Abstract
Toll-like receptor 4 (TLR-4) is well known for its host innate immunity. Despite the fact that TLR-4 activation confers antitumor responses; emerging evidence suggests that TLR-4 is associated with tumor development and progression. It is now clear that overactivation of TLR-4, through various immune mediators, may cause immune response dysfunction, resulting in tumorigenesis. Different cancers could have different extents of TLR-4 involvement during tumorigenesis or tumor progression. In this review, we focus on infection- and inflammation-related TLR-4 activation in noncancer and cancer cells, as well as on the current evidence about the role of TLR-4 in ten of the most common cancers, viz, head and neck cancer, lung cancer, gastrointestinal cancer, liver cancer, pancreatic cancer, skin cancer, breast cancer, ovarian cancer, cervical cancer, and prostate cancer.
Introduction
Toll-like receptors (TLRs) are a recently discovered family of pattern recognition receptors that show homology with the Drosophila Toll protein and the human interleukin (IL)-1 receptor family. The first member of the TLR family to be identified was a Drosophila proteinCitation1 implicated in dorsoventral patterning during embryonal development. TLRs are evolutionarily conserved proteins characterized by an extracellular leucine-rich repeat (LRR) domain, the transmembrane domain, and the cytoplasmic intracellular Toll/IL-1 receptor-like (TIR) domain. LRRs, found in both cytoplasmic and transmembrane proteins, play a vital role in ligand recognition and signal transduction. There are 12 TLRs, in which ten human isoforms of TLRs (TLR-1 to 10) have been identified. The LRR, which is deputed to recognition of the ligand, is composed of 19–25 tandem repeats of 24–29 amino acids, folded in strands and in helices that are linked by loops.Citation2 The transmembrane domain and TIR domain are highly conserved among the TLRs.Citation3
TLR-4 structure
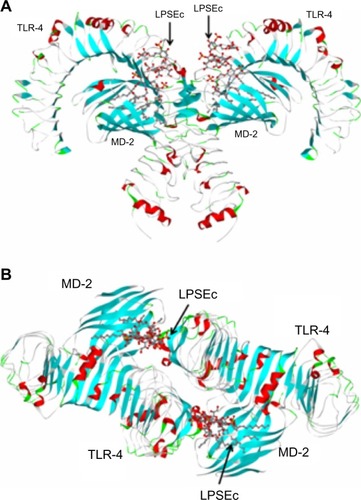
Recently, TLR-4 was the first identified TLR whose crystal structure (Protein Data Bank [PDB] ID: 3FXI) was solved (),Citation4 leading to the derivation of computational simulation models that predict the mechanism of its interaction with its cognate ligands. The TIR domain, which shares homology with the IL-1 receptor (IL-1R), is responsible for the propagation of the signal within the cell, through interaction with a complex signaling cascade. Human TLR-4 is located on chromosome 9q32–q33 and contains four exons.Citation5
Figure 1 Structure of the TLR-4/MD-2/LPSEc complex.
Abbreviations: LPSEc, lipopolysaccharide from Escherichia coli; MD-2, myeloid differentiation protein-2; PDB, Protein Data Bank; TLR-4, Toll-like receptor 4.
TLR-4 signaling pathway
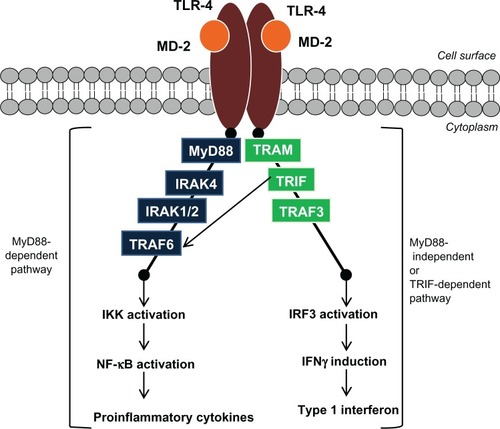
TLR-4 regulates the inflammatory responses against gram-negative bacteria, as shown in . Lipopolysaccharide (LPS) is the major component of the outer membrane of gram-negative bacteria and triggers TLR-4 signaling.Citation6,Citation7 TLR-4 requires myeloid differentiation factor 2 (MD-2) for its activation.Citation8 LPS will first bind to the LPS-binding protein (LBP) and then to the cluster of differentiation (CD)-14 before binding to TLR-4/MD-2 complex. The role of CD-14 is to enhance the sensitivity of the TLR-4/MD-2 complex.Citation9,Citation10 The binding of LPS to the TLR-4/MD-2 complex triggers conformational changes in the structure of the TLR-4/MD-2 complex, leading to TLR-4/MD-2 homodimerization (activation) and resulting in the production of proinflammatory cytokines, through the myeloid differentiation primary response protein 88 (MyD88)-dependent pathway, and the production of type 1 interferons, through a MyD88-independent pathway (via the interaction of TIR domains with adaptor molecules).Citation11
Figure 2 Schematic illustration of the TLR-4 signaling pathway.
Abbreviations: IFN, interferon; IKK, inhibitory kappa B alpha kinase; IL-1R, interleukin 1 receptor; IRAK, interleukin-1 receptor-associated kinase; IRF3, interferon regulatory factor 3; MD-2, myeloid differentiation protein-2; MyD88, myeloid differentiation protein 88; NF-κB, nuclear factor-kappaB; TLR-4, Toll-like receptor 4; TRAF, tumor necrosis factor receptor-associated factor; TRAM, TRIF-related adapter protein; TRIF, Toll/IL-1R-domain containing adapter-inducing IFN-β.
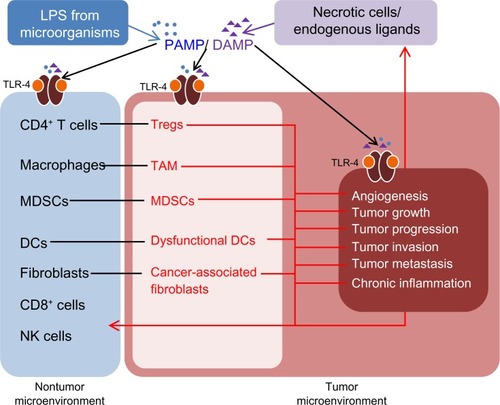
TLR-4 plays an important role in innate immunity as the first line of host defense. TLR-4 is expressed in normal epithelial cells, immune cells, and in cancer cells.Citation12 Most human cells express a low level of TLR-4 and high levels of TLR- antagonist proteins, such as Toll-interacting protein (TOLLIP), which prevent the overexpression of TLR.Citation13–Citation16 The normal immune and epithelial cells present in the skin, digestive, respiratory, and reproductive systems activate the host’s immune systems pathways through pathogen-associated molecular patterns (PAMPs) and danger associated molecular patterns (DAMPs). TLR-4 responds to the various invading exogenous pathogens through PAMPs and recognizes the endogenous ligands from necrotic cells through DAMPs ().Citation17 Although innate immunity appears to be a nonspecific response, it could differentiate “self” molecules and pathogens through the pattern recognition receptors on TLR-4.Citation18
Figure 3 TLR-4 signaling induces the transformation of the nontumor microenvironment to a tumor microenvironment.
Abbreviations: CD, cluster of differentiation; DAMP, danger-associated molecular patterns; MDSCs, myeloid derived suppressor cells; NK, natural killer; PAMP, pathogen-associated molecular patterns; TAM, tumor associated macrophages; TLR-4, Toll-like receptor 4; Tregs, regulatory T cells; DCs, dendritic cells; LPS, lipopolysaccharide.
Despite the promising innate immune responses from TLR-4, there is growing evidence that TLR-4 activation appears to act as a double-edged sword in cancers, ie, TLR-4 activation has been linked to both cancer inhibition and growth. Given the links of infection and inflammation with cancer, further evaluation of the role of TLR-4 in cancer is warranted. The detailed biological relationships between TLR-4 and cancers are still poorly understood. Therefore, in this review article, we gather the knowledge of TLR-4 activation in infection and inflammation related cancers. Also, we surveyed TLR-4 expression and its effects in various cancers (), viz, head and neck cancer, lung cancer, gastrointestinal cancer, liver cancer, pancreatic cancer, skin cancer, breast cancer, ovarian cancer, cervical cancer, and prostate cancer. Ultimately we wish to establish the therapeutic indication for whether TLR-4 agonist or antagonist is required in treating cancers. The details of the role of TLR-4 as one of the key mediators in innate immunity responsesCitation19–Citation21 and of the brief relationship between TLRs and inflammatory diseases,Citation22–Citation25 TLR-4 and infections,Citation26,Citation27 and potential TLR-4 ligandsCitation28,Citation29 are not the focus of this review paper, as these topics have been covered by other authors.
