Abstract
The expression and clinical significance of insulin-like growth factor 1 (IGF-1), insulin-like growth factor binding protein 3 (IGFBP-3), and insulin-like growth factor binding protein 7 (IGFBP-7) were investigated in serum and lung cancer tissues from 57 patients with non-small cell lung cancer (NSCLC). Lung cancer tissues at different pathologic stages (27 patients at stages I–II and 30 patients at stages III–IV), normal lung tissues from 17 patients with benign pulmonary disease, and serum samples from both lung cancer and benign pulmonary disease patients were collected during surgery. Enzyme-linked immunosorbent assay and avidin-biotin-peroxidase complex immunohistochemical staining were used to detect IGF-1, IGFBP-3, and IGFBP-7 expression in serum and tissues, respectively. The results show that expression of IGF-1 in lung cancer tissues and serum from NSCLC patients were significantly higher than in the control (P < 0.05). However, expression of IGFBP-3 and IGFBP-7 in cancer tissues and serum from NSCLC patients was significantly lower than in the control (P < 0.05). These results suggest that upregulation of IGF-1 and downregulation of IGFBP-3 and IGFBP-7 may be potential diagnostic biomarkers for NSCLC.
Introduction
Lung cancer is the second most common cancer in men and women and a leading cause of cancer-related deaths worldwide, with non-small cell lung cancer (NSCLC) representing approximately 85% of all cases.Citation1 In addition, more than one half of affected patients have advanced metastasis with a poor prognosis. According to the International Agency for Research on Cancer of World Health Organization, lung cancer is the most common cancer in the world, with an estimated 1.6 million new cases in 2008, representing one in eight of all new cancers. It is also the most common form of cancer death in the world, comprising nearly one in five of all deaths from cancer.Citation1 In the People’s Republic of China, lung cancer has been the predominant type of cancer in males.Citation2
Tumorigenesis can be induced by out-of-control cell proliferation and apoptosis or cell cycle dysfunction. In recent years, studies have demonstrated that, by binding to receptors, the insulin-like growth factor family (IGFs) can initiate the cell signal transduction pathway, promote cell mitosis, proliferation, and differentiation, and inhibit cell apoptosis. This, in turn, may play an important role in tumor occurrence and development.Citation3 The biological effect of IGFs after binding to their receptors is regulated by a series of specific insulin-like growth factor binding proteins (IGFBPs).Citation4 Research evidence shows that IGFs have mitogenic and antiapoptotic properties; they have been implicated in the development of lung cancer, and the effects of IGFs are modulated by IGFBPs.Citation5,Citation6 The rationale for measuring the fasting level of these proteins in peripheral blood is that, due to the anabolic nature of these proteins, fasting allows for normalization. To elucidate the relationship between IGF-1/IGFBPs and NSCLC, the expression of IGF-1, IGFBP-3, and IGFBP-7 in serum and lung tissues from NSCLC patients and controls were analyzed in this study.
Materials and methods
Materials
Enzyme-linked immunosorbent assay (ELISA) kits for IGF-1 and IGFBP-3, a hematoxylin and eosin staining kit, Mayer’s hematoxylin, and avidin-biotin complex stain were purchased from Wuhan Boster Biological Technology, Ltd (Wuhan, People’s Republic of China). An IGFBP-7 ELISA kit was purchased from Shanghai Jintai Biological Technology, Ltd. (Shanghai, People’s Republic of China). The ELISA reader was a MultiskanMK3, purchased from Shanghai Zhili Scientific Instrument Company Ltd., China (Shanghai, People’s Republic of China). Rabbit anti-human IGF-1, IGFBP-3, and IGFBP-7 polyclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). ELISA kits were purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, People’s Republic of China).
