Abstract
Background
The purpose of this study was to validate B7-H3 as a new cancer-specific endothelial marker in clear cell renal cell carcinoma.
Methods
B7-H3 expression patterns were compared between cancer and paired adjacent normal renal parenchyma by immunohistochemistry in paraffin-embedded specimens from 200 consecutive patients with clear cell renal cell carcinoma from January to December 2010. Four corpus luteum specimens were used as physiologic angiogenesis controls. B7-H3 messenger (m)RNA levels representing circulating endothelial cells were analyzed in 24 peripheral blood samples using real-time polymerase chain reaction. Collection and processing of tissue and peripheral blood samples was performed in compliance with the Declaration of Helsinki.
Results
Cancer cell-specific expression of B7-H3 was detected in 19% of clear cell renal cell carcinoma specimens, and tumor vasculature B7-H3 expression was confirmed in 98% (196) of cases. A diffuse pattern of vascular B7-H3 expression was associated with multiple adverse clinical and pathologic features (P<0.001). B7-H3 expression was not detected in paired adjacent normal renal parenchyma or vessels, or in luteal blood vessels. The B7-H3 mRNA level of circulating endothelial cells in peripheral blood was significantly higher in metastatic clear cell renal cell carcinoma (P<0.001).
Conclusion
This pilot study indicates that B7-H3 is a cancer-specific endothelial marker of potential importance for the development of tumor-specific, vascular-targeted therapies, and is a prognostic marker in clear cell renal cell carcinoma.
Introduction
Renal cell carcinoma (RCC) accounts for 2%–3% of all adult malignant neoplasms and is the most lethal of the urologic cancers. RCC comprises a number of histologic subtypes, among which clear cell RCC is the most common type. Surgery is an effective treatment for renal cancer if the disease is diagnosed at an early stage, whereas cases of inoperable or metastatic disease are not curable. Targeted therapy, which has radically altered the treatment of late-stage renal cancer in recent years, relies on two main groups of agents, ie, vascular endothelial growth factor-targeting drugs including sunitinib, sorafenib, bevacizumab, and pazopanib, and mammalian target of rapamycin (mTOR) inhibitors such as temsirolimus and everolimus. Both types of agents are based on a common effect, ie, inhibition of angiogenesis, which may explain their significant activity in RCC.Citation1 Despite recent achievements in targeted therapies, two significant challenges remain in the management of RCC patients: the disruption of both physiologic and pathologic angiogenesis by these agents makes them less targeted, and new markers are needed for the evaluation of antiangiogenic effects.
B7-H3, first identified in 2001, is a member of the B7 ligand family and is thought to serve as an accessory coregulator of T-cell responses following initial antigen priming.Citation2 At present, there is no consensus regarding the physiologic or pathophysiologic roles of B7-H3 because both immune stimulatory and inhibitory effects have been described for this ligand. Seaman et al showed that B7-H3 expression was increased in colon and lung tumors, and staining with an anti-B7-H3 antibody revealed a vessel-like pattern in human colorectal, lung, breast, esophageal, and bladder cancers, but not in the corresponding normal tissues. B7-H3 was not detectable in the human corpus luteum, which is a useful control for physiologic angiogenesis, indicating that B7-H3 is specifically overexpressed in the blood vessels of human tumors.Citation3 More recent research reported almost universal expression of B7-H3 in the tumor vasculature of patients with clear cell RCC.Citation4 These findings led us to hypothesize that the specific expression of B7-H3 in tumors and tumor vasculature in clear cell RCC makes it a useful target and prominent biomarker for tumor-specific antiangiogenic therapies. The present study was designed to validate B7-H3 as a new cancer-specific endothelial marker in clear cell RCC.