Table 1 Summary of TLR-4 expression and its effects on various tumors
TLR-4 in infection and its relation to cancer
The recognition of PAMPs by TLR-4 during microorganism invasion occurs at the plasma membrane, endosomes, lysosomes, and endolysosomes.Citation30 The innate immunity usually enhances epithelial proliferation, wound healing, and the acute inflammatory responses.Citation31 In normal host homeostasis, host immune cells activate various antitumor activities to prevent noncarcinogenic cells from evolving into carcinogenic cells. In acute pathogenic infection, CD8+ T-cells are activated and in turn, induce natural killer cells activation. The activated CD8+ T-cells eliminate any tumor through tumor-associated antigen-specific immunity, while the activated natural killer cells stimulate dendritic cells (DCs) to modulate adaptive antitumor immunity. However, studies have suggested that in a tumor microenvironment, the antitumor activities of the infiltrating immune cells were downregulated, due to the activated TLR-4 present in cancer cells.Citation32–Citation36
A recent human study reported that Helicobacter pylori, one of the common pathogenic gram-negative bacteria, induced a high expression of TLR-4 in normal gastric mucosa at the initial exposure. The authors suggested that early exposure to LPS in the gastric mucosa reduces the expression of TOLLIP and leads to the production of proinflammatory cytokines. The overexpression of TLR-4 and the chronic production of these inflammatory cytokines contributes to aberrant transcription of caudal type homeobox 2 (CDX-2), phenotypic change to intestinal metaplasia, and a lower TOLLIP expression in the cells. The progressive increase in the levels of CDX-2 activates pro-oncogenic intracellular pathways and may lead to cancer.Citation16
An animal study supported that TLR-4-deficient mice were protected from colitis-associated tumors.Citation37 The authors found that the MyD88 pathway of TLR-4 contributed to microbiota-induced colitis-associated cancer. The severity of chronic colitis was directly correlated with its colorectal cancer development.Citation38 Several other studies have also suggested that MyD88 promoted carcinogenesis in epithelial cells, in several in vivo studies.Citation39–Citation41
Bacterial-induced inflammation via TLR-4 can also induce tumor progression.Citation38 Killeen et al reported that LPS increases the expression of urokinase plasminogen activator and urokinase plasminogen activator receptor in colorectal cancer cells.Citation42 LPS also enhances the colorectal cancer cell adhesion and invasion, but not in the presence of TLR-4 blocking antibody.Citation42 LPS helps the cancer cells evade immune surveillance via the production of IL-6, IL-12, inducible nitric oxide synthase as well as via the expression of antiapoptotic proteins, such as X-linked inhibitor of apoptosis.Citation43,Citation44 Studies have also suggested that the chronic activation of TLR-4 from microorganism invasion induces oncogenic potential in the host, through the chronic activation of nuclear factor-kappaB (NF-κB) and cyclooxygenase-2 (COX-2).Citation31,Citation45,Citation46
TLR-4 in inflammation and its relation to cancer
The association between cancers and inflammation induced by PAMPs is clearly evident; however, the association between PAMPs and TLR-4 expression in cancers is minimal. This suggests nonpathogenic TLR-4-induced inflammation also plays an important role in cancers. In the noncancerous condition, cell death is usually driven by phagocytosis or apoptosis. On the other hand, in cancer cells, necrosis-induced cell death is related to the release of DAMP (). Certain DAMP, such as high mobility group box-1 protein (HMGB1), could potentially promote cancer progression.Citation19,Citation47 HMGB1 is a deoxyribonucleic acid (DNA)-binding protein secreted from cells upon cytokine stimulation or necrotic cell death. Cancer cells have been found to have high expression of HMGB1.Citation48,Citation49 HMGB1 has also been reported to induce cancer cell invasion, migration, and metastasisCitation50,Citation51 as well as in vitro endothelial cell proliferation and in vivo neovascularization.Citation52 Anti-HMGB1 antibody was also shown to inhibit angiogenesis.Citation53 This evidence collectively suggests HMGB1 as an endogenous TLR-4 ligand that induces carcinogenesis.Citation28,Citation54 In one study, it was found that the levels of these DAMP-derived molecules were high in the tumor microenvironment and that they induced TLR-4-related chronic inflammation, leading to carcinogenesis, cancer progression, and metastasis.Citation12 Another study highlighted that the TLR-4/MD-2 complex enhanced the formation of regions of hyperpermeability, through upregulation of C-C chemokine receptor type 2 (CCR2) expression, in inflamed mice and thus increased the rate of lung metastasis.Citation55 Metastasis was found to be initiated also by serum amyloid, through TLR-4-dependent NF-κB inflammation pathways. Citation56 All these latest findings clearly suggested the pivotal role of the TLR-4/MD-2 complex in inflammation-associated cancers.
TLR-4 signaling in tumor cells versus immune cells
The hallmarks of inflammation-associated cancers are the presence of inflammatory cells and cytokines in the tumor microenvironments that leads to tumor growth, angiogenesis, tumor invasion, and/or metastasis.Citation57,Citation58 TLR-4 activation upregulates the proinflammatory cytokines and chemokines (CCL, CXCL, CCR, CXCR, IL-6, IL-18, tumor necrosis factor [TNF]-α, etc) as well as immunosuppressive cytokine (transforming growth factor [TGF-β]1, IL-10 etc), especially in tumor microenvironment ().Citation12 CD4+, CD25+, and Foxp3+ regulatory T cells in the tumor microenvironment secrete IL-10 and TGF-β, which limit the antitumor effect of non-regulatory T-cells.Citation12,Citation59 In tumors, associated macrophages have been shown to release angiogenic and lymphangiogenic factors that promote metastasis.Citation12,Citation60 Myeloid-derived suppressor cells recruited to the tumor microenvironment under the influence of IL-1β, IL-6, and prostaglandin (PG)E2 were also shown to impair the antitumor response of the host, through the release of nitric oxide synthase and TGF-β.Citation12 DCs too, were found to be dysfunctional in the tumor microenvironment, due to the suppressive effects of vascular endothelial growth factor (VEGF), IL-6, IL-1, TGF-β, COX-2 and PGE2.Citation12,Citation61 Elsewhere, fibroblast was transformed into cancer-associated fibroblast (CAF) in the tumor microenvironment, and TGF-β-induced CAF activation promoted tumor growth and proliferation. TLR-4 activation has also been related to TGF-β signaling–related cancer growth.Citation12,Citation62–Citation64
As a result of these findings, these infiltrating immune cells were hypothesized to support cancer progression, angiogenesis, and metastasis.Citation32–Citation36 All the above suggest that the release of various immunosuppressive cytokines, proinflammatory cytokines, and chemokines during the activation of TLR-4 could contribute to cancer formation in normal epithelial cells, immune cells, and cancer cells.
TLR-4 in modulation of angiogenesis and its relation in limiting tumor progression
Despite the fact that activated TLR-4 has procarcinogenic effects, the antitumor effect induced by activated TLR-4 should not be neglected. One study reported that the Mycobacterium bovis bacillus Calmette–Guérin cell wall skeleton enhanced the cytotoxic activity of T-cells and macrophages against cancer cells, probably through TLR-4. Bacillus Calmette–Guérin cell wall skeleton induced TNF-α secretion from DCs through TLR-2 and TLR-4 signaling and thus induced the maturation of DCs.Citation65 Elsewhere, OK-432, a penicillin-killed and lyophilized preparation of Streptococcus pyogenes exhibited potent immunotherapeutic effect in cancers.Citation66 Studies supported that OK-PSA, the molecule isolated from a butanol extract of OK-432, induced anticancer immunity and DC maturation, via TLR-4.Citation67 It was also found that OK-PSA-induced cytokine production was inhibited by anti-TLR-4 monoclonal antibody. In other work, the compound isolated from Aeginetia indica L. (AILb-A) induced NF-κB activation in NF-κB-dependent reporter cells containing TLR-4 plasmid; AILb-A also did not induce any cytokine production in TLR-4 deficient mice.Citation67 Finally, synthetic serine-based glycolipid (CCL-34)-activated macrophages were shown to induce cancer cell death through TLR-4 dependent pathways.Citation68 These findings suggest TLR-4 activators induce antitumor immunity through TLR-4 signaling.
Role of MD-2 in cancer
The expression of MD-2 in most cancer cells has not been well studied. Limited evidence has concluded that MD-2 plays a role in cancer progression only. One study highlighted that MD-2 was overexpressed in highly invasive colorectal cancer cells (SW837), in poorly differentiated, moderately invasive colorectal cancer cells (HT-29), and in well-differentiated but non-invasive colorectal cancer cells (Caco-2).Citation69 Another study reported that serum amyloid A 3, a major component of acute phase inflammation, binds to MD-2 and activates the MyD88-dependent TLR-4/MD-2 pathway and thus facilitates lung metastasis.Citation70 Therefore, MD-2 could be related to the degree of differentiation, proliferation, and migration capacity of cancers.
