005 (from 0.3% to 0.6%, P < 0.0001).Citation9 In a chart review of the past 18 years in our institution, there were 18 cases of bladder SCC, which accounted for 0.74% of all bladder malignancy. Among the 18 patients from our institution, six of the cases occurred between 1995 and 2003, and the other twelve fell between 2004 and 2012. From this data, we found that there was an increasing trend for bladder SCC, which matches Koay et al’s findings.
The pathogenesis of bladder SCC is still unknown. The possible mechanism may be linked to loss of genetic material, hypermethylation of tumor suppressors, and amplification of the chromosomal regions carrying oncogenes.Citation10 Environmental and genetic risk factors in an aging population may also contribute to this disease.Citation11 Previous studies postulated that the exposure to tobacco smoke may contribute to SCC.Citation11 According to previous literature, most patients were in the sixth to seventh decade of life, males predominated, and many had a history of cigarette smoking. The study by Church et al revealed that cigarette smoking is a risk factor for SCC of the bladder with 50% to 70% of patients reporting a smoking history.Citation12 The most common presentation was gross hematuria. In our study, this disease is male predominant, with the mean age of 70.67 years, and more than 70% of the patients had a history of cigarette smoking. All patients presented with symptoms of gross hematuria, and three of them had bladder tamponade requiring blood clot evacuation by cystoscopy. The mean tumor size was 4.29 cm in diameter, which was far larger than the size of average bladder tumors at the time of discovery.Citation13
The diagnosis of bladder SCC is based on the criteria established by the WHO classification system (2004), which was used for the diagnosis of lung SCC.Citation14 On light microscopy, the cancer cells stained with haematoxylin and eosin showed packed cells having scant cytoplasm containing few organelles. Tumors were composed of nests of small round malignant cells with pyknotic round to oval nuclei and evenly dispersed “salt and pepper chromatin”.Citation14 Immunohistochemistry plays an important role in the diagnosis of bladder SCC through the staining of tumor components by antibody markers targeting the following antigens: neuron-specific enolase (NSE), chromogranin, synaptophysin, serotonin, cytokeratin, S-100 protein, thyroid transcription factor 1 (TTF1), epidermal growth factor receptor (EGFR) and C-KIT.Citation15–Citation17 SCC may occur in other genitourinary organs; however, the bladder is the most common site for genitourinary extrapulmonary SCC.Citation18 Most of the bladder SCC cases were mixed with other histologic types of carcinoma.Citation18 In our study, the combination of SCC with UC was the most frequent type, and this accounted for 61.11% of our patients. Cramer et al proposed that SCC of the urinary bladder originates from metaplastic urothelium.Citation3 The frequent association of SCC of the urinary bladder with UC suggests that SCC may originate from a urothelial stem cell rather than from a specific neuroendocrine precursor cell. Other studies suggest that the origin of bladder SCC might be a multi-potential common stem cell, which has the ability to differentiate into various cell types depending on the influence of a specific transformation or progression-related gene. These hypotheses might explain the coexistence of bladder SCC with UC, and the heterogeneity of immune staining.Citation19–Citation21
Our study revealed that, when the diagnosis was confirmed, most of the bladder SCC patients were staged as advanced disease. Even though some patients were staged as organ confined disease at the beginning, the disease had a strong trend to progress and became metastatic. In our study, the average duration between the time of tumor discovery and the time of metastasis was only 8.03 months. This explains why most treatment results for bladder SCC were unsatisfactory. Because of the rarity of bladder SCC, there was no standard treatment for the disease. According to the literature, treatment options vary greatly. It has been a debated issue whether receiving radical cystectomy provides a better overall survival for bladder SCC patients. In a review reported by the MD Anderson Cancer Center, radical cystectomy was favored, and 52% of the patients who underwent cystectomy had a better survival.Citation22 Furthermore, patients who received neoadjuvant chemotherapy had significantly better survival than those who did not receive it.Citation22 However, in a multi-centered review of localized bladder SCC, the efficacy of cystectomy has been questioned. No survival difference