Methods
Selection of NSCLC and control cases
Fifty-seven surgical inpatients with NSCLC (March through November 2010) from the department of thoracic surgery at the Affiliated Hospital of Guangdong Medical College (Zhanjiang, Guangdong, People’s Republic of China) were selected as NSCLC cases, including 38 males and 19 females aged 27–84 years. According to the Union for International Cancer Control 2009 tumor, node, metastasis (TNM) staging standard for lung cancers and the World Health Organization histologic type classification standard for lung cancer, 19 cases were identified as squamous cell carcinoma and 38 cases as adenocarcinoma, with 27 patients being at stage I–II and 30 patients being at stage III–IV. During the same time period, seven male and ten female surgical inpatients aged 33–75 years with benign pulmonary disease were selected as the control group. These included three cases of bronchiectasis, five cases of inflammatory pseudotumor, five cases of tuberculosis ball, three cases of bronchial cyst, and one case of pulmonary sequestration. All NSCLC and control cases were diagnosed by pathologic examination (double-blind assessment by three pathologists) in the pathology department of the Affiliated Hospital of Guangdong Medical College. No case had preoperative chemotherapy or radiotherapy. Patients with diabetes or other metabolic disorders were excluded. In addition, human specimens collected for this study after surgical operations had been approved by the ethics committee of the Affiliated Hospital of Guangdong Medical College. All patients had provided their written consent to participate in this study.
Serum collection and ELISA
Before treatment, fasting peripheral venous blood (5 mL) was collected from all participants in the morning. Blood samples were kept at room temperature for 30 minutes, and then centrifuged at 150 g for 20 minutes. Supernatant serum was collected and stored at −20°C until use. Serum IGF-1, IGFBP-3, and IGFBP-7 levels were then analyzed using ELISA according to the manufacturer’s instructions.
Lung tissue collection, avidin-biotin complex immunohistochemistry, and hematoxylin and eosin staining
The tumorigenic tissues were harvested from the NSCLC patients during surgery. Normal lung tissues were harvested from the outer region of the lesions from control patients during surgery. All tissues were immediately fixed in 4% formalin for histopathologic examination. The formalin-fixed tissues were stored at 4°C until examination. Tissues were processed using standard histology laboratory techniques. Using a microtome, 3–4 μm sections were cut. These were then stained with avidin-biotin complex immunohistochemical stain following a standard staining protocol to detect expression of IGF-1, IGFBP-3, and IGFBP-7. Briefly, sections were treated with 3% peroxide in methanol for 15 minutes to block endogenous peroxidase activity, followed by overnight incubation with the primary antibody at 4°C. Mouse primary monoclonal antibodies directed against IGF-I, IGFBP-3, and IGFBP-7 (1:100 dilution; Santa Cruz Biotechnology) were used in these experiments. After rinsing with phosphate-buffered saline, secondary biotinylated antisera were followed by the avidin-biotin complex (Wuhan Boster Biological Technology); the reaction was developed with 3-39-diaminobenzine-tetrahydrochloride and counterstained with Mayer’s hematoxylin. IGF-I, IGFBP-3, and IGFBP-7 immunoreactivity was graded according to the relative immunostaining intensity (IGF-I, IGFBP-3, and IGFBP-7 stain intensity). Hematoxylin and eosin staining was conducted according to the manufacturer’s instructions for indicating the pathology.
Statistical analysis
Statistical analyses were performed using Statistical Package for the Social Sciences version 17 software (SPSS Inc., Chicago, IL, USA). Two-samples mean comparison was analyzed by t-test. Multiple-sample mean comparison was analyzed by one-way analysis of variance. According to homogeneity variance results, the least squares difference test was used for data analyses when variance was equal, but Tamhane’s T2 test was used when variance was unequal. The mutual relationship between two growth factors was analyzed by Pearson correlation analysis. The intragroup and intergroup expression difference detected by immunohistochemistry assay was analyzed by the rank-sum test. The correlation of two ranked data was analyzed by Spearman correlation analysis. The mean method was used to compare the means of measurement data among different groups. Eta and Eta square values were used to measure the correlation. Statistical significance was set at P < 0.05.