TLR-4 in head and neck cancer
The laryngeal carcinomas predominate in this category.Citation71 The treatment of head and neck cancers remains a big challenge for oncologists. A study in Poland of 20 laryngeal cancer patients with no distant metastasis (M0=100%) reported TLR-2-, TLR-3-, and TLR-4-expression in the laryngeal carcinoma microenvironment. Although TLR-4 is the least frequently expressed TLR on laryngeal tumors, it is still the most frequently expressed TLR on inflammatory cells in all tumor masses and tumor stroma. This fact suggests the possibility of TLR-4 involvement in the escape of tumors from immune surveillance. The detection of TLR-2, TLR-3, TLR-4, and major histocompatibility complex (MHC) class II antigens in all laryngeal carcinoma suggests the role of TLRs in the activation of adaptive immunity.Citation72
TLR-4 was also detected in laryngeal tumor tissue (n=27), oral cavity tumor tissue (n=10), and cancer cells (PCI-1, PCI-13, and PCI-30).Citation73 Moreover, TLR-4 levels in tumors were correlated with tumor differentiation. TLR-4 was shown to enhance the release of tumor progression mediators, resulting in tumor proliferation and progression.Citation73,Citation74 A study reported cancer cells express significantly higher levels of TLR-4 and NF-κB compared with noncancerous cells. A positive correlation was also established between TLR-4 levels in laryngeal tumor central cells, with tumor front grading.Citation74 Another study, published in 2012, suggested the increased activity of TLR-4, TNF receptor-associated factor (TRAF) 6, and IL-1 receptor-associated kinase (IRAK) 1 in advanced laryngeal carcinoma, suggesting the TLR-4/MyD88-dependent signaling pathway involvement in laryngeal carcinoma progression.Citation75
TLR-4 in lung cancer
Worldwide, lung cancer is the most common cause of cancer-related deaths in men and women. Lung cancer mortality rates have been rising in recent decades. Chronic inflammatory disease, such as chronic obstructive pulmonary disease (COPD), has been identified as a risk factor for lung cancer.Citation76 Since TLR-4 is also actively involved in the immune response against cancers, researchers have postulated that TLR-4 exerts both a defensive role in normal cells and a negative role in cancer cells. However, the available evidence is still not conclusive on the link between TLR-4 and lung cancer.
A study of functional TLR-4 and mutated TLR-4 in mice found that mice with the former had less lung capillary permeability, less weight loss, leukocyte inflammation, and primary tumor formation. Thus, the authors, Bauer et al, postulated that TLR-4 inhibits lung carcinogenesis by inhibiting tumor progression.Citation77 The researchers proved TLR-4 activation could protect the lungs from being inflamed during any potential tumorigenesis.Citation78 Elsewhere, a lower level of TLR-4 in the nasal epithelium of a smoker compared with a nonsmoker was observed, with a profound reduction in patients with severe COPD.Citation79 This finding suggests the potential role of TLR-4 for airway inflammation and lung cancer progression.
In vitro studies have also found that TLR-4 is constantly expressed and upregulated on human lung cancer cells.Citation33,Citation80 In one study, the level of TLR-4 was significantly linked with the production of immunosuppressive cytokines, production of proangiogenic chemokine, and with resistance to apoptosis by lung cancer cells.Citation33 Despite the reported significance of TLR-9 in lung cancer progression,Citation81–Citation84 a positive correlation (P<0.05) of TLR-4, but not TLR-9, with tumor differentiation in lung cancer patients was reported.Citation80
TLR-4 in gastrointestinal cancer
The gastrointestinal tract (esophagus, stomach, and small and large intestine) is often exposed to pathogens and carcinogens. Epidemiological studies suggest chronic inflammation, whether pathogen-related or not, increases the risk of gastrointestinal cancer.Citation31,Citation85
Globally, there has been an increase in esophageal cancer.Citation86 Esophageal cancer usually occurs in Barrett’s esophagus. The link or relationship between inflammation and tumorigenesis in esophageal cancer remains controversial. Esophageal motility allows pathogen transit from the oral cavity to the stomach, making it difficult to quantify PAMP and bacteria exposure. Single nucleotide polymorphism arrays have suggested that in patients with esophageal squamous cell carcinoma, genetic alterations in TLR-4 (9q32–q33) along with other chromosomal mutations are associated with higher cancer proliferation and metastasis.Citation87 Immunohistochemistry and reverse transcription polymerase chain reaction of samples from 87 esophageal cancer patients revealed that TLR-4 as well as TLR-3, TLR-7, and TLR-9 are overexpressed in esophageal cancer. The isolated mononuclear inflammatory cells associated with higher lymph node metastasis and invasion were also shown to express a high level of TLR-4.Citation88 The level of mucosal expression of TLRs in the esophagus has not yet been elucidated.Citation31
H. pylori infection has been identified as the root cause of stomach cancer. Gastric inflammation is an invariable finding in patients infected with H. pylori and represents the host immune response to the organism. @@H. pylori infection leads to gastric inflammation, characterized histologically by surface epithelial degeneration and infiltration of the gastric mucosa, through acute and chronic inflammatory cells. In H. pylori infections, TLR-4 on the gastric epithelial cells regulates the LPS response.Citation89–Citation91 Gastric biopsy samples have suggested H. pylori infections accelerate TLR-4 and MD-2 expression in human gastric epithelial cells,Citation90 which in turn enables the gastric carcinoma cells to further interact with H. pylori and consequently, induce the secretion of gastric carcinoma promoting factors.Citation92 Elsewhere, anti-TLR-4 antibody inhibited H. pylori-induced messenger ribonucleic acid (mRNA) expression of human β-defensin in gastric cancer cells (MKN 45).Citation93 These results further strengthened the hypothesis that H. pylori induce the expression of TLR-4 in gastric epithelial cells.
Based on the report of TLR-4 signaling in colonic COX-2 expression and PGE2 production, Fukata et al suggested that there is a high correlation between TLR-4 signaling and the expression of COX-2 as well as PGE2 in H. pylori-associated gastric cancers.Citation94 The expression of NF-κB in association with COX-2 and TNF-α was postulated to be mediated through TLR-4, but not through TLR-2 or -9, based on the findings from previous research, in which guinea pig gastric epithelial cells were preincubated with H. pylori LPS.Citation89
Patients with poorly differentiated gastric adenocarcinomas have been shown to have Thr35 Ala polymorphism in the LRR of TLR-4, but not TLR-2, TLR-6, or TLR-9.Citation95 This finding supports the hypothesis that TLR-4 gene polymorphism is related to poorly-differentiated gastric adenocarcinoma. In addition, a significantly higher risk of gastric carcinoma has been found with TLR-4 Ala896Gly polymorphism.Citation96 A case-control study of 171 Italian gastric cancer patients and 151 controls reported TLR-4 Thr399Ile polymorphism, but not TLR-4 Asp299Gly polymorphism, is linked with increased risk of gastric cancer (P=0.023, hazard ratio [HR] =3.62). Further, an increased risk of intestinal gastric cancer (P=0.006, HR=5.38), but not diffuse gastric cancer (P=0.612, HR=1.85) was reported in carriers of TLR-4 Thr399Ile allele.Citation97 Similar results were reported by repeated studies on the role of TLR-4 Asp299Gly/Thr399Ile single nucleotide polymorphism in relation to gastric carcinogenesis.Citation98,Citation99 However, another case-control study carried out in Mexico reported neither TLR-4 Asp299Gly polymorphism (P=0.82) nor TLR-4 Thr399Ile polymorphism (P=0.2) was associated with significant incidence of gastric cancer.Citation100
TLR-4 in colorectal cancer can be a double-edged sword, enhancing the host anticancer immunity and promoting tumor growth at the same time. In acute intestinal mucosa injury, in response to LPS, TLR-4 expression induces COX-2 expression, which leads to wound healing. Thus, mice that lack MyD88 or TLR-4 signaling have been shown to have a reduced healing ability after an acute injury. However, in chronic intestinal inflammation, TLR-4 induces COX-2 and PGE2 production, which may result in early colorectal carcinogenesis, inhibition of apoptosis, and increase of angiogenesis.Citation94,Citation101 Thus, blocking of TLR-4 signaling can prevent colon cancer cell (MC26 cells) proliferation and reverse tumor-mediated suppression of T-cell proliferation. Just as in other cancers, chronic inflammation in intestinal epithelial cells is closely related to the incidence rate of colorectal cancer. Healthy intestinal epithelial cells constitutively express TLR-3 and TLR-5, where TLR-2 and TLR-4 are lower in quantity.Citation102 However, altered expression of TLRs is observed in chronically inflamed intestinal epithelial cells.Citation101–Citation103 TLR-4 expression is significantly upregulated in inflammatory bowel disease (IBD), but expression of TLR-2 and TLR-5 remains unchanged.Citation102 A significant induction of TLR-2 and TLR-4 expression has been observed on the submucosa of inflamed intestinal epithelial cells.Citation104 Also, a significant increase in TLR-4 expression was identified in the colon cancer cells.Citation105,Citation106 Patients with higher levels of TLR-4 in colon tumor stroma were shown to have an earlier relapse (14 months) compared with those who had lower expression (40 months).Citation105 This clearly suggests the role of TLR-4 in colorectal tumorigenesis and progression.