was found between patients undergoing surgery and those without surgery (5-year survival was 16% versus 18%, respectively).Citation23 Most of our patients received radical cystectomy, and according to their post-operative conditions, they were either followed with radiation therapy or adjuvant chemotherapy, as shown in . Two of our patients had already reached critical status when they arrived at our hospital; therefore, they only received biopsy instead of further treatments in order to prove that their tissue sample was cancerous - both died soon after. Nevertheless, our data showed that radical cystectomy did not help improve the overall survival, which is different from bladder UC. As shown in , for patients with >T3 staging, those with bladder UC who received radical cystectomy had a much better overall survival in the end. On the contrary, significance was not seen among bladder SCC patients. Platinum-etoposide combination chemotherapy has been employed as the main systemic treatment option for bladder SCC. Chemotherapy was usually combined with other therapeutic modalities, especially in patients whose disease was limited to the regional area. Owing to the rarity of this malignancy, no prospective study has been carried out to establish the efficacy and duration of chemotherapy or the relative efficacy of platinum-etoposide versus other chemotherapeutic regimens.Citation24 Since the outcomes of treating bladder SCC remain poor, the improvement in survival may rely on the identification of new molecular markers for early diagnosis and novel targeted therapies. Targeted therapy against the c-kit proto-oncogene has been successful for treating gastrointestinal stromal tumors. A significant proportion of SCcs of the urinary bladder express c-kit, suggesting that it may be useful as a therapeutic target in the future.
As shown in , our study revealed that there was no remarkable predictive value for the overall survival of primary bladder SCC. Although the patients with lymph nodes or distant metastasis (N or M stage) had worse overall survival, the difference was not statistically significant (P = 0.062). Cheng et al reviewed the data of 64 patients, and found that none of the clinicopathologic parameters, including age, gender, presenting symptoms, smoking history, the presence of a coexisting nonsmall cell carcinoma component, cystectomy, chemotherapy, or radiation therapy are relative to the patients’ overall survival.Citation23 It has been a debated issue whether AJCC stage of bladder SCC impacts the overall survival or not. Podesta and True reported that staging was correlated with survival in their review.Citation25 However, Mackey and associates did not observe a correlation when their patients were analyzed retrospectivelyCitation26 Explanations for this finding can be attributed to the small number of current studies on bladder UC, which is also why the finding was not statistically significant. Apart from this, it may also be possible that the categorization of AJCC is for bladder UC instead of bladder SCC. For example, current AJCC staging for bladder cancer classifies any lymph node involvement as stage IV. However, Koay et al observed that patients who had regional lymph node metastasis but no distant metastases had a better overall survival than patients who had distant metastasis, while these were all grouped as stage IV disease.Citation9 Therefore, according to Koay’s opinion, staging bladder SCC differently from UC should be investigated further.Citation9
Conclusion
Since the incidence of bladder SCC has increased in the past two decades, we should pay more attention to this malignant disease with very poor prognosis. Although this survey was performed in a single medical center, and there were only 18 cases, this is the first reported study to describe primary bladder SCC in a Taiwanese population. All other studies done in East Asia were mostly small-scale case reports, which lack systematic data compilation. Therefore, even though findings from our current study cannot be generalized for the entire Asian population, we still consider the results to be of great significance. Our study demonstrated that primary bladder SCC incidence was male predominant, and presented at a later stage and a worst prognosis than UC. The treatment modalities varied widely in the literature because of the shortage of previous treatment experience. Identification of new molecular markers for early diagnosis and novel targeted therapies may play an important role in the future. Furthermore, prospective study will be needed to elucidate a more effective treatment for bladder SCC.
Disclosure
The authors report no conflicts of interest in this work.
