Abstract
Precision medicine is increasingly recognized as a promising approach to improve disease treatment, taking into consideration the individual clinical and biological characteristics shared by specific subgroups of patients. In specific fields such as oncology and hematology, precision medicine has already started to be implemented in the clinical setting and molecular testing is routinely used to select treatments with higher efficacy and reduced adverse effects. The application of precision medicine in psychiatry is still in its early phases. However, there are already examples of predictive models based on clinical data or combinations of clinical, neuroimaging and biological data. While the power of single clinical predictors would remain inadequate if analyzed only with traditional statistical approaches, these predictors are now increasingly used to impute machine learning models that can have adequate accuracy even in the presence of relatively small sample size. These models have started to be applied to disentangle relevant clinical questions that could lead to a more effective management of psychiatric disorders, such as prediction of response to the mood stabilizer lithium, resistance to antidepressants in major depressive disorder or stratification of the risk and outcome prediction in schizophrenia. In this narrative review, we summarized the most important findings in precision medicine in psychiatry based on studies that constructed machine learning models using clinical, neuroimaging and/or biological data. Limitations and barriers to the implementation of precision psychiatry in the clinical setting, as well as possible solutions and future perspectives, will be presented.
Introduction
Coinciding with the sequencing of the human genome, and the resulting delineation of the genetic architectures of many complex diseases, precision medicine has emerged as a novel player in healthcare.Citation1,Citation2 Although its popularity among providers, users, and public and private stakeholders has increased constantly in the last decade, the adequate comprehension of its characteristics (and of its exact definition), as well as of the complex operational approaches accompanying its implementation, remains limited. For instance, there is consensus that precision medicine is changing the paradigm of clinical care from the traditional evidence-based approach (founded on data gathered in large populations of patients), to an individual-based deep knowledge of clinical (phenotypic) and biological characteristics.Citation3 Yet, accurate descriptions of the potential impact of precision medicine on healthcare, even in economic terms, remain scant. In general, precision medicine “prioritizes the individualization of care and focuses attention on unique characteristics of a particular patient”.Citation3 This is clearly in line with the traditional, clinically oriented approach that has historically permeated medicine, where physicians base their intervention on an accurate dissection of the signs and the symptoms manifested by each individual. Psychiatry, in this regard, is a perfect testament of the potential of a personalized clinical approach. Its foundations are based on descriptive psychopathology and phenomenology, which in turn originate from the accurate analysis and understanding of the internal abnormal processes expressed by each individual.Citation4 However, even if psychiatry is deeply rooted in a personalized approach, the transition toward precision medicine, which needs other sources of information such as neuroimaging and/or biological measures, is still lagging behind compared to other fields of medicine.Citation5 For instance, cancer and haematology have experienced tremendous advancement in this area.Citation6,Citation7 In oncology, the SHIVA trial assessed whether histology-agnostic use of marketed molecularly targeted agents outside their indications based on tumour molecular profiling could improve outcomes for patients with any kind of cancer for whom standard of care had failed, compared with treatment at the physician’s choice.Citation6 Although the trial did not show a significant difference in progression-free survival between molecularly targeted agents compared with treatment at physician’s choice, it did show the potential for a precision medicine approach in oncology.Citation6 Concerning haematology, in 2018 the US Food and Drug Administration (FDA) approved the chimeric antigen receptor T cell (CAR T) for treatment of refractory pre-B cell acute lymphoblastic leukaemia and diffuse large B cell lymphoma.Citation7 This treatment is based on the application of sophisticated ex vivo culture and cellular engineering approaches to autologous T cells that can then express a CAR specific for the CD19 B lymphocyte molecule.Citation7 Thus, CAR T is the perfect exemplification of an intervention designed according to individual biological characteristics.
These are more optimistic scenarios than the one observable in psychiatry. However, it was not so long ago that psychiatry saw attempts to implement precision medicine approaches. One of the examples was the dexamethasone suppression test, which showed a moderate sensitivity (50–65%) but a high specificity (96%) in the prediction, not only of future episodes of depression, but also of response to antidepressant treatment.Citation8 However, the presence of an altered function of the hypothalamus–pituitary–adrenal (HPA) axis is a feature shared by almost all severe psychiatric disorders, limiting the clinical utility of these findings in terms of precision medicine.