Patients suffering with IBD do have increased risk of cancer (0.5%–1% yearly).Citation31 The impact of TLR-4 polymorphism on IBD is controversial. No association was reported with TLR-4 Asp299Gly and Thr399Ile polymorphism, in a group of IBD patients from Southern Italy,Citation107 New Zealand,Citation108 Germany, and Hungary.Citation109 However, some studies have suggested a significant link between TLR-4 Asp299Gly polymorphism and either ulcerative colitis or Crohn’s disease or both.Citation110–Citation115 In addition, a meta-analysis reported in 2010, showed a significant association between TLR-4 (Asp299Gly and Thr399Ile) polymorphism with ulcerative colitis (odds ratio [OR] =1.08, 95% confidence interval [CI]: 1.08–1.51), Crohn’s disease (OR: 1.29, 95% CI: 1.08–1.54), and IBD (OR: 1.25, 95% CI: 1.06–1.48).Citation116 As well, TLR-4 Asp299Gly polymorphism was significantly higher in colorectal patients compared with normal healthy adults (P=0.0269).Citation117 Also, studies report that LPS enhanced colorectal cancer cell adhesion and invasion, through TLR-4- and NF-κB-dependent activation of the urokinase plasminogen activator system and beta-1 integrin, which ultimately leads to tumor progression.Citation64,Citation118,Citation119 In vivo data suggests TLR-4 inhibition has prolonged the survival rate of tumor-bearing mice (BALB/c).Citation43 Stimulation of the TLR-4/MD-2 complex by LPS can activate phosphoinositide-3-kinase (PI3 K) signaling and thus promotes the adhesiveness and metastatic capacity of colorectal cancer cells.Citation120 All these findings have consolidated the role of TLR-4 in colorectal cancer progression.
TLR-4 in liver cancer
Dysregulated innate immunity is an integral component in liver disease. Chronic liver diseases, such as alcoholic liver cirrhosis, have also been established as the major cause of liver cancer.Citation121 Many liver cells (Kupffer cells, hepatocytes, stellate cells, biliary epithelium, and sinusoidal endothelium) constantly express TLR-4.Citation122 The levels of TLR-4 have been found to be upregulated in hepatitis B– and hepatitis C–infected cellsCitation123,Citation124 and further upregulated in alcohol-induced liver damage and tumors.Citation125,Citation126 On the other hand, diethylnitrosamine, a carcinogen, was shown to stimulate TLR-4 signaling in mice, resulting in an increase in the size and number of tumors, while the size and number of tumors were found be reduced in MyD88 deficient mice.Citation40
TLR-4 in pancreatic cancer
It is very difficult to detect pancreatic ductal adenocarcinoma at early stage because of its anatomic location and insidious nature. LPS could be a triggering factor in the initiation and progression of pancreatitis and pancreatic cancer.Citation7,Citation127 This suggests the possible role of TLR-4 in pancreatic cancer since LPS is a well-established agonist of TLR-4.
Significant expression of TLR-4 (P=0.002) was detected in a study of pancreatic ductal adenocarcinoma as compared with adjacent normal tissues.Citation128 In this study, there was no correlation found between the levels of TLR-4 and age, gender, location and differentiation of tumor; however, TLR-4 levels were correlated with tumor size, lymph node involvement, venous invasion, and pathological stage. Positive correlations were also observed between TLR-4 and hypoxia-inducible transcription factor-1α (HIF-1α). The expression of NF-κB phosphorylated p65 was also higher in the tumor cells. The patients with overexpressed TLR-4 or overexpressed HIF-1α had a significantly shorter survival period than did the patients with normal expression (P=0.011 and P=0.005, respectively). Longer survival (P=0.014) was noted among patients with neither TLR-4 nor HIF-1α over-expressed compared with patients who had both TLR-4 and HIF-1α overexpressed.Citation130 A separate in vitro study on human pancreatic cancer cells (Panc-1 and AsPC-1) revealed that TLR-4 was responsible for the invasive ability of cancer cells, mostly due to TLR-4-dependent NF-κB activation.Citation6 These results demonstrated the importance of TLR-4 in pancreatic cancer proliferation.
TLR-4 in skin cancer
Not only is skin the largest organ in our human body, it also protects us from pathogen invasion through its innate and adaptive immunity. A study has drawn attention to the role of TLRs in atopic dermatitis, psoriasis, acne vulgaris, and skin infection and has suggested the potential of TLRs as a therapeutic target to treat skin cancer.Citation129 Melanoma cells have a significantly higher amount of TLR-4 levels.Citation130–Citation133 An in vitro analysis showed the consistent expression of TLR-4 on 13 out of 15 established human metastatic melanoma cells (ME1, ME2, ME5, ME7, ME8, ME9, ME16, ME17, ME18, ME19, ME20, ME21, and ME22) tested. The expression of TLR-2 and -3 was also detected in some, but not in all. TLR-1,-5,-6,-7,-8,-9, and -10 were either absent or weakly expressed. The TLRs, including TLR-4, were upregulated in melanoma,Citation32 and it was noted that the coadministration of paclitaxel and icariside II (isolated from Herba Epimedii) enhanced apoptosis and decreased the levels of IL-8 and VEGF in human melanoma A375 cells, through the inhibition of TLR-4/MyD88 signaling.Citation134 A group of German scientists found that TLR-4 was involved in melanoma responses to hyaluronic acid-induced tumor invasion and metastasis.Citation132
Another study suggested that the chemical carcinogen 7,12-dimenthylbenz(a)anthracene (DMBA) is associated with endotoxin hypersensitivity mediated through TLR-4-triggered T-cell activation, resulting in cell-mediated immunity. In that study, TLR-4-deficient mice were found to have more tumors compared with normal mice.Citation135 Another study showed that melanoma inhibits macrophage activation by suppressing TLR-4 signaling.Citation136 An interesting theory, proposed by Sanchez-Perez et al, is that the intentional generation of an autoimmune response by normal cells could generate a potential antitumor response against tumors of the same type cells. This would explain their finding that the heat shock protein (Hsp)70, a potent immune adjuvant acting through TLR-4 activation, kills inflammatory melanocytes.Citation131 Thus, TLR-4 activation could be beneficial, at the initial stage, to control melanoma progression. However, the persistent activation of TLR-4 may be harmful to the host.
TLR-4 in breast cancer
Breast cancer is one of the most common cancers affecting women worldwide. The current available therapy has yet to meet the desired outcomes. Two research teams have suggested the role of TLR-2Citation137 and TLR-9Citation138 in breast cancer proliferation. In 2010, Yang et al reported that ten TLRs were expressed in MDA-MB-231 cells, the estrogen receptor–negative human breast cancer cells. Among all the TLRs, TLR-4 expression was the highest and was five times higher than TLR-3 expression (the least expressed among the remaining TLR-1 to TLR-10). Functional analyses of ribonucleic acid interference (RNAi) against TLR-4 revealed this successfully inhibited the growth and proliferation of MDA-MB-231 cells and resulted in a significant (P<0.05) reduction of inflammatory cytokines.Citation139
In other work, 4T1 (spontaneously metastasizing mammary adenocarcinoma) cells challenged with lipopolysaccharide induced tumor growth and metastasis, by increasing angiogenesis, vascular permeability, and tumor invasion.Citation140,Citation141 An immunohistochemical study on clinical carcinomas showed a significant association of high TLR-4 expression with local cancer proliferation and lymph node metastasis.Citation142 A total of 74 breast carcinomas were collected from patients to study the clinical relevance of TLRs in breast cancer. Tumors with high TLR-4 expression, but not TLR-9 expression, in mononuclear cells were found to have a higher probability of metastasis.Citation143 In metastatic breast cancer cells, activated TLR-4 regulates the expression of mediators that promote cancer adhesion and invasion. In addition to this, TLR-4 signaling increases microRNA 21 (miR-21) in breast cancer cells, through NF-κB.Citation144
Incidents of relapses have been shown to be high in breast cancer patients harboring the TLR-4 Asp299Gly polymorphism who were treated with anthracycline-based chemotherapeutic drugs.Citation145 This polymorphism also confers to an increased risk of breast cancer progression.Citation146 However, the levels of the TLR-4 Ala896Gly allele in breast cancer patients were not found to be significantly different from those levels in normal healthy Caucasian women.Citation147 These studies suggest TLR-4 involvement in breast cancer progression. Future studies are warranted, in developing novel therapeutic approaches targeting TLR-4 against breast cancer.