Results
Comparison of serum IGF-1, IGFBP-3, and IGFBP-7 expression between NSCLC and controls
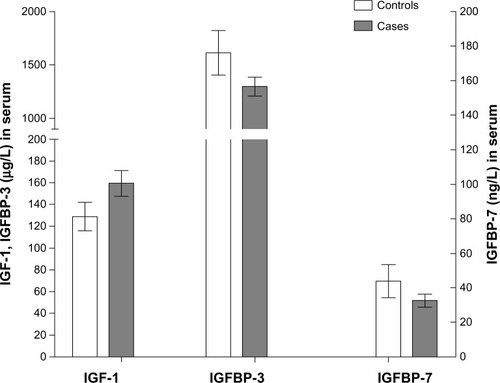
The t-test showed that the concentration of IGF-1 in the serum from NSCLC cases (159.24 ± 44.74 μg/L) was significantly higher than that from control cases (128.79 ± 25.40 μg/L, P = 0.034). However, the concentration of IGFBP-3 in the serum from NSCLC cases (1299.58 ± 328.87 μg/L) was significantly lower than that of control cases (1611.56 ± 405.08 μg/L, P = 0.042). The concentration of IGFBP-7 in serum from NSCLC cases (32.51 ± 14.05 ng/L) was also significantly lower than that from control cases (43.81 ± 18.32 ng/L, P = 0.026, ).
Stratification analysis of pathologic parameters and serum IGF-1, IGFBP-3, and IGFBP-7 concentrations in NSCLC cases
Stratification analysis showed that concentrations of IGF-1, IGFBP-3, and IGFBP-7 in the serum of NSCLC cases had no significant (P > 0.05) association with the location of NSCLC, diameter of the tumor, or pathologic grade or type, but was significantly (P < 0.05) associated with levels of lymph node metastasis, local invasion, distant metastasis, and TNM stage ().
Table 1 Stratification analysis on pathologic parameters and serum concentrations of IGF-1, IGFBP-3, and IGFBP-7 of NSCLC cases (mean ± standard deviation)
Correlation analysis of serum IGF-1 and IGFBP-3 or IGFBP-7 concentrations in NSCLC cases
Pearson correlation analysis showed that the concentrations of IGF-1 and IGFBP-3 in the serum of NSCLC cases had a significant negative correlation (r = −0.253, P = 0.023), but there was no correlation between the concentrations of IGF-1 and IGFBP-7 in serum from NSCLC cases (r = −0.151, P = 0.272).
IGF-1, IGFBP-3, and IGFBP-7 expression in NSCLC tissue and normal control lung tissue
The intensity of IGF-1, IGFBP-3, or IGFBP-7 expression in lung tissues from NSCLC cases was detected by avidin-biotin complex immunohistochemical staining (). Pathology was shown by hematoxylin and eosin staining (). IGF-1 expression in lung cancer tissues from NSCLC cases progressed from weakly positive to strongly positive, which was significantly stronger than that (mainly from negative to weak positive) in normal lung tissues from control cases (40.57 versus 27.21, P < 0.05). IGFBP-3 expression in lung cancer tissues from NSCLC cases progressed from negative to positive, and was significantly weaker than its expression in control cases (from positive to strong positive) (34.62 versus 47.15, P < 0.05). IGFBP-7 expression in lung cancer tissues from NSCLC cases progressed from negative to positive, also significantly weaker than its expression in control cases (30.77 versus 40.40, P < 0.05, rank-sum test).