One additional introductory remark should be made before starting our exposition. This concerns the need to use proper terminology, and methodology, in precision medicine in general, and even more so in psychiatry. Although a detailed review of these aspects lies outside the scope of this paper, we believe that a general overview of the terms might help the reader in familiarizing with the concepts of precision psychiatry. A list of the main terms is outlined in . Of particular relevance here is the concept of clinical significance. This is strictly related to, but somewhat independent, from statistical significance. Indeed, a statistically significant finding can or cannot be clinically relevant.Citation9 Conversely, clinical significance relates precisely to its own impact and importance for a patient population.Citation10 Findings with clinical significance are starting to being applied to dosing recommendations for psychotropic drugs, including selective serotonin reuptake inhibitors (SSRI),Citation11 tricyclic antidepressants (TCAs),Citation12 atomoxetine,Citation13 and carbamazepine,Citation14 based on genetic information. Specifically, information on genotypes of CYP2D6 (atomoxetine) and/or CYP2C19 (SSRIs and TCAs), two genes encoding enzymes that contribute to the metabolism of several antidepressants, can be used to adjust the dosage or select an alternative treatment based on the recommendations made by the Clinical Pharmacogenetics Implementation Consortium (CPIC)Citation15 or the Dutch Pharmacogenetics Working Group (DPWG).Citation16 Although translation of CYP2D6 genotype to metabolizing phenotypes is not standardized across laboratories, CPIC and DPWG have recently started to adopt a standardized system in order to develop more consistent dosing guidelines.Citation17 In the case of carbamazepine, recommendations based on HLA genotypes were formulated on the basis of a large body of evidence supporting an association between specific alleles and the risk of severe adverse drug reactions.Citation14 These initiatives represent a precious effort to overcome one of the barriers to precision psychiatry, ie, the difficulty in translating pharmacogenetic results into actionable treatment decisions.
Table 1 Glossary of Relevant Terms in Precision Psychiatry
However, despite the aforementioned examples, while there is a general consensus on the definition of statistical significance, clinical significance remains a vague concept, relying heavily on the subjective interpretations and assumptions made by the researchers.Citation10 This quandary has started to be resolved with the implementation of data analysis methods, such as machine learning, that allow to make inferences even in the presence of non-linear correlations between the independent variables of a specific outcome, and of relatively small sample sizes, using supervised and unsupervised methods.Citation18 Indeed, machine learning deals with the creation and evaluation of algorithms that facilitate pattern recognition, classification, and prediction, based on models derived from existing data.Citation19 Two main approaches, supervised and unsupervised learning, can be used when applying this analytical method.Citation19 While in supervised learning, specific set of attributes are used to classify data, unsupervised learning discovers the similarity patterns existing in the datasets allowing to identify distinct clusters of objects.Citation19 Although there are challenges, including reproducibility, management of missing data, and overfitting,Citation20,Citation21 that might dampen the enthusiasm for machine learning approaches, these have shown their utility in building clinically relevant predictive models. The classification performance of these models is typically measured using area under the receiver operating characteristic curve (ROC-AUC), accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). As we will show in the following sections, precision medicine, including precision psychiatry, is increasingly relying on the application of this methodology.
In this context, we reckon timely a narrative review of the most significant findings in precision psychiatry on predictive models based on clinical (and partly neuroimaging) data, and on biological measures. We will then discuss the barriers that are still present in the implementation of precision psychiatry, concluding with its potential and future perspectives.
Clinical Predictive Models in Precision Psychiatry
Precision medicine in psychiatry can be enriched by deep knowledge of the biological characteristics of each individual. Yet, there have been several attempts, partly successful, to implement predictive models of specific outcomes (for instance treatment response) based purely on clinical data (). These studies have mainly focused on: 1) response to lithium,Citation22–Citation24 the mainstay of treatment of bipolar disorder (BD), a recurrent mood disorder characterized by alternating episodes of depression and mania; 2) prediction of resistance to antidepressants in major depressive disorder;Citation25,Citation26 and 3) stratification of the riskCitation27 and outcome predictionCitation28–Citation30 in schizophrenia.