TLR-4 in ovarian cancer
A proinflammatory environment in the ovary, such as with ovarian endometriosis, predisposes women to ovarian cancer. Epithelial ovarian cancer cells have been found to express TLR-4, but not the adjacent nondysplasia cells.Citation34,Citation148 It has been found that ovarian tumors derived from the surface epithelium of normal ovaries express TLR-4, which leads to NF-κB inhibitor (IkB) degradation and the activation of NF-κB for its proinflammatory responses. The MyD88 pathway, a downstream signal of TLR-4, is reported to be essential for LPS-induced ovarian tumor growth.Citation34 The tumor growth and survival could be due to the interaction between the inducible HspA1 A from ovarian cancer cells with TLR-4 expressed on the neutrophil surface. These neutrophils enhance the production of reactive oxygen species and induce tumor progression as well as tumor lysis.Citation149
Paclitaxel has been the drug of choice for the treatment of ovarian cancer. A recent study has proven the association of MyD88 expression with paclitaxel resistance, and paclitaxel-induced proinflammatory cytokine release.Citation34 Paclitaxel resistance is probably due to the activation of the protein kinase B (Akt) survival pathways and the expression of the antiapoptotic protein X-linked inhibitor of apoptosis protein (XIAP) following TLR-4 ligation.Citation34,Citation150 The tumors have been found to be resistant to paclitaxel, a TLR-4 ligand, but not to carboplatin.Citation34 Another laboratory finding has suggested that five ovarian cancer (OVCAR3, SKOV3, AD10, A2780, CP70) cells with TLR-4 ligation induced IL-1 receptor-associated kinase (IRAK)-4 activation, c-Jun phosphorylation, NF-κB activation, and IL-8, IL-6, VEGF, and monocyte chemotactic protein-1 (MCP)-1 production, all of which promote tumor survival and chemoresistance.Citation148 These findings suggest that further research on the role of TLR-4 in relation to management and chemotherapy for ovarian cancer should be carried out.
TLR-4 in cervical cancer
The female reproductive tract is constantly exposed to pathogens and carcinogens. Cervical cancer is one of the most common cancers closely linked to infection and inflammation. Human papillomavirus (HPV) has been identified as the leading cause of cervical cancer. HPV cervical infection results in cervical morphological lesions ranging from normal to invasive cancer.Citation151 The presence of other infectious agents, such as bacteria, protozoa, and viruses, in the female genital tract induce the inflammation and cancer.Citation152 A multi-center case-control study revealed that Chlamydia trachomatis infection induced the cervical cancer.Citation153 Also, pathogenic Escherichia coli and Pseudomonas aeruginosa infection, but not the nonpathogenic Lactobacillus reuteri infection, caused the upregulation of TLR-4 in cervical cancer cells.Citation154 In human cervical cancer (HeLa) cells, TLR-4 was found to be the highest expressed TLR, more than 100 times higher compared with the other TLRs.Citation126 This observation provides proof of the linkage between TLR-4 and the progression of cervical cancer.
Unlike other studies linking higher levels of TLR-4 with cancer, it was found that TLR-4 is downregulated in cervical intraepithelial neoplasia patients compared with healthy women. TLR-4 expression was found to decrease as the histopathologic grade of cervical intraepithelial neoplasia increased. TLR-4 expression was found to be inversely proportional (P<0.001) with the expression of p16INK4A, a marker of high-risk HPV infection.Citation155,Citation156 There are limited studies that conclude on the role of TLR-4 in cervical cancer progression; thus, the TLR-4 Thr399Ile polymorphism (P=0.044, OR=2.51, 95% CI: 1.03–6.12) was, again, found to be significantly associated with early stages of cervical cancers among North Indian women.Citation157 Therefore, further research is needed to fully understand the role of TLR-4 in cervical cancer.
TLR-4 in prostate cancer
Prostate cancer is one of the most common causes of morbidity and mortality in men. Prostate epithelial cells are actively involved in inflammatory processes.Citation158 Higher levels of proinflammatory cytokines, produced through TLR-3, -4, and -9 downstream signaling pathways, were observed in the prostate tissues of cancer patients. In this study, TLR-3 -4 and -9 were highly expressed in prostate cancer tissues but not in benign tissues. Only TLR-3 levels, and not TLR-4 or TLR-9 levels, were found to have statistical significance (P=0.016) with levels of preoperative serum prostate-specific antigen, in prostate cancer patients.Citation159
However, in vitro studies have shown the expression of higher levels of TLR-4 on human prostate adenocarcinoma (DU-145) cells and its activation, leading to NF-κB and proinflammatory cytokine production through the MyD88- dependent pathway.Citation160 Also, TLR-4 activation was found to increase the proangiogenic factor (VEGF) and immunosuppressive cytokine (TGF-β1) secretion in human prostate adenocarcinoma (PC3) cells.Citation161 Further, a knockdown of TLR-4 in PC3 cells resulted in the reduction of tumor cell migration and invasion.Citation162 These results support the negative impact of TLR-4 upregulation in prostate cancer.
In addition, single nucleotide polymorphism in the TLR-4 gene is suspected to be associated with the risk of prostate carcinoma.Citation163 The sequence variant (11381 G/C, also known as rs11536889) in the 3′-untranslated region of the TLR-4 gene was found to be higher in patients with prostate carcinoma, in studies conducted on 1,383 Swedish patientsCitation164 and 157 Korean patients.Citation165 In one study, a significantly higher risk of prostate cancer (OR: 1.26; 95% CI: 1.01–1.57) was detected among men who had a single nucleotide polymorphism of TLR-4 (GC or CC) compared with the wild-type genotype (GG).Citation164 However, other studies found no association of prostate cancer (OR: 1.01; 95% CI: 0.79–1.29) with this rs11536889 sequence variant of TLR-4;Citation166,Citation167 one of these, a study involving 700 prostate cancer patients found that homozygosity of the variant alleles of these eight single-nucleotide polymorphisms of TLR-4 including rs2149356 were found to have a lower risk of prostate cancer.Citation166 A case-control study of 506 incident advanced prostate cancer patients found two single nucleotide polymorphisms of TLR-4 (rs10759932 and rs2149356) were associated with a higher cancer risk.Citation167 Nevertheless, Lindstrom et al have suggested that the association of TLR-4 with prostate cancer risk is a chance finding and that to ascertain the relationship, large sample sizes are needed.Citation168 The inconsistent findings of the association of genetic polymorphism of TLRs with cancer progression clearly supports the need for further investigation in this field.
Conclusion
The activation of TLR-4 is required for host defense against gram-negative bacteria. However, TLR-4 activation may be a double-edged sword, with both antitumor and protumor responses. The general expression of TLR-4 by all the tumor cells, suggesting TLR-4 signaling, may be continually activated and contribute to tumor initiation, progression, and also invasion. Tumor progression involves TLR-4-mediated irregular and uninhibited production of proinflammatory cytokines, chemokines, and also immunosuppressive cytokines; suggesting that the discovery of TLR-4 antagonists might be an ideal strategy to treat cancer. However, TLR-4 antagonists could pose the risk of the compromise of host immunity. Hence, it is a scientific dilemma whether a TLR-4 agonist or antagonist should be targeted for the treatment of cancer. Further studies need to be carried out to fully elucidate the effects of TLR-4 agonists and antagonists in various cancers.
Acknowledgments
Authors wish to thank International Medical University for financial support (IMU 226/2010).
Disclosure
The authors report no conflicts of interest in this work.