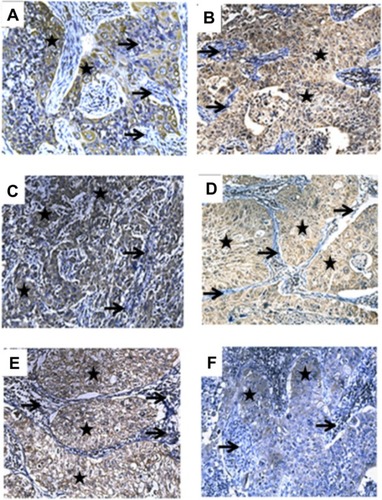
Figure 2 Intensity of IGF-1, IGFBP-3, or IGFBP-7 expression in lung tissues at different stages of squamous carcinoma of the lung. (A) IGF-1 expression in stage Ia squamous carcinoma of the lung, slightly positive (yellow-brown staining). (B) IGF-1 expression in stage IIb squamous carcinoma of the lung, strongly positive. (C) IGFBP-3 expression in stage Ia lung squamous carcinoma, strong positive. (D) Expression of IGFBP-3 in IIb stage squamous carcinoma of the lung, slightly positive. (E) IGFBP-7 expression in stage Ia squamous carcinoma of the lung, strongly positive. (F) IGFBP-7 expression in stage IIb squamous carcinoma of the lung, slightly positive. All figures were captured under the microscope at 200× magnification. ★Lung tumor tissue areas; → normal lung tissue areas.
IGF-1, IGFBP-3, and IGFBP-7 expression in lung cancer tissues and stratification analysis with pathologic parameters of NSCLC cases
Stratification analysis showed that the intensity of IGF-1, IGFBP-3 and IGFBP-7 expression had no significant association (P > 0.05) with location of the lung tissue, tumor diameter, histologic type, or pathologic grade, but had a significant (P < 0.05) association with stage III–IV TNM, lymph node metastasis, and distance metastasis parameters ().
Table 2 Stratification analysis on association of expression of IGF-1, IGFBP-3, and IGFBP-7 in lung tissues and its pathologic parameters in NSCLC cases
Correlation analysis of intensity of IGF-1 with IGFBP-3 or IGFBP-7 expression in lung tissues from NSCLC cases
Spearman’s rank correlation analysis showed that there was no significant correlation between the expression intensity of IGF-1 and IGFBP-3 (rs = 0.224, n = 57, P = 0.062). However, there was a significant negative correlation between the expression intensities of IGF-1 and IGFBP-7 (rs = −0.437, n = 57, P = 0.001).
Association of IGF-1, IGFBP-3, or IGFBP-7 concentrations in serum and expression intensity in lung tissues from NSCLC cases
Serum IGF-1 concentrations were significantly different (P < 0.05), with a positive correlation (Eta = 0.369) at different levels of IGF-1expression intensity in lung cancer tissues from NSCLC cases shown by the measures of association test. Serum IGFBP-3 concentrations showed no significant differences (P > 0.05), with a slight positive correlation (Eta = 0.079) at different levels of IGFBP-3 expression intensity in lung tissues from NSCLC cases. Serum IGFBP-7 concentrations showed no significant differences (P > 0.05), with a slight positive correlation (Eta = 0.144) at different levels of IGFBP-7 expression intensity in lung tissues from NSCLC cases.
Discussion
IGF-1, a growth hormone, was first identified by Salmon and Daughaday in 1957, and since then the function of growth hormone-IGF in regulating cell growth and apoptosis has been widely investigated.Citation3 IGF-1 is produced primarily by the liver as an endocrine hormone as well as in target tissues in a paracrine/autocrine fashion.Citation7 Its primary action is mediated by binding to its receptor, the insulin-like growth factor 1 receptor (IGF-1R), present on many cell types in many tissues. IGF-1R then stimulates systemic body growth, and has growth-promoting effects on almost every cell in the human body. In addition to its insulin-like effects, IGF-1R can also regulate cell growth and development. IGF-1R seems to have assumed an important role, especially in cancer biology.Citation8 In recent years, studies have shown that IGF-1 can enhance expression and activity of the latent form of urokinase-type plasminogen activator, matrix metalloproteinase-2, matrix metalloproteinase-9, and promotes cancer cell invasion.Citation9,Citation10 The IGFBPs are a family of homologous proteins that have coevolved with the IGFs.Citation11 Normally, the amount of IGFBP-3 in the serum is very low, but it can be increased under certain physiologic or pathologic conditions like pregnancy, surgery, or malignancy.Citation12 A study by Oh et al showed that IGFBP-3 can effectively prevent urokinase-type plasminogen activator and matrix metalloproteinase-9 from stimulating the invasion pathways, and ultimately reduce metastasis of lung cancer cells.Citation13 Chen et alCitation14 investigated 138 patients with lung cancer and 20 cases of normal lung tissue by immunohistochemistry and confirmed that insulin-like growth factor binding protein-related protein 1 (IGFBP-rP1) had high expression levels in normal tissues and low expression levels in cancer tissues. Moreover, by polymerase chain reaction sequencing and in nude mice experiments, they found that IGFBP-rP1 could inhibit tumor growth rate, increase the number of apoptotic cells, and activate expression of caspase-3.Citation14 IGFBP-rPl plays a role as a tumor suppressor in human lung cancer by downregulating the methylation of DNA.Citation14