Table 2 Clinical Predictive Models in Precision Psychiatry
The prediction of response to lithium therapy is a clear example of the significance of precision psychiatry. One-third of lithium-treated patients shows a pattern of complete clinical response, with absence of mood recurrences and, importantly, return to normal levels of functioning as well as to good quality of life.Citation31,Citation32 Thus, lithium modifies substantially the trajectory of illness in BD patients, a characteristic that makes this drug a unique therapeutic tool in the field of psychiatry. The identification of reliable clinical predictors of lithium response has therefore gained attention. Quantitative data synthesis has pointed to several clinical factors as possible predictors.Citation33,Citation34 Among these are the presence of an episodic clinical course, the absence of psychotic symptoms, a positive family history of BD and later age of onset.Citation33,Citation34 However, the predictive power of these variables, either singularly or cumulatively, remains inadequate if based only on standard univariate statistical approaches. Proper analytical methods, such as machine learning, could be instrumental in determining, even in the presence of relatively small sample sizes, the accuracy of predictive models and their eventual clinical significance. In this context, Kim et al used data from a randomized clinical trial of outpatients with BD type I or II who received adjunctive personalized treatment plus either lithium or quetiapine.Citation22 In the analysis of lithium response data, the authors split the total sample (n = 240) in a training set (n = 192) and a test set (n = 48), finding that the predictive accuracy of the model was around 17%.Citation22 The most significant predictors of lithium response were the presence of non-suicidal self‐injurious behavior, of attention‐deficit/hyperactivity disorder, of high level of mania, of social phobia/social anxiety disorder, and of suicide risk.Citation22 Although potentially clinically relevant, these findings were based on a clinical outcome (response to lithium with a concomitant evidence-based stabilizing treatment) different from monotherapy efficacy, making the comparison with previous literature difficult. In addition, the model was based on data collected in a trial with a limited duration, which differs from the classical phenotypic definition of long-term lithium response and included also nonclinical features such as serum levels of laboratory markers (creatinine, sodium, potassium, and so on). More recently, a collaborative initiative led by Canadian investigators,Citation23 made use of data from seven international specialist clinics, with a minimum duration of lithium treatment of 1 year, using a validated scale for the assessment of clinical response to lithium.Citation35 This study suggested that lithium response could be predicted with a low rate of false positives (specificity 0.9), although the sensitivity was comparatively less strong (0.53).Citation23 Importantly, even considering the substantial between-site heterogeneity, completely episodic clinical course was the most informative feature in the prediction model of lithium response,Citation23 in line with prior evidence.Citation33,Citation34 It should be noted that attempts of predicting lithium response have been made also using neuroimaging data.Citation24 The proof of concept study of Fleck et al showed that a machine learning system applied to functional magnetic resonance imaging (fMRI) and proton magnetic resonance spectroscopy (1H-MRS) inputs were able to predict post-treatment symptom reductions at 8 weeks of lithium treatment with at least 88% accuracy in training and 80% accuracy in validation.Citation24 However, the outcome chosen for the analysis (short-term lithium response) was again different from the one typically used in clinical and genetic studies.