References
- MedzhitovRPreston-HurlburtPJanewayCAA human homologue of the Drosophila Toll protein signals activation of adaptive immunityNature199738866403943979237759
- JinMSLeeJOStructures of the toll-like receptor family and its ligand complexesImmunity200829218219118701082
- O’NeillLABowieAGThe family of five: TIR-domain- containing adaptors in Toll-like receptor signallingNat Rev Immunol20077535336417457343
- ParkBSSongDHKimHMChoiBSLeeHLeeJOThe structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complexNature200945872421191119519252480
- OpalSMEsmonCTBench-to-bedside review: functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsisCrit Care200371233812617738
- IkebeMKitauraYNakamuraMLipopolysaccharide (LPS) increases the invasive ability of pancreatic cancer cells through the TLR4/MyD88 signaling pathwayJ Surg Oncol2009100872573119722233
- Del PozoJLPrimers on molecular pathways: lipopolysaccharide signaling – potential role in pancreatitis and pancreatic cancerPancreatology2010102–311411820460943
- NagaiYAkashiSNagafukuMEssential role of MD-2 in LPS responsiveness and TLR4 distributionNat Immunol20023766767212055629
- GioanniniTLTeghanemtAZhangDIsolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrationsProc Natl Acad Sci U S A2004101124186419115010525
- OhtoUFukaseKMiyakeKSatowYCrystal structures of human MD-2 and its complex with antiendotoxic lipid IVaScience200731658311632163417569869
- LoiarroMRuggieroVSetteCTargeting TLR/IL-1R signalling in human diseasesMediators Inflamm2010201067436320396389
- SatoYGotoYNaritaNHoonDSCancer Cells Expressing Toll-like Receptors and the Tumor MicroenvironmentCancer Microenviron20092 Suppl 120521419685283
- Pimentel-NunesPSoaresJBRoncon-AlbuquerqueRDinis-RibeiroMLeite-MoreiraAFToll-like receptors as therapeutic targets in gastrointestinal diseasesExpert Opin Ther Targets201014434736820146632
- OtteJMCarioEPodolskyDKMechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cellsGastroenterology200412641054107015057745
- MansellASmithRDoyleSLSuppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradationNat Immunol20067214815516415872
- Pimentel-NunesPGonçalvesNBoal-CarvalhoIHelicobacter pylori induces increased expression of Toll-like receptors and decreased Toll-interacting protein in gastric mucosa that persists throughout gastric carcinogenesisHelicobacter2013181223223061653
- TsanMFToll-like receptors, inflammation and cancerSemin Cancer Biol2006161323716153858
- KimbrellDABeutlerBThe evolution and genetics of innate immunityNat Rev Genet20012425626711283698
- KawaiTAkiraSThe role of pattern-recognition receptors in innate immunity: update on Toll-like receptorsNat Immunol201011537338420404851
- LuYCYehWCOhashiPSLPS/TLR4 signal transduction pathwayCytokine200842214515118304834
- UnderhillDMToll-like receptors: networking for successEur J Immunol20033371767177512811836
- SoEYOuchiTThe application of Toll like receptors for cancer therapyInt J Biol Sci20106767568121060729
- FrenchSWOlivaJFrenchBALiJBardag-GorceFAlcohol, nutrition and liver cancer: role of Toll-like receptor signalingWorld J Gastroenterol201016111344134820238401
- Rakoff-NahoumSMedzhitovRToll-like receptors and cancerNat Rev Cancer200991576319052556
- HuangBZhaoJUnkelessJCFengZHXiongHTLR signaling by tumor and immune cells: a double-edged swordOncogene200827221822418176603
- MiyakeKRoles for accessory molecules in microbial recognition by Toll-like receptorsJ Endotoxin Res200612419520416953972
- MillerSIErnstRKBaderMWLPS, TLR4 and infectious disease diversityNat Rev Microbiol200531364615608698
- OblakAJeralaRToll-like receptor 4 activation in cancer progression and therapyClin Dev Immunol2011201160957922110526
- ParkSHKimNDJungJKLeeCKHanSBKimYMyeloid differentiation 2 as a therapeutic target of inflammatory disordersPharmacol Ther2012133329129822119168
- AkiraSUematsuSTakeuchiOPathogen recognition and innate immunityCell2006124478380116497588
- FukataMAbreuMTRole of Toll-like receptors in gastrointestinal malignanciesOncogene200827223424318176605
- GotoYArigamiTKitagoMActivation of Toll-like receptors 2, 3, and 4 on human melanoma cells induces inflammatory factorsMol Cancer Ther20087113642365319001446
- HeWLiuQWangLChenWLiNCaoXTLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistanceMol Immunol200744112850285917328955
- KellyMGAlveroABChenRTLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancerCancer Res20066673859386816585214
- LiHHanYGuoQZhangMCaoXCancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1J Immunol2009182124024919109155
- StraussLBergmannCWhitesideTLHuman circulating CD4+CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosisJ Immunol200918231469148019155494
- FukataMChenAVamadevanASToll-like receptor-4 promotes the development of colitis-associated colorectal tumorsGastroenterology200713361869188118054559
- UronisJMMühlbauerMHerfarthHHRubinasTCJonesGSJobinCModulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibilityPLoS One200946e602619551144
- SwannJBVeselyMDSilvaADemonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesisProc Natl Acad Sci U S A2008105265265618178624
- NauglerWESakuraiTKimSGender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 productionScience2007317583412112417615358
- Rakoff-NahoumSMedzhitovRRegulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88Science2007317583412412717615359
- KilleenSDWangJHAndrewsEJRedmondHPBacterial endotoxin enhances colorectal cancer cell adhesion and invasion through TLR-4 and NF-kappaB-dependent activation of the urokinase plasminogen activator systemBr J Cancer2009100101589160219436306
- HuangBZhaoJLiHToll-like receptors on tumor cells facilitate evasion of immune surveillanceCancer Res200565125009501415958541
- DanHCSunMKanekoSAkt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP)J Biol Chem200427975405541214645242
- AkiraSTakedaKToll-like receptor signallingNat Rev Immunol20044749951115229469
- FukataMAbreuMTPathogen recognition receptors, cancer and inflammation in the gutCurr Opin Pharmacol20099668068719828376
- LotzeMTZehHJRubartelliAThe grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunityImmunol Rev2007220608117979840
- EllermanJEBrownCKde VeraMMasquerader: high mobility group box-1 and cancerClin Cancer Res200713102836284817504981
- BrezniceanuMLVölpKBösserSHMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinomaFASEB J200317101295129712759333
- PoserIGolobMBuettnerRBosserhoffAKUpregulation of HMG1 leads to melanoma inhibitory activity expression in malignant melanoma cells and contributes to their malignancy phenotypeMol Cell Biol20032382991299812665595
- SasahiraTKiritaTOueNHigh mobility group box-1-inducible melanoma inhibitory activity is associated with nodal metastasis and lymphangiogenesis in oral squamous cell carcinomaCancer Sci20089991806181218616526
- SchlueterCWeberHMeyerBAngiogenetic signaling through hypoxia: HMGB1: an angiogenetic switch moleculeAm J Pathol200516641259126315793304
- van BeijnumJRPetersenKGriffioenAWTumor endothelium is characterized by a matrix remodeling signatureFront Biosci (Schol Ed)2009121622519482697
- SimsGPRoweDCRietdijkSTHerbstRCoyleAJHMGB1 and RAGE in inflammation and cancerAnnu Rev Immunol20102836738820192808
- HiratsukaSIshibashiSTomitaTPrimary tumours modulate innate immune signalling to create pre-metastatic vascular hyperpermeability fociNat Commun20134185323673638
- HiratsukaSWatanabeASakuraiYThe S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phaseNat Cell Biol200810111349135518820689
- MantovaniAAllavenaPSicaABalkwillFCancer-related inflammationNature2008454720343644418650914
- CandidoJHagemannTCancer-related inflammationJ Clin Immunol201333 Suppl 1S79S8423225204
- PerroneGRuffiniPACatalanoVIntratumoural FOXP3-positive regulatory T cells are associated with adverse prognosis in radically resected gastric cancerEur J Cancer200844131875188218617393
- MartinezFOSicaAMantovaniALocatiMMacrophage activation and polarizationFront Biosci20081345346117981560
- ZouWImmunosuppressive networks in the tumour environment and their therapeutic relevanceNat Rev Cancer20055426327415776005
- ZhouYHLiaoSJLiDTLR4 ligand/H2O2 enhances TGF-β1 signaling to induce metastatic potential of non-invasive breast cancer cells by activating non-Smad pathwaysPLoS One201385e6590623734265
- SekiEDe MinicisSOsterreicherCHTLR4 enhances TGF-beta signaling and hepatic fibrosisNat Med200713111324133217952090
- BhowmickNAChytilAPliethDTGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epitheliaScience2004303565984885114764882
- AzumaISeyaTDevelopment of immunoadjuvants for immunotherapy of cancerInt Immunopharmacol2001171249125911460306