Materials and methods
The medical records of 200 consecutive patients with clear cell RCC were collected from January to December 2010. B7-H3 expression patterns were examined by immunohistochemistry in paraffin-embedded specimens. Vascular expression patterns of B7-H3 were compared with that of the pan-endothelial cell specific marker CD34;Citation5 paired normal renal parenchyma specimens from all patients and four corpus luteum specimens were also analyzed. Blood vessels in the tissues analyzed were first detected by CD34 staining (Dako, Glostrup, Denmark); adjacent layers from the same samples were then stained with goat anti-human B7-H3 monoclonal antibody (R&D Systems, Minneapolis, MN, USA), and compared with the expression patterns of CD34. Immunohistochemical analyses were performed as follows. Formalin-fixed, paraffin-embedded tissues were cut into 5 μm sections, deparaffinized, and rehydrated in a graded series of ethanols. Antigen retrieval was done by heating tissue sections in ethylenediaminetetraacetic acid (EDTA) 1 mmol/L (pH 8) to 121°C using a Digital Decloing Chamber (Biocare Medical, Concord, CA, USA), cooling to 90°C, and incubating for 5 minutes, and treating with peroxidase blocking reagent (Dako). Sections were incubated with a biotin-labeled polyclonal antibody against B7-H3 (R&D Systems) and CD34 (Dako) followed by a horseradish peroxidase-conjugated anti-biotin antibody (Dako) and visualization by diaminobenzidine staining. Sections were lightly counterstained with hematoxylin.
The percentages of tumor cells that stained positive for B7-H3 were quantified in 10% increments. Tumors with <10% of cells staining positive were scored negative. In addition, B7-H3 expression in the tumor vasculature and the vasculature of adjacent nontumor renal tissue was evaluated and recorded as absent, focal, moderate, or diffuse in comparison with the universal expression of CD34 in endothelial cells. Absent expression of B7-H3 in the tumor vasculature was determined after a second analysis of samples obtained from another site. Tumor vascular expression patterns of B7-H3 were compared with patients’ demographic characteristics and clinical and pathologic features, such as Eastern Cooperative Oncology Group (ECOG) performance status and TNM Classification of Malignant Tumors stage.
B7-H3 messenger (m)RNA levels were analyzed in another 24 peripheral blood samples using real-time polymerase chain reaction (PCR). Of these peripheral blood samples, 12 were from localized clear cell RCC, eight were from metastatic clear cell RCC, and the other four were from three simple renal cysts and one infection after nephrectomy, which were used as the normal control. Collection and processing of tissue and peripheral blood samples was performed in compliance with the Declaration of Helsinki.
RNA isolation
For RNA isolation, 0.5 mL EDTA blood was mixed with 14 mL of erythrocyte lysis buffer (0.899% [w/v] ammonium chloride, 0.1% [w/v] potassium bicarbonate, and 0.0037% [w/v] EDTA, pH 7.3) and incubated for 10 minutes at room temperature. After 10 minutes of centrifugation at 500×g, the buffer was removed and the cell pellet was resuspended in 350 μL RLT buffer (Qiagen, Basel, Switzerland) and stored at −20°C until RNA isolation. RNA was isolated using the RNeasy® kit plus additional DNase digestion (Qiagen) according to the manufacturer’s instructions. RNA was eluted in 50 μL of RNase-free water. The quality of the isolated RNA was determined by gel electrophoresis and confirmed to contain no genomic DNA contamination.
Reverse transcription
Samples for real-time PCR were reverse transcribed with the Taqman reverse transcription kit from Applied Biosystems (Rotkreuz, Switzerland) according to the manufacturer’s protocol. Samples were reverse-transcribed with random hexamer primers and the maximal allowed volume of RNA sample that can be added to the reverse transcription reaction was used. To verify that the primers did not amplify genomic DNA, some RNA samples were diluted and incubated as normal samples in the absence of reverse transcriptase.
Primers
PCR primers for B7-H3 (NCBI reference sequence NM_001024736/ NM_025240) were designed with Primer Express software (Applied Biosystems) and checked with Primer Blast (National Center for Biotechnology Information, Bethesda, MD, USA). The following primers for B7-H3 were used: forward primer 5′-CTTGTTCGATGTTCACAGCG-3′ and reverse primer 5′-GCCGTAGAGCTGTCTTGGATC-3′. The resulting amplification had a size of 302 base pairs.