Another set of findings concerns the testing of predictive algorithms of treatment resistance to antidepressants.Citation25,Citation26 Treatment-resistant depression is a common clinical phenomenon, with about 30% of patients with major depressive disorder showing lack of clinical improvement.Citation36 In addition, a much larger proportion of antidepressant-treated patients show suboptimal responses.Citation36 Predicting with adequate accuracy treatment-resistant depression could translate into a more effective management of the disorder, with a more expedite relief of the symptoms, and a reduction of the costs determined by long-term disability. Using data drawn from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, Perlis applied different analytical approaches (logistic regression and machine learning algorithms) to test the predictive power of a series of clinical variables selected on the basis of manual and automated approaches.Citation25 Both approaches led to a comparable performance in the prediction of treatment resistance depression with an area under the curve (AUC) of 0.7 in training, testing, and validation datasets.Citation25 Specifically, several variables, such as years of education, presence of recurrent illness, presence of psychosis, African-American ethnicity, presence of Post-Traumatic Stress Disorder, and higher total score at the Quick Inventory of Depressive Symptoms, increased the risk of treatment-resistant depression.Citation25 These findings suggest that it is possible to rely solely on patient self-reported measures to identify at least a subset of individuals at greatest risk for treatment resistance.Citation25 More recently, Nie and coauthors applied five different machine learning approaches to clinical and socio-demographic data from the STAR*D cohort and an independent cohort, the latter used as an external test dataset to predict treatment-resistant depression after two trials of treatment regimens.Citation26 The authors found that, using the top 30 clinical predictors, the gradient boosting decision tree (GBDT) model was able to predict both treatment resistance and non-treatment resistance with comparable accuracy (about 0.7).Citation26 Several variables, including more severe 16-item Quick Inventory of Depressive Symptomatology, clinician-rated (QIDS-C16) symptom severity total score at week 2, presence of anxiety/chronicity as identified with the Psychiatric Diagnostic Screening Questionnaire (PDSQ) questions, lower levels of Physical Health Composite Scores (PCS), presence of nervous system symptom (PRISE question) and Work and Social Adjustment Scale (WSAS) total score were associated with increased odds of being treatment resistant to antidepressants.Citation26
Finally, a few studies focused 1) on the ability of machine learning algorithms to identify subgroups of patients affected by schizophrenia with more homogenous characteristics, that could be targeted by ad hoc interventions, either pharmacological or non-pharmacological, and 2) on the predictive accuracy of diagnostic conversion in at-risk subjects or of symptomatic recurrences.Citation27–Citation30 The implementation of accurate deep phenotyping is a necessary condition to reduce the impact of heterogeneity on prediction accuracy.Citation37 But this might not be sufficient if adequate analytical approaches are not implemented. The work of Talpalaru et al used different classifiers, logistic regression, random forest and support vector machine (SVM), to identify subgroups of schizophrenic patients on the basis of symptomatic and magnetic resonance imaging (MRI) data of 167 subjects were used.Citation27 The subgroups included patients at 1) high symptom burden, 2) predominantly positive symptom burden, and 3) mild symptom burden. These authors found that all three classifiers predicted the high symptom burden group with an AUC higher than the case–control comparison. Additionally, the RF classifier also outperformed the case–control study in predicting the mild symptom burden group.Citation27 Even if not including solely clinical information, these findings show the ability of machine learning algorithms to perform clinical prediction. Furthermore, they demonstrate how the integration of different types of data can increase the performance of predictive algorithms even with relatively small sample sizes. The study of Brodey et alCitation28 tested whether machine learning analysis of data from a self-report screener for early psychosis was able to predict with accuracy the diagnostic conversion to psychosis. The SVM algorithm applied on data from 353 participants showed that this assessment tool had cross-validated PPV of 76.5% at separating individuals who would not convert to psychosis within 12 months from those who either would convert within 12 months or who had already experienced a first-episode psychosis.Citation28 Fond et al showed the ability of a classification and regression tree to predict psychotic relapse in a cohort of 315 patients followed up for 2 years.Citation29 Several clinical characteristics, such as high anger and/or physical aggressiveness, the number of lifetime hospitalizations in psychiatry, low education level, and high positive symptoms at baseline were found to be the best predictors of psychotic relapse at 2 years.Citation29 The work of Koutsouleris et al applied non-linear SVM machine learning to clinical data from 334 patients in the European First-Episode Schizophrenia Trial to predict outcome after 4 weeks and 52 weeks of treatment.Citation30 The accuracy of the predictive models was adequate (75% for 4-week outcomes and 73.8% for 52-week outcomes), showing the feasibility of generalizable, individual-patient prediction of treatment outcomes in first-episode psychosis using pre-treatment clinical information.Citation30 Importantly, the most useful predictors of poor 4-week and 52-week treatment outcome were unemployment, unmet needs in the Camberwell Assessment of Needs (CAN) questionnaire about relationships, daytime activities, and psychological distress.Citation30 Educational difficulties were predictive at 52-week, and low educational status of the patient and the patient’s mother at 4-weeks.Citation30 Poor 1-year outcome was predicted by a series of variables including the Mini-International Neuropsychiatric Interview (MINI) diagnostic items [recurrent major depression, schizophrenia (present and lifetime)], suicidality, male sex, and lower baseline scores on the PANSS positive subscale, presence of conceptual disorganization and hyperactivity (P2 and P4 items of PANSS, respectively).Citation30 Poor 4-week outcome was predicted by higher clinical global impression (CGI) and lower global assessment of functioning (GAF) score at baseline, haloperidol treatment, and unmet needs in the CAN’s accommodation, information, money, and sexual expression domains.Citation30
In summary, these findings show that precision psychiatry have a solid base on clinical data, provided that accurate phenotyping, preferably in a longitudinal perspective, is performed. This can be empowered by the integration of other sources of data, such as neuroimaging and, as we will discuss below, biological measures.