- OkamotoMFuruichiSNishiokaYExpression of toll-like receptor 4 on dendritic cells is significant for anticancer effect of dendritic cell-based immunotherapy in combination with an active component of OK-432, a streptococcal preparationCancer Res200464155461547015289356
- OkamotoMSatoMToll-like receptor signaling in anti-cancer immunityJ Med Invest2003501–292412630564
- LinYSHuangLDLinCHIn vitro and in vivo anticancer activity of a synthetic glycolipid as Toll-like receptor 4 (TLR4) activatorJ Biol Chem201128651437824379221949133
- GrondinVSeksikPDumontSRegulation of colon cancer cell proliferation and migration by MD-2 activityInnate Immun201117441442220699280
- DeguchiATomitaTOmoriTSerum Amyloid A3 Binds MD-2 To Activate p38 and NF-κB Pathways in a MyD88-Dependent MannerJ Immunol201319141856186423858030
- HartmannEWollenbergBRothenfusserSIdentification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancerCancer Res200363196478648714559840
- SzczepańskiMStelmachowskaMStryczyńskiLAssessment of expression of toll-like receptors 2, 3 and 4 in laryngeal carcinomaEur Arch Otorhinolaryngol2007264552553017165086
- SzczepanskiMJCzystowskaMSzajnikMTriggering of Toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attackCancer Res20096973105311319318560
- StarskaKFormaELewy-TrendaIThe expression of SOCS1 and TLR4-NFkappaB pathway molecules in neoplastic cells as potential biomarker for the aggressive tumor phenotype in laryngeal carcinomaFolia Histochem Cytobiol200947340141020164024
- StarskaKFormaEBryśMThe expression of TLR pathway molecules in peripheral blood mononuclear cells and their relationship with tumor invasion and cytokine secretion in laryngeal carcinomaAdv Med Sci201257112413522240197
- YaoHRahmanICurrent concepts on the role of inflammation in COPD and lung cancerCurr Opin Pharmacol20099437538319615942
- BauerAKDixonDDeGraffLMToll-like receptor 4 in butylated hydroxytoluene-induced mouse pulmonary inflammation and tumorigenesisJ Natl Cancer Inst200597231778178116333033
- BauerAKFostelJDegraffLMTranscriptomic analysis of pathways regulated by toll-like receptor 4 in a murine model of chronic pulmonary inflammation and carcinogenesisMol Cancer2009810719925653
- MacRedmondREGreeneCMDorscheidDRMcElvaneyNGO’NeillSJEpithelial expression of TLR4 is modulated in COPD and by steroids, salmeterol and cigarette smokeRespir Res200788418034897
- ZhangYBHeFLFangMIncreased expression of Toll-like receptors 4 and 9 in human lung cancerMol Biol Rep20093661475148118763053
- ManegoldCGravenorDWoytowitzDRandomized phase II trial of a toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancerJ Clin Oncol200826243979398618711188
- RenTWenZKLiuZMTargeting toll-like receptor 9 with CpG oligodeoxynucleotides enhances anti-tumor responses of peripheral blood mononuclear cells from human lung cancer patientsCancer Invest200826544845518568766
- WangHRayburnERWangWKandimallaERAgrawalSZhangRChemotherapy and chemosensitization of non-small cell lung cancer with a novel immunomodulatory oligonucleotide targeting Toll-like receptor 9Mol Cancer Ther2006561585159216818518
- DroemannDAlbrechtDGerdesJHuman lung cancer cells express functionally active Toll-like receptor 9Respir Res20056115631627
- ParkinDMPisaniPFerlayJGlobal cancer statisticsCA Cancer J Clin19994913364 110200776
- McCullochPWardJTekkisPPASCOT group of surgeons; British Oesophago-Gastric Cancer GroupMortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort studyBMJ200332774251192119714630753
- ChattopadhyayISinghAPhukanRGenome-wide analysis of chromosomal alterations in patients with esophageal squamous cell carcinoma exposed to tobacco and betel quid from high-risk area in IndiaMutat Res2010696213013820083228
- SheyhidinINabiGHasimAOverexpression of TLR3, TLR4, TLR7 and TLR9 in esophageal squamous cell carcinomaWorld J Gastroenterol201117323745375121990957
- KawaharaTKuwanoYTeshima-KondoSToll-like receptor 4 regulates gastric pit cell responses to Helicobacter pylori infectionJ Med Invest2001483–419019711694959
- IshiharaSRumiMAKadowakiYEssential role of MD-2 in TLR4-dependent signaling during Helicobacter pylori-associated gastritisJ Immunol200417321406141615240737
- ChochiKIchikuraTKinoshitaMHelicobacter pylori augments growth of gastric cancers via the lipopolysaccharide-toll-like receptor 4 pathway whereas its lipopolysaccharide attenuates antitumor activities of human mononuclear cellsClin Cancer Res200814102909291718483357
- SchmausserBAndrulisMEndrichSMüller-HermelinkHKEckMToll-like receptors TLR4, TLR5 and TLR9 on gastric carcinoma cells: an implication for interaction with Helicobacter pyloriInt J Med Microbiol2005295317918516044857
- KawauchiKYagihashiATsujiNHuman beta-defensin-3 induction in H. pylori-infected gastric mucosal tissuesWorld J Gastroenterol200612365793579717007044
- FukataMChenAKlepperACox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestineGastroenterology2006131386287716952555
- OharaTMorishitaTSuzukiHHibiTHeterozygous Thr 135 Ala polymorphism at leucine-rich repeat (LRR) in genomic DNA of toll-like receptor 4 in patients with poorly-differentiated gastric adenocarcinomasInt J Mol Med2006181596316786156
- HoldGLRabkinCSChowWHA functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursorsGastroenterology2007132390591217324405
- SantiniDAngelettiSRuzzoAToll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms in gastric cancer of intestinal and diffuse histotypesClin Exp Immunol2008154336036418826495
- Trejo-de laOATorresJPérez-RodríguezMTLR4 single-nucleotide polymorphisms alter mucosal cytokine and chemokine patterns in Mexican patients with Helicobacter pylori-associated gastroduodenal diseasesClin Immunol2008129233334018755634
- RigoliLDi BellaCFedeleFTLR4 and NOD2/CARD15 genetic polymorphisms and their possible role in gastric carcinogenesisAnticancer Res201030251351720332463
- Garza-GonzalezEBosques-PadillaFJMendoza-IbarraSIFlores-GutierrezJPMaldonado-GarzaHJPerez-PerezGIAssessment of the toll-like receptor 4 Asp299Gly, Thr399Ile and interleukin-8 -251 polymorphisms in the risk for the development of distal gastric cancerBMC Cancer200777017462092
- FukataMAbreuMTTLR4 signalling in the intestine in health and diseaseBiochem Soc Trans200735Pt 61473147818031248
- CarioEPodolskyDKDifferential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel diseaseInfect Immun200068127010701711083826
- AbreuMTFukataMArditiMTLR signaling in the gut in health and diseaseJ Immunol200517484453446015814663
- HausmannMKiesslingSMestermannSToll-like receptors 2 and 4 are up-regulated during intestinal inflammationGastroenterology200212271987200012055604
- CammarotaRBertoliniVPennesiGThe tumor microenvironment of colorectal cancer: stromal TLR-4 expression as a potential prognostic markerJ Transl Med2010811221059221
- FurrieEMacfarlaneSThomsonGMacfarlaneGTMicrobiology and Gut Biology GroupTayside Tissue and Tumour BankToll-like receptors-2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteriaImmunology2005115456557416011525
- RigoliLRomanoCCarusoRAClinical significance of NOD2/CARD15 and Toll-like receptor 4 gene single nucleotide polymorphisms in inflammatory bowel diseaseWorld J Gastroenterol200814284454446118680223
- HongJLeungEFraserAGMerrimanTRVishnuPKrissansenGWTLR2, TLR4 and TLR9 polymorphisms and Crohn’s disease in a New Zealand Caucasian cohortJ Gastroenterol Hepatol200722111760176617914947
- BaumgartDCBuningCGeerdtsLThe c.1–260C.T promoter variant of CD14 but not the c.896A.G (p.D299G) variant of toll-like receptor 4 (TLR4) genes is associated with inflammatory bowel diseaseDigestion2007763–419620218174680
- FranchimontDVermeireSEl HousniHDeficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299 gly polymorphism is associated with Crohn’s disease and ulcerative colitisGut200453798799215194649
- BrandSStaudingerTSchnitzlerFThe role of Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms and CARD15/NOD2 mutations in the susceptibility and phenotype of Crohn’s diseaseInflamm Bowel Dis200511764565215973118
- GazouliMMantzarisGKotsinasAAssociation between polymorphisms in the Toll-like receptor 4, CD14, and CARD15/NOD2 and inflammatory bowel disease in the Greek populationWorld J Gastroenterol200511568168515655821
- TörökHPGlasJTonenchiLMussackTFolwacznyCPolymorphisms of the lipopolysaccharide-signaling complex in inflammatory bowel disease: association of a mutation in the Toll-like receptor 4 gene with ulcerative colitisClin Immunol20041121859115207785
- OostenbrugLEDrenthJPde JongDJAssociation between Toll-like receptor 4 and inflammatory bowel diseaseInflamm Bowel Dis200511656757515905704
- OuburgSMallant-HentRCrusiusJBThe toll-like receptor 4 (TLR4) Asp299Gly polymorphism is associated with colonic localisation of Crohn’s disease without a major role for the Saccharomyces cerevisiae mannan-LBP-CD14-TLR4 pathwayGut200554343944015710998