Real-time PCR
For amplification of B7-H3, 1 μL complementary (c)DNA was added to QuantiTect SYBR Green PCR Master Mix (Qiagen) containing 400 nM forward and reverse primers. PCR was performed in a Taqman 7700 (Applied Biosystems) using the following thermal settings: one cycle of 5 minutes at 95°C, 30 seconds at 94°C, 30 seconds at 60°C, and 30 seconds at 72°C, repeat 39–49 cycles, 10 minutes at 72°C, and 4°C thereafter. Relative mRNA expression was calculated with the comparative cycle threshold (Ct) method. As a calibrator, the sample with the median Ct value was used and set to 100%. The relative level of B7-H3 mRNA was calculated as the median Ct value minus the individual Ct value.
To test the variations in our method, RNA was prepared twice individually from the same blood sample, and real-time PCR analysis was performed with these separate samples.
Results
B7-H3 expression in cancer cells was detected in 19% of clear cell RCC cases; the staining was heterogeneous within individual tumors with a median level of expression of 25% (range 10%–100%). Positively stained tumors showed similar staining intensity. No statistically significant association was observed between clinical and pathologic features and tumor cell B7-H3 expression.
B7-H3 expression in the tumor vasculature was confirmed in 98% (196) of cases, with focal expression in 29% (58), moderate expression in 28% (56), diffuse expression in 41% (82), and absent expression in four of the samples after a second check. Diffuse B7-H3 vascular expression was detected in large, medium, and small blood vessels, whereas focal B7-H3 expression was mostly detected in large tumor blood vessels, with positive expression rarely found in the endothelial cells of small blood vessels (). Diffuse vascular expression of B7-H3 was associated with multiple adverse clinical and pathologic features, such as symptomatic presentation and presence of aggressive tumor pathology, including large tumor size, advanced TNM stage, high nuclear grade, coagulative tumor necrosis, and capsular invasion (χ2 test, P<0.001, ).
Figure 1 Tumor vasculature expression patterns for B7-H3 in clear cell renal cell carcinoma. (A) ×100, (C) ×100, and (E) ×200: universal expression of CD34 in endothelial cells. (B) ×100, (D) ×100, and (F) ×200: expression patterns of B7-H3 in endothelial cells in adjacent layers. According to the patterns of CD34, diffuse vasculature expression of B7-H3 was detected in large, medium, and small blood vessels (B). Focal expression of B7-H3 was mainly located in the larger tumor blood vessels, and positive expression was rarely found in the endothelial cells of small blood vessels (F). (D) shows moderate expression of B7-H3.
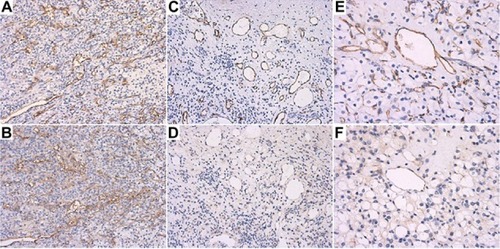
Table 1 Tumor vasculature expression of B7-H3 in clear cell renal cell carcinoma (RCC)
No obvious positive expression was detected in paired adjacent normal renal parenchyma specimens or their vessels (). Luteal blood vessels did not express B7-H3.
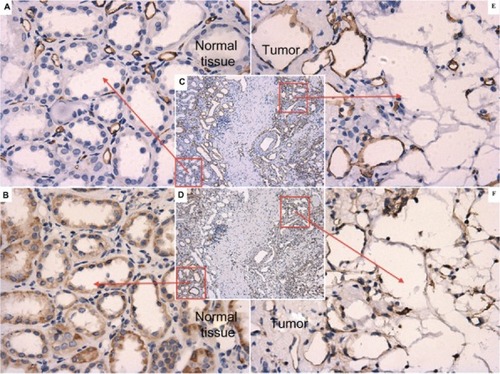
Figure 2 B7-H3 expression patterns in paired adjacent normal renal parenchyma and their vessels. (A) ×400, normal renal parenchyma area from image C. (C) ×100 and (E) ×400, cancerous part from image C: universal expression of CD34 in endothelial cells. (B) ×400, normal renal parenchyma part from image D. (D) ×100 and (F) ×400, cancerous part from image D: expression patterns of B7-H3 in endothelial cells in adjacent layers. According to the patterns of CD34, no obvious positive expression was detected in paired adjacent normal renal parenchyma or their vessels.