Biological Predictive Models in Precision Psychiatry
The majority of studies assessing whether biological variables are able to increase the accuracy of predictive models of treatment response to psychotropic medications focused on antidepressants (). A recent meta-analysis evaluated previous studies using machine learning-devised models to predict therapeutic outcomes in unipolar and bipolar depression.Citation38 The quantitative evaluation of 20 studies showed an overall accuracy, clinically relevant, of 0.82. Importantly, the models with the best performance were those informed by multiple data types which included neuroimaging, genetic and clinical predictors.Citation38 In particular, the latter included overall mood symptom severity, anxiety, anhedonia, global functioning, and the number of previous mood episodes.Citation38 In addition, socio-demographic variables, such as employment status, level of education, and household income, contributed significantly to the performance of the predictive models.Citation38 This evidence highlights the importance of including multiple source of data to increase the accuracy of predictive models.
Table 3 Biological Predictive Models in Precision Psychiatry
Among the first studies using genetic data to predict response to antidepressants, Serretti and Smeraldi (2004) applied a neural network approach in a sample of 121 patients with major depressive disorder treated with fluvoxamine for 6 weeks.Citation39 Although this study only included genotypic data for two variants [serotonin transporter gene-linked functional polymorphic region (5-HTTLPR) and a polymorphism located in the promoter of the TPH gene, it reached a promising accuracy equal to 0.71 in predicting response to fluvoxamine defined as an Hamilton Depression Rating Scale (HAMD) 21 items (HAMD-21) ≤8.Citation39 An important effort to define algorithms for treatment-resistant depression is currently being made by the European Group for the Study of Resistant Depression (GSRD).Citation40 In a recent work, using random forest and k-means clustering, the authors identified a signature of three genetic variants related to treatment outcome [rs6265 in the gene encoding for the brain-derived neurotrophic factor (BDNF), rs7430 in the Protein Phosphatase 3 Catalytic Subunit Gamma (PPP3CC) gene, and rs6313 in the gene encoding for the serotonin transporter receptor 2A (HTR2A)] that, combined with the absence of melancholy, were associated with a decline in the HAMD score <17 (62% of the patients with this combination compared to 34% in the whole study population).Citation41 A more comprehensive list of genetic variants was investigated by Maciukiewicz et al (2018).Citation42 The authors used LASSO regression to identify the most promising predictors and SVM to predict treatment outcome in a sample of 186 major depressive disorder patients treated with duloxetine for 8 weeks and for which genome-wide association data (GWAS) were available.Citation42 Response was defined as a 50% increase in the Montgomery–Asberg Depression Rating Scale (MADRS) score from baseline, while remission was reached with a MADRS score ≤10 at end point. While no model showed a good performance for response, a slightly better performance was achieved for remission, although substantially below the threshold for clinical applicability (accuracy=0.52, sensitivity=0.58, and specificity=0.46).Citation42 Although innovative in its approach, the limited number of participants and the choice not to apply any filter or weight according to the biological relevance of the variants might have influenced the results of this study. Besides genotypic data, peripheral messenger RNA (mRNA) levels of selected genes might also inform machine learning models. This approach was applied by a recent study showing that baseline expression levels of six genes (IFITM3, RPL5, GZMA, RPL24, MATR3 and RPL17) selected from genome-wide transcriptome data reached 0.76 accuracy in predicting non-remission after 8 weeks of treatment with citalopram.Citation43
Other studies investigated the use of genetic variants together with imaging or metabolomic information.Citation44,Citation45 Specifically, an SVM model integrating clinical variables with resting-state fMRI and 13 selected single nucleotide polymorphisms (SNPs) was used to predict early response to antidepressants defined as a 50% reduction of the 6-item HAMD (HAMD-6) score after 2 weeks in a sample of 98 Chinese patients with major depressive disorder treated with SSRI (n=63) or SNRI (n=35).Citation45 In this study, the model based on multimodal features achieved a better performance (accuracy = 0.86) compared to models including only fMRI (accuracy = 0.81) or genetic (accuracy = 0.73) data. Another recent study was conducted by Athreya et al (2018), who developed a learning-augmented clinical assessment workflow.Citation44 The aim of this model was to integrate metabolomics and genomics