- ShenXShiRZhangHLiKZhaoYZhangRThe Toll-like receptor 4 D299G and T399I polymorphisms are associated with Crohn’s disease and ulcerative colitis: a meta-analysisDigestion2010812697720093834
- Boraska JelavićTBarisićMDrmic HofmanIMicrosatelite GT polymorphism in the toll-like receptor 2 is associated with colorectal cancerClin Genet200670215616016879199
- WangJHManningBJWuQDBlanksonSBouchier-HayesDRedmondHPEndotoxin/lipopolysaccharide activates NF-kappa B and enhances tumor cell adhesion and invasion through a beta 1 integrin-dependent mechanismJ Immunol2003170279580412517943
- AndrewsEJWangJHWinterDCLaugWERedmondHPTumor cell adhesion to endothelial cells is increased by endotoxin via an upregulation of beta-1 integrin expressionJ Surg Res2001971141911319874
- HsuRYChanCHSpicerJDLPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasisCancer Res20117151989199821363926
- FriedmanSLMolecular regulation of hepatic fibrosis, an integrated cellular response to tissue injuryJ Biol Chem200027542247225010644669
- TestroAGVisvanathanKToll-like receptors and their role in gastrointestinal diseaseJ Gastroenterol Hepatol200924694395419638078
- WangJPZhangYWeiXCirculating Toll-like receptor (TLR) 2, TLR4, and regulatory T cells in patients with chronic hepatitis CAPMIS2010118426127020402671
- WeiXQGuoYWLiuJJWenZFYangSJYaoJLThe significance of Toll-like receptor 4 (TLR4) expression in patients with chronic hepatitis BClin Invest Med2008313E123E13018544275
- MachidaKTsukamotoHMkrtchyanHToll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker NanogProc Natl Acad Sci U S A200910651548155319171902
- NishimuraMNaitoSTissue-specific mRNA expression profiles of human toll-like receptors and related genesBiol Pharm Bull200528588689215863899
- VonlaufenAXuZDanielBBacterial endotoxin: a trigger factor for alcoholic pancreatitis? Evidence from a novel, physiologically relevant animal modelGastroenterology200713341293130317919500
- ZhangJJWuHSWangLTianYZhangJHWuHLExpression and significance of TLR4 and HIF-1alpha in pancreatic ductal adenocarcinomaWorld J Gastroenterol201016232881288820556833
- MillerLSToll-like receptors in skinAdv Dermatol200824718719256306
- MolteniMMarabellaDOrlandiCRossettiCMelanoma cell lines are responsive in vitro to lipopolysaccharide and express TLR-4Cancer Lett20062351758315922507
- Sanchez-PerezLKottkeTDanielsGAKilling of normal melanocytes, combined with heat shock protein 70 and CD40L expression, cures large established melanomasJ Immunol200617764168417716951382
- VoelckerVGebhardtCAverbeckMHyaluronan fragments induce cytokine and metalloprotease upregulation in human melanoma cells in part by signalling via TLR4Exp Dermatol200817210010718031543
- Saint-JeanMKnolACNguyenJMKhammariADrénoBTLR expression in human melanoma cellsEur J Dermatol201121689990521926036
- WuJGuanMWongPFYuHDongJXuJIcariside II potentiates paclitaxel-induced apoptosis in human melanoma A375 cells by inhibiting TLR4 signaling pathwayFood Chem Toxicol20125093019302422743248
- YusufNNastiTHLongJAProtective role of Toll-like receptor 4 during the initiation stage of cutaneous chemical carcinogenesisCancer Res200868261562218199559
- ClarkeJHChaJYWalshMDMelanoma inhibits macrophage activation by suppressing toll-like receptor 4 signalingJ Am Coll Surg2005201341842516125076
- XieWWangYHuangYYangHWangJHuZToll-like receptor 2 mediates invasion via activating NF-kappaB in MDA-MB-231 breast cancer cellsBiochem Biophys Res Commun200937941027103219141294
- MerrellMAIlvesaroJMLehtonenNToll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activityMol Cancer Res20064743744716849519
- YangHZhouHFengPReduced expression of Toll-like receptor 4 inhibits human breast cancer cells proliferation and inflammatory cytokines secretionJ Exp Clin Cancer Res2010299220618976
- HarmeyJHBucanaCDLuWLipopolysaccharide-induced metastatic growth is associated with increased angiogenesis, vascular permeability and tumor cell invasionInt J Cancer2002101541542212216068
- AhmedAWangJHRedmondHPSilencing of TLR4 increases tumor progression and lung metastasis in a murine model of breast cancerAnn Surg Oncol Epub2012814
- EhsanNMuradSAshiqTSignificant correlation of TLR4 expression with the clinicopathological features of invasive ductal carcinoma of the breastTumour Biol20133421053105923338716
- González-ReyesSMarínLGonzálezLStudy of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasisBMC Cancer20101066521129170
- LiaoSJZhouYHYuanYTriggering of Toll-like receptor 4 on metastatic breast cancer cells promotes αvβ3-mediated adhesion and invasive migrationBreast Cancer Res Treat2012133385386322042369
- ApetohLTesniereAGhiringhelliFKroemerGZitvogelLMolecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapiesCancer Res200868114026403018519658
- TheodoropoulosGESaridakisVKarantanosTToll-like receptors gene polymorphisms may confer increased susceptibility to breast cancer developmentBreast201221453453822560646
- EtokebeGEKnezevićJPetricevićBPavelićJVrbanecDDembićZSingle-nucleotide polymorphisms in genes encoding toll-like receptor -2, -3, -4, and -9 in case-control study with breast cancerGenet Test Mol Biomarkers200913672973419810822
- SzajnikMSzczepanskiMJCzystowskaMTLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancerOncogene200928494353436319826413
- KlinkMNowakMKielbikMThe interaction of HspA1A with TLR2 and TLR4 in the response of neutrophils induced by ovarian cancer cells in vitroCell Stress Chaperones201217666167422528050
- WangACSuQBWuFXZhangXLLiuPSRole of TLR4 for paclitaxel chemotherapy in human epithelial ovarian cancer cellsEur J Clin Invest200939215716419200169
- StanleyMAPettMRColemanNHPV: from infection to cancerBiochem Soc Trans200735Pt 61456146018031245
- Platz-ChristensenJJSundströmELarssonPGBacterial vaginosis and cervical intraepithelial neoplasiaActa Obstet Gynecol Scand19947375865888079612
- SmithJSBosettiCMuñozNIARC multicentric case-control studyChlamydia trachomatis and invasive cervical cancer: a pooled analysis of the IARC multicentric case-control studyInt J Cancer2004111343143915221973
- WernerJDecarloCAEscottNZehbeIUlanovaMExpression of integrins and Toll-like receptors in cervical cancer: effect of infectious agentsInnate Immun2012181556921239458
- YuLWangLLiMZhongJWangZChenSExpression of toll-like receptor 4 is down-regulated during progression of cervical neoplasiaCancer Immunol Immunother20105971021102820177675
- HasanUABatesETakeshitaFTLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16J Immunol200717853186319717312167
- PandeySMittalRDSrivastavaMImpact of Toll-like receptors [TLR] 2 (–196 to –174 del) and TLR 4 (Asp299Gly, Thr399Ile) in cervical cancer susceptibility in North Indian womenGynecol Oncol2009114350150519541348
- GattiGRiveroVMotrichRDMaccioniMProstate epithelial cells can act as early sensors of infection by up-regulating TLR4 expression and proinflammatory mediators upon LPS stimulationJ Leukoc Biol200679598999816522744
- González-ReyesSFernándezJMGonzálezLOStudy of TLR3, TLR4, and TLR9 in prostate carcinomas and their association with biochemical recurrenceCancer Immunol Immunother201160221722620978888
- GattiGQuintarAAAndreaniVExpression of Toll-like receptor 4 in the prostate gland and its association with the severity of prostate cancerProstate200969131387139719496069
- PeiZLinDSongXLiHYaoHTLR4 signaling promotes the expression of VEGF and TGFbeta1 in human prostate epithelial PC3 cells induced by lipopolysaccharideCell Immunol20082541202718649875
- HuaDLiuMYChengZDSmall interfering RNA-directed targeting of Toll-like receptor 4 inhibits human prostate cancer cell invasion, survival, and tumorigenicityMol Immunol200946152876288419643479
- VidasZPolymorphisms in Toll-like receptor genes – implications for prostate cancer developmentColl Antropol201034277978320698170
- ZhengSLAugustsson-BälterKChangBSequence variants of toll-like receptor 4 are associated with prostate cancer risk: results from the CAncer Prostate in Sweden StudyCancer Res20046482918292215087412
- SongJKimDYKimCSThe association between Toll-like receptor 4 (TLR4) polymorphisms and the risk of prostate cancer in Korean menCancer Genet Cytogenet20091902889219380025
- ChenYCGiovannucciELazarusRKraftPKetkarSHunterDJSequence variants of Toll-like receptor 4 and susceptibility to prostate cancerCancer Res20056524117711177816357190
- ChengIPlummerSJCaseyGWitteJSToll-like receptor 4 genetic variation and advanced prostate cancer riskCancer Epidemiol Biomarkers Prev200716235235517301271
- LindströmSHunterDJGrönbergHSequence variants in the TLR4 and TLR6-1-10 genes and prostate cancer risk. Results based on pooled analysis from three independent studiesCancer Epidemiol Biomarkers Prev201019387387620200442