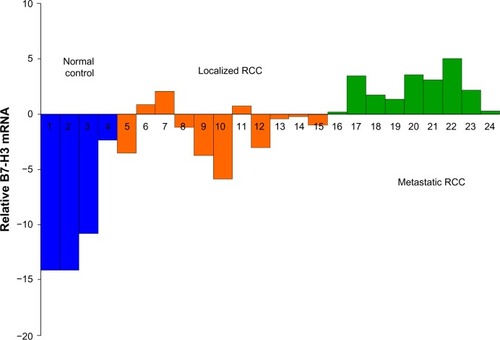
The B7-H3 mRNA level was generally limited in peripheral blood, with a median Ct value of 30.91 for all samples. The median Ct values were 28.27, 31.65, and 43.36 for metastatic cancers, localized cancers, and normal controls, respectively. The B7-H3 mRNA level was significantly higher in clear cell RCC cases than in normal controls, and was higher in metastatic clear cell RCC than in localized cases (Kruskal–Wallis test, P<0.001, ). The variations between these samples of two separate real-time PCR were of no statistical significance (P=0.30), as determined by the paired t-test.
Discussion
Current targets of antiangiogenic therapy are critical for both physiologic and pathologic angiogenesis. Given that angiogenesis is also required for normal physiologic processes, the clinical side effects of antiangiogenesis therapy are beginning to emerge. Cross-reactivity with normal tissues is an important consideration in the development of vascular disrupting agents. On the other hand, biomarkers or imaging modalities predictive of the clinical benefit of specific agents have not been identified to date.Citation1 Therefore, in the era of targeted therapy for RCC, markers that can distinguish pathologic from physiologic angiogenesis are urgently needed to selectively deliver antiangiogenic or vascular disrupting agents to diseased tissues, minimize the potential side effects, and predict the clinical outcome of using different targeted agents. In the present study, we investigated B7-H3 as a potent, new, cancer-specific endothelial marker in clear cell RCC.
B7-H3 mRNA is found in various normal tissues and in several tumor cell lines; however, two independent northern blots showed no detectable B7-H3 mRNA in peripheral blood leukocytes. The B7-H3 protein is not constitutively expressed, because it is undetectable in resting immune cells. Its expression can be induced in dendritic cells and monocytes by inflammatory cytokines and phorbol myristate acetate plus ionomycin.Citation2,Citation6 In addition to its presence in immune cells, B7-H3 is expressed in several human cancers, including prostate cancer, clear cell RCC, non-small cell lung cancer, pancreatic cancer, gastric cancer, ovarian cancer, colorectal cancer, and urothelial cell carcinoma. Increasing data support an association between high expression of B7-H3 and multiple adverse clinicopathologic features and poor prognosis, as indicated in our own study. B7-H3 has been proposed to be involved in the regulation of antitumor immunity.Citation7
In the present study, we were unable to establish a relationship between B7-H3 expression and survival in clear cell RCC because of the lack of long-term follow-up; however, our data indicate that diffuse vascular expression of B7-H3 was associated with multiple adverse clinical and pathologic features, such as higher TNM stage and nuclear grade. In a previous study, B7-H3 expression in tumor cells and tumor vasculature was detected in 17% and 95% of specimens, respectively, whereas 46% of specimens showed either tumor cell or diffuse tumor vasculature expression of B7-H3, which was associated with multiple adverse clinical and pathologic features. After multivariable adjustment, the presence of either tumor cell or diffuse tumor vasculature B7-H3 expression was significantly associated with an increased risk of death from RCC. These results indicate that tumor cell and tumor vasculature B7-H3 expression is an important predictor of clear cell RCC outcomes.Citation4
In preclinical studies, an anti-B7-H3 monoclonal antibody was shown to mediate potent antibody-dependent cellular cytotoxicity against a broad range of tumor cell types. Further, in human CD16 A-bearing transgenic mice, an anti-B7-H3 monoclonal antibody exhibited potent antitumor activity in B7-H3-expressing xenograft models of RCC and bladder carcinoma. Toxicology studies in cynomolgus monkeys revealed no significant test article-related safety findings.Citation8 Therefore, targeted therapy against B7-H3 could be a potential antitumor strategy. However, the total probability of cancer cell-specific B7-H3 expression in the clear cell RCC population was <20% in our study and in another report,Citation4 suggesting that induction of cytotoxicity or antitumor immunity by agents against B7-H3 expressed in cancer cells may not be sufficient in clinical practice.