measures derived from peripheral blood with clinical data, to increase the accuracy in the prediction of treatment outcomes. The workflow was designed using data from about 300 patients with major depressive disorder treated with citalopram/escitalopram for 8 weeks and included in the Mayo Clinic Pharmacogenomics Research Network Antidepressant Medical Pharmacogenomic Study (Mayo PGRN-AMPS) and for whom biological measures were available. Response to SSRIs at 4 and 8 weeks was evaluated using the QIDS-C.Citation46 In this study, the analysis of GWAS data and plasma metabolomic concentrations for 31 metabolites, integrated with psychometric measures and sociodemographic factors, increased the prediction accuracy of treatment response from around 52% to 64% compared to a model using only clinical predictors.Citation44 In the first step, unsupervised learning techniques were used to identify the cluster of patients and metabolites associated with symptom severity (eg, serotonin, kynurenine and tryptophan). GWAS data were also used to identify SNPs associated with concentrations of the previously identified metabolites (such as SNPs located in the DEFB1, AHR, TSPAN5 and ERICH3 genes). The final model included 65 variables among SNPs, metabolites and clinical predictors.Citation44 Along this line of research, recently, Chang et alproposed the Antidepressant Response Prediction Network for Major Depressive Disorder (ARPNet) initiative, a platform aiming to predict whether the patient will reach clinical remission as well as the degree of response to antidepressants (expressed as the HAM-D score).Citation47 In this model, elastic net was used to select the most informative features among clinical and biological predictors including demographic variables, brain MRI features, genetic (35 genes related to antidepressants) and epigenetic data (136 CpG sites). The model was applied to predict response to antidepressants (HAMD scores of the patients measured at 1, 4, 8 and 24 weeks after the initial visit) in a sample of 121 Korean patients with major depressive disorder treated with various antidepressants. From a methodological point of view, the novelty of this model consisted of being composed of three distinct layers (a patient representation, an antidepressant prescription representation and a prediction layer).Citation47 The first two layers capture informative features to create the patient and antidepressant representation vectors, respectively, which are used by the prediction layer based on a linear regression approach. ARPNet reached high sensitivity (0.80), specificity (0.87) and accuracy (0.85), being able to outperform six other machine learning models, including SVM regressor with a linear kernel and random forest regressor.Citation47
Fewer studies applied machine learning models including biological features to predict response to antipsychotics. Rather than focusing on response to single antipsychotics, most studies focused on predicting treatment-resistant schizophrenia.Citation48 A recent study from Vivian-Griffiths et al (2019) used an SVM model to compare its predictive performance to that of a polygenic risk score (PRS) prediction in discriminating patients with treatment-resistant schizophrenia from healthy controls.Citation49 The study was conducted in the CLOZUK sample, including 5554 patients with treatment-resistant schizophrenia and 6299 healthy controlsCitation50 and the PRS included 4998 SNPs from the Psychiatric Genomics Consortium (PGC) wave 2 GWAS meta-analysis.Citation51 In this study, the SVM model showed a worse accuracy compared to PRS, with neither of the two approaches showing a prediction accuracy adequate for clinical implementation [median area under the receiver operating characteristics curve (AUC-ROC): PRS = 0.644, SVM = 0.634].Citation49
Based on the high heritability of antipsychotics-induced side effects (0.60–0.80), a recent study developed a model based on genetic data to predict extrapyramidal symptoms induced by antipsychotics,Citation52 which represented a refinement of a previous model proposed by the same authors.Citation53 In light of the evidence implicating the mTOR signaling pathway in antipsychotics-induced extrapyramidal symptomsCitation53 as well as in L-DOPA-induced dyskinesia,Citation54 Boloc et al evaluated 12 functional SNPs located in four genes of the mTOR signaling pathway (AKT1, FCHSD1, RPTOR and DDIT4). Four of these SNPs (rs33925946 and rs1130214 in the AKT1 gene; rs3476568 and rs9915667 in the RPTOR gene) were selected on the basis of their nominal association with extrapyramidal symptoms in a discovery sample including 131 patients treated with risperidone (of which 48 with and 83 without extrapyramidal symptoms). Naive Bayes learner achieved the best performance among three machine learning algorithms applied to the discovery sample and was therefore used to predict the extrapyramidal symptoms status in two replication samples, showing good specificity (0.81 and 0.79) but low sensitivity (0.39 and 0.38).Citation52 Taken together, these data show that, although still falling short of the clinical significance threshold, predictive models based on purely biological data, or integrating them with clinical information, are: 1) starting to reach the accuracy needed to become implantable in clinical settings; 2) showing in general low sensitivity, but high specificity, implying that, at least for predictive models of treatment response, it may be of importance to avoid a premature suspension of a drug trial given that an eventual response would be highly probable.