References
- BlomjousCEVosWDe VoogtHJVan der ValkPMeijerCJSmall cell carcinoma of the urinary bladder. A clinicopathologic, morphometric, immunohistochemical, and ultrastructural study of 18 casesCancer1989646134713572548704
- LopezJIAnguloJCFloresNToledoJDSmall cell carcinoma of the urinary bladder. A clinicopathological study of six casesBr J Urol199473143497507782
- CramerSFAikawaMCebelinMNeurosecretory granules in small cell invasive carcinoma of the urinary bladderCancer19814747247306261916
- WangXMacLennanGTLopez-BeltranAChengLSmall cell carcinoma of the urinary bladder – histogenesis, genetics, diagnosis, biomarkers, treatment, and prognosisAppl Immunohistochem Mol Morphol200715181817536302
- SvedPGomezPManoharanMCivantosFSolowayMSSmall cell carcinoma of the bladderBJU Int2004941121715217423
- TriasIAlgabaFCondomESmall cell carcinoma of the urinary bladder. Presentation of 23 cases and review of 134 published casesEur Urol2001391859011173944
- MostofiFKDavisCJSesterhennIAWHO histologic typing of urinary bladder tumorsBerlinSpringer1999
- EdgeSBByrdDRComptonCCFritzAGGreeneFLTrottiAAJCC cancer staging manual7th edNew York, NYSpringer2010
- KoayEJTehBSPaulinoACBuderEBA Surveillance, Epidemiology, and End Results analysis of small cell carcinoma of the bladder: epidemiology, prognostic variables, and treatment trendsCancer2011117235325533321567387
- Pant-PurohitMLopez-BeltranAMontironiRMacLennanGTChengLSmall cell carcinoma of the urinary bladderHistol Histopathol201025221722120017108
- SorianoPNavarroSGilMLlombart-BoschASmall-cell carcinoma of the urinary bladder. A clinico-pathological study of ten casesVirchows Arch2004445329229715248064
- ChurchDNBahlAClinical review – small cell carcinoma of the bladderCancer Treat Rev200632858859317008012
- Millán-RodríguezFChéchile-TonioloGSalvador-BayarriJPalouJVicente-RodríguezJMultivariate analysis of the prognostic factors of primary superficial bladder cancerJ Urol20001631737810604317
- AbrahamsNAMoranCReyesAOSiefker-RadtkeAAyalaAGSmall cell carcinoma of the bladder: a contemporary clinicopathological study of 51 casesHistopathology2005461576315656887
- JonesTDKemekKMYangXJThyroid transcription factor 1 expression in small cell carcinoma of the urinary bladder: an immunohistochemical profile of 44 casesHum Pathol200536771872316084939
- PanCXYangXJLopez-BeltranAc-kit Expression in small cell carcinoma of the urinary bladder: prognostic and therapeutic implicationsMod Pathol200518332032315502806
- TeradaTAutopsy case of primary small cell carcinoma of the urinary bladder: KIT and PDGFRA expression and mutationsPathol Int200959424725019351368
- MukeshMCookNHollingdaleAEAinsworthNLRussellSGSmall cell carcinoma of the urinary bladder: a 15-year retrospective review of treatment and survival in the Anglian Cancer NetworkBJU Int2009103674775219076139
- TerraccianoLRichterJTornilloLChromosomal imbalances in small cell carcinomas of the urinary bladderJ Pathol1999189223023510547580
- ChristopherMESeftelADSorensonKRe snickMISmall cell carcinoma of the genitourinary tract: an immunohistochemical, electron microscopic and clinicopathological studyJ Urol199114623823881713277
- van HoevenKHArtymyshynRLCytology of small cell carcinoma of the urinary bladderDiagn Cytopathol19961442922978725127
- Siefker-RadtkeAODinneyCPAbrahamsNAEvidence supporting preoperative chemotherapy for small cell carcinoma of the bladder: a retrospective review of the MD. Anderson cancer experienceJ Urol2004172248148415247709
- ChengLPanCXYangXJSmall cell carcinoma of the urinary bladder: a clinicopathologic analysis of 64 patientsCancer2004101595796215329903
- PanCXZhangHLaraPNChengLSmall-cell carcinoma of the urinary bladder: diagnosis and managementExpert Rev Anticancer Ther20066121707171317181484
- PodestaAHTrueLDSmall cell carcinoma of the bladder. Report of five cases with immunohistochemistry and review of the literature with evaluation of prognosis according to stageCancer19896437107142545325
- MackeyJRAuHJHughJVennerPGenitourinary small cell carcinoma: determination of clinical and therapeutic factors associated with survivalJ Urol19981595162416299554367