A comparison of gene expression patterns between endothelial cells derived from normal and malignant colorectal tissues identified 46 tumor endothelial markers.Citation9 Further study showed that while most of them are upregulated in the vessels of the corpus luteum during physiologic angiogenesis, expression patterns restricted to tumor vasculature are also observed.Citation10 Blocking expression of these markers inhibits pathologic angiogenesis and potentiates tumoricidal responses against multiple cancer types.Citation11 Seaman et al have identified several genes that are selectively upregulated only in tumor blood vessels and are therefore potential specific targets for tumor angiogenesis. B7-H3 mRNA was undetectable by in situ hybridization in normal human colonic mucosa, but prominent in blood vessels from malignant colorectal tissues. Further, B7-H3 expression was increased in colon and lung tumors, and staining with an anti-B7-H3 antibody revealed a vessel-like pattern in human colorectal, lung, breast, esophageal, and bladder cancers, but not in the corresponding normal tissues. B7-H3 was not detectable in the human corpus luteum, which is a useful control for physiologic angiogenesis, indicating that B7-H3 is specifically overexpressed in the blood vessels of human tumors. Therefore, B7-H3 might be a useful target for tumor-specific antiangiogenic therapies.Citation3 Our results in clear cell RCC provide evidence supporting the notion that B7-H3 is specific to tumors and tumor vasculature.
Tumor vasculature B7-H3 expression patterns were further examined by comparison with those of the pan-endothelial cell-specific marker CD34.Citation5 Consistent with previous data, our results show almost universal expression of vascular B7-H3 in clear cell RCC. However, B7-H3 expression was not detected in paired adjacent normal renal parenchyma and their vessels or in luteal blood vessels.Citation3,Citation4 Diffuse vascular expression of B7-H3 was observed in large, medium, and small blood vessels, whereas focal B7-H3 expression was limited to large tumor blood vessels, and positive expression was rarely detected in the endothelial cells of small blood vessels. Diffuse vascular expression of B7-H3 was associated with multiple adverse clinical and pathologic features. Although the underlying mechanism remains unknown, the heterogeneity of B7-H3 expression patterns within individual tumors and different tissues indicates that B7-H3 expression may be regulated by both host factors and the tumor microenvironment.
Studies have indicated that circulating endothelial cells, which are vessel wall cells detached from activated or damaged vessels, show significant increases in number and viability in untreated cancer patients compared with healthy subjects, whereas they are limited in peripheral blood.Citation12,Citation13 In the present study, we showed positive tumor vasculature expression of B7-H3 in almost all clear cell RCC cases and an association between diffuse vascular expression of B7-H3 and multiple adverse clinical and pathologic features. Further, B7-H3 was not expressed in paired adjacent normal renal parenchyma and their vessels or in luteal blood vessels. However, because of the heterogeneous expression patterns of B7-H3 in blood vessels, we presumed that the absolute number of B7-H3-expressing circulating endothelial cells was limited, which jeopardized their detection in peripheral blood. One promising approach is the application of real-time PCR to quantify endothelial cell-specific mRNA in blood samples, which provides a highly sensitive method for detection of circulating endothelial cells,Citation14 and could be applied for comparison of B7-H3 mRNA levels in peripheral blood. Despite the limitations of a small population and possible selection bias, our pilot study found that B7-H3 mRNA levels in peripheral blood were significantly higher in metastatic clear cell RCC cases.
The potential contribution of circulating tumor cells to the B7-H3 mRNA level in peripheral blood must also be considered. However, the amount of circulating tumor cells in peripheral blood is limited,Citation15 and is significantly lower than that of circulating endothelial cells; further, not all the clear cell RCC cancer cells expressed B7-H3, which is detected in <20% of cases, with a median level of B7-H3 expression <30%.Citation3 Therefore, the B7-H3 mRNA detected in peripheral blood is likely to be mostly derived from circulating endothelial cells.