Barriers to Precision Medicine in Psychiatry
There are great expectations for the successful implementation of precision psychiatry. However, a series of hurdles might delay or even impede this paradigm shift. These involve ethical aspects, the impact of stigma and the possible lack of cost-effectiveness. Ethical components of a correct application of precision psychiatry include self-determination (autonomy), nonmaleficence, clinician competency, justice and veracity.Citation55,Citation56 For instance, self-determination implies that a patient, duly informed on the development of novel diagnostic and prognostic approaches, can freely exert his right to choose (or refuse) them.Citation56 To guarantee this, a clinician should be properly informed on the methodology, and should not have any conflict of interest (such as personal financial benefit) related to it.Citation56 In addition, given that precision psychiatry is strongly related to the analysis of massive datasets (either phenotypic, neuroimaging, or biological), confidentiality and privacy concerns are becoming increasingly relevant. In this context, it is crucial to develop an appropriate ethical-legal framework, which would facilitate safe data sharing. Closely related to the concept of self-determination is the essential need of an active role of the patient to access precision psychiatry instruments (electronic monitoring tools, neuroimaging, genotyping, laboratory tests in general). While this is easily achievable in less severe psychiatric disorders (anxiety disorders, persistent depressive disorder), patients with severe depression, and presenting social withdrawal and/or disorganized behaviour, as in schizophrenia, might self-exclude from precision psychiatry. This should be taken into account in the organization of future mental health services when precision psychiatry will be implemented. Another aspect which has a fundamental impact on mental healthcare in general, and in precision psychiatry specifically, is stigma. Both public (the prejudice disseminated in the general population toward patients affected by mental illness) and self-stigma (occurring when this prejudice is internalized by the patient) can impact on the implementation of precision psychiatry. Public stigma could negatively orient public health policies diminishing the relevance of mental health as a potential target for precision medicine. In addition, self-stigmatized patients could withdraw their participation in precision psychiatry approaches to avoid the spotlight. The influence of stigma should not be neglected, as it is known to influence several important outcomes in psychiatry, such as, for instance, suicide.Citation57
A final remark concerns the possibility that precision psychiatry tools may not be cost-effective, at least in the first phases of their implementation. Although there are examples of cost-effectiveness, such as in pharmacogenetic testing of polymorphisms within the HLA region for the onset of Stevens–Johnson syndrome/toxic epidermal necrolysis in Asian patients treated with carbamazepine (HLA-A*31:01), or agranulocytosis/neutropenia in clozapine treated patients [HLA-B (158T) and HLA-DQB1 (126Q)],Citation58 these remain limited to the prediction of side effects with lack of evidence with regard to the prediction of clinical response. For instance, CAR-T is extremely effective but is priced amongst the most expensive cancer therapies to date, up to $475,000 per treatment.Citation59 This raises doubts on the long-term sustainability of these approaches for public healthcare. However, promising preliminary considerations can be drawn from studies investigating cost savings associated with available pharmacogenetics panels, such as the Assurex GeneSight Psychotropic test.Citation60 Recent studies suggested significant cost savings in medication costs for patients whose antidepressant and antipsychotic prescribing was congruent with the test recommendations.Citation61–Citation63 However, as these studies were funded by Assurex Health, further independent investigations are needed to draw definitive conclusions. In addition, the presence of economic disparities at a global level might also impact the implementation of precision medicine approaches in mental health.