The spread of cancer involves multiple steps, including cell adhesion to extracellular matrix-specific glycoprotein, prevention of the degraded extracellular matrix composition migrating out of the blood vessels via tumor-related protein, and local invasion.Citation15,Citation16 Our results show that IGF-1 expression in NSCLC cases was significantly higher than that in the benign lung lesion cases, and expression was increased as malignant lung cancer progression occurred, suggesting that IGF-1 has a greater capacity for promoting mitosis in lung cancer.Citation17 The high level of IGF-1 expression may play an important role in the development of lung cancer, promoting proliferation of cancer cells and inhibiting their apoptosis. In this study, we also found that, in cases with lymph node metastasis, distant metastasis, invasion of the chest wall, pericardium, or great vessels, or at TNM stages III–IV, the concentrations or expression of IGF-1 in serum or lung cancer tissues was significantly higher than in patients without lymph node metastasis, distant metastasis, local invasion, invasion of the visceral pleura, or at TNM stage I–II. This result suggests that IGF-1 may be involved in local invasion and distant metastasis of lung cancers. Therefore, the serum IGF-1 concentration and intensity of its expression in lung cancer tissues may have clinical significance in early diagnosis and prediction of NSCLC. A new molecular target therapy that regulates the level of IGF-1 expression or blocks IGF-1 from binding with its cell surface receptor might be useful in the treatment of NSCLC.
In addition, we found that the concentration of IGFBP-3 in serum in NSCLC cases was significantly lower than in controls, and there were a negative correlation between the concentrations of IGF-1 and IGFBP-3 in serum. Under circumstances of lymph node metastasis, distant metastasis, invasion of the chest wall, pericardium, or great vessels, or TNM stage III–IV, the concentration or expression of IGFBP-3 in serum and lung cancer tissue was significantly lower than that in patients without lymph node metastasis, distant metastasis, local invasion or only invasion of the visceral pleura, or TNM stage I–II. These results suggest that regulation of IGFBP-3 by IGF-1 may be through binding with IGF-1 and then inhibiting its effect of promoting differentiation and proliferation of tumor cells. In an in vitro experiment, Alami et alCitation18 found that recombinant human IGFBP-3 could inhibit proliferation of M-3LL lung cancer cells in a dose-dependent manner, and could also significantly inhibit tumor growth after M-3LL cell transplantation in mice. Although no correlation between concentrations of IGF-1 and IGFBP-7 in the serum from NSCLC cases was found in this study, the expression of IGFBP-7 decreased as the lung cancer progressed, suggesting that IGFBP-7 may also be a lung cancer inhibitor.
In conclusion, our results suggest that expression of IGF-1, IGFBP-3, and IGFBP-7 in serum and lung tissues may play an important role in the development of lung cancer. Detection of IGF-1, IGFBP-3, and IGFBP-7 in the peripheral serum could be significant in risk assessment of lung cancer patients, early prediction of the presence or absence of potential lymph node metastasis and distant metastasis, and thereby guide clinical treatment. Upregulation of IGF-1 and downregulation of IGFBP-3 and IGFBP-7 may be potential diagnostic biomarkers for NSCLC. To reduce the circulating and local expression of IGF-1 and enhance IGFBP-3 and IGFBP-7 levels in the body may become one of the targets of lung cancer treatment in future.