In the real-time approach used in the present study, we did not normalize to any housekeeping genes, such as β-actin or glyceraldehyde 3-phosphate dehydrogenase, which are used to eliminate differences in cell numbers, RNA preparation, and cDNA synthesis. This normalization strategy is not useful for quantification of circulating endothelial cells because normalization to a housekeeping gene would result in calculation of a ratio of circulating endothelial cells to leukocytes, since the housekeeping genes expressed in all cells and leukocytes are at least in a 1,000-fold surplus compared with circulating endothelial cells. Such a ratio would not reflect absolute circulating endothelial cell numbers in blood samples because of inclusion of leukocytes, which are strongly influenced by infections or cancer therapies, including chemotherapy or radiation. Therefore, accurate assessment of the number of circulating endothelial cells in peripheral blood requires calculation of leukocyte counts in blood samples.Citation14 To test the variations in our method, RNA was prepared twice individually from the same blood sample, and real-time PCR analysis was performed with these separate samples. Variations between these samples of two separate real-time PCR was minimal, so normalization to a housekeeping gene and inclusion of leukocyte numbers is not necessary in this analysis and would only result in increased inaccuracy instead of reduced variation.
Although the limited sample size and heterogeneity of expression within individual tumors prevented further analysis of the relationship between tumor cell expression of B7-H3 and the clinical and pathologic features of clear cell RCC, our study provides enough data to validate B7-H3 as a new cancer-specific endothelial marker in clear cell RCC. Further, our pilot study showed that the mRNA level of B7-H3 in peripheral blood was significantly higher in metastatic clear cell RCC than in nonmetastatic disease. Our results warrant further investigations to evaluate B7-H3 as a potentially important tool for development of tumor-specific vascular-targeted therapies in clear cell RCC.
Acknowledgments
This research was supported by grants from the Shanghai Science Committee of Basic Research in Key Projects (10 JC1403000). The authors thank the following colleagues for their technical help: Yan Chen, Xiaomeng Jiang, Yijun Shen, Yiping Zhu, Wenjun Xiao, Chunguang Ma, Guowen Lin, Xiaoli Zhu, and Yongming Lu.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- LainakisGBamiasATargeting angiogenesis in renal cell carcinomaCurr Cancer Drug Targets20088534935818690841
- ChapovalAINiJLauJSB7-H3: a costimulatory molecule for T cell activation and IFN-gamma productionNat Immunol20012326927411224528
- SeamanSStevensJYangMYLogsdonDGraff-CherryCSt CroixBGenes that distinguish physiological and pathological angiogenesisCancer Cell200711653955417560335
- CrispenPLSheininYRothTJTumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinomaClin Cancer Res200814165150515718694993
- PusztaszeriMPSeelentagWBosmanFTImmunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissuesJ Histochem Cytochem200654438539516234507
- SteinbergerPMajdicODerdakSVMolecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domainsJ Immunol200417242352235914764704
- LoosMHedderichDMFriessHKleeffJB7-H3 and its role in antitumor immunityClin Dev Immunol2010201068387521127709
- LooDAldersonRFChenFZDevelopment of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activityClin Cancer Res201218143834384522615450
- St CroixBRagoCVelculescuVGenes expressed in human tumor endotheliumScience200028954821197120210947988
- NandaACarson-WalterEBSeamanSTEM8 interacts with the cleaved C5 domain of collagen alpha 3(VI)Cancer Res200464381782014871805
- ChaudharyAHiltonMBSeamanSTEM8/ANTXR1 blockade inhibits pathological angiogenesis and potentiates tumoricidal responses against multiple cancer typesCancer Cell201221221222622340594
- MancusoPBurliniAPruneriGGoldhirschAMartinelliGBertoliniFResting and activated endothelial cells are increased in the peripheral blood of cancer patientsBlood200197113658366111369666
- BertoliniFShakedYMancusoPKerbelRSThe multifaceted circulating endothelial cell in cancer: towards marker and target identificationNat Rev Cancer200661183584517036040
- FurstenbergerGvon MoosRSennHJBonebergEMReal-time PCR of CD146 mRNA in peripheral blood enables the relative quantification of circulating endothelial cells and is an indicator of angiogenesisBr J Cancer200593779379816160694
- HiokiTSugimuraY[Detection of circulating cancer cells by nested reverse transcription-polymerase chain reaction of cytokeratin-19 in patients with renal cell carcinoma.]Hinyokika Kiyo1999458577581 Japanese10500966