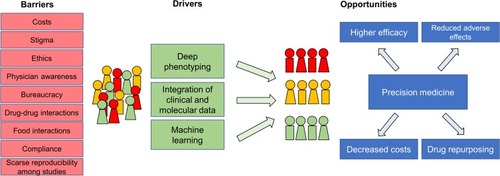
Other obstacles can hinder the implementation of precision psychiatry in clinical settings. Among these are the lack of an adequate knowledge of precision medicine tools, including pharmacogenetics/pharmacogenomics, in clinicians and mental health operators, and compliance toward precision medicine tools. There are extensive data on the impact that inadequate education and training can exert on the proper implementation of precision medicine.Citation64,Citation65 It has been proposed that to realize the translation of precision medicine concepts and instruments into clinical care, specific training packages should be implemented at undergraduate and graduate levels.Citation66 Furthermore, a specific set of skills are needed in healthcare providers involved in precision medicine implementation.Citation67 In this context, initiatives such as those spearheaded by the Electronic Medical Records and Genomics Network show the relevance of ongoing education to ensure provider acceptance and adoption of precision medicine tools.Citation68 Another relevant component that might delay the implementation of precision psychiatry is users’ adherence to tests and procedures. It is conceivable that adherence to precision medicine tools can be as common as that for pharmacological treatment in individuals with mental illness. As several factors, including access to scientifically reliable and valid evidence,Citation69 might substantially influence adherence, disseminating information and involving patients in shared decision-making could favor the implementation of precision psychiatry tools.Citation70 illustrates some of the main barriers and opportunities for precision psychiatry.
Finally, a concluding remark should be made on the protagonists of the implementation of precision psychiatry. As the primary responsibility for the care of patients, psychiatrists will need to take the lead in the implementation of precision psychiatry tools. As previously mentioned, adequate training and education will be key to achieve successful results. In addition, the involvement of patients in specific brief training activities might be an achievable and useful objective, as recent evidence shows that brief community educational program can improve knowledge of complex genomic concepts in the general population.Citation71
In summary, stakeholders will need to assess the impact of these barriers on the application of precision psychiatry. Changes in the organization of mental healthcare as well as of funding policies and creation of proper legislation will be crucial to address them.
Perspectives and Potential of Precision Medicine in Psychiatry
We have argued that precision psychiatry has its foundation in accurate deep phenotyping. However, this can be achieved only if a dynamic longitudinal perspective is taken fully into account. Indeed, most severe psychiatric disorders show developmental trajectories that start in childhood or early adolescence. Antecedents of the disorders are not only psychopathological but also neurocognitive, neuroanatomical, and biological.Citation72 In this context, precision psychiatry might have also a preventative value. The integration of omics approach with neuroimaging and phenotypic data can increase the accuracy in the prediction of diagnostic conversion in at-risk population. Attempts of implementing these approaches have been made in the population at clinical high risk for psychosis.Citation73 Yet, the longitudinal component in psychiatric disorders remains neglected. This is of relevance if we think that even genetic associations with a specific disorder might differ according to different trajectories or patterns of antecedents.Citation74 Even in well-characterized phenotypes, such as lithium response, there is scarce knowledge on the temporal dynamics of those neurobiological changes that underscore the onset of a long-term efficacy of the treatment. And it remains to be established whether immediate neurobiological changes correspond to long-term changes and whether the latter are predictable from the former. In this context, an ongoing large