Acknowledgments
This work was partly supported by grants from the Zhanjiang Scientific Project (2010004), Guangdong Scientific Project (2011B080701039), the National Nature Science Foundation of China (81273111), the Ningbo Scientific Project (2012C5019), and the KC Wong Magna Fund in Ningbo University.
Disclosure
The authors report no conflicting interests in this work.
References
- FerlayJShinHRBrayFFormanDMathersCParkinDMCancer Incidence and Mortality Worldwide: IARC CancerBase No 10Lyon, FranceInternational Agency for Research on Cancer2010 Available from: http://globocan.iarc.frAccessed August 24, 2013
- WangYCWeiLJLiuJTLiSXWangQSComparison of cancer incidence between China and the USACancer Biol Med20129212813223691468
- GrimbergACohenPRole of insulin-like growth factors and their binding proteins in growth control and carcinogenesisJ Cell Physiol200018311910699960
- HermaniAShuklaAMedunjaninSWernerHMayerDInsulin-like growth factor binding protein-4 and -5 modulate ligand-dependent estrogen receptor-alpha activation in breast cancer cells in an IGF-independent mannerCell Signal20132561395140223499909
- LeeHYChunKHLiuBInsulin-like growth factor binding protein-3 inhibits the growth of non-small cell lung cancerCancer Res200262123530353712068000
- SueokaNLeeHYWiehleSInsulin-like growth factor binding protein-6 activates programmed cell death in non-small cell lung cancer cellsOncogene200019384432443610980619
- HausmanDBDiGirolamoMBartnessTJHausmanGJMartinRJThe biology of white adipocyte proliferationObes Rev20012423925412119995
- BasergaRPeruzziFReissKThe IGF-1 receptor in cancer biologyInt J Cancer2003107687387714601044
- DunnSETorresJVOhJSCykertDMBarrettJCUp-regulation of urokinase-type plasminogen activator by insulin-like growth factor-I depends upon phosphatidylinositol-3 kinase and mitogen-activated protein kinase kinaseCancer Res20016141367137411245436
- BredinCGLiuZKlominekJGrowth factor-enhanced expression and activity of matrix metalloproteases in human non-small cell lung cancer cell linesAnticancer Res2003236C4877488414981939
- KelleyKMOhYGargoskySEInsulin-like growth factor-binding proteins (IGFBPs) and their regulatory dynamicsInt J Biochem Cell Biol19962866196378673727
- BaciuchkaMRemacle-BonnetMGarrousteFFavreRSastreBPommierGInsulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) proteolysis in patients with colorectal cancer: possible association with the metastatic potential of the tumorInt J Cancer19987954604679761113
- OhSHLeeOHSchroederCPAntimetastatic activity of insulin-like growth factor binding protein-3 in lung cancer is mediated by insulin-like growth factor-independent urokinase-type plasminogen activator inhibitionMol Cancer Ther20065112685269517121915
- ChenYPacyna-GengelbachMYeFInsulin-like growth factor binding protein-related protein 1 (IGFBP-rP1) has potential tumour-suppressive activity in human lung cancerJ Pathol2007211443143817236181
- EngersRGabbertHEMechanisms of tumor metastasis: cell biological aspects and clinical implicationsJ Cancer Res Clin Oncol20001261268269211153140
- ReuningUMagdolenVHapkeSSchmittMMolecular and functional interdependence of the urokinase-type plasminogen activator system with integrinsBiol Chem200338481119113112974381
- FurstenbergerGSennHJInsulin-like growth factors and cancerLancet Oncol20023529830212067807
- AlamiNPageVYuQRecombinant human insulin-like growth factor-binding protein 3 inhibits tumor growth and targets the Akt pathway in lung and colon cancer modelsGrowth Horm IGF Res200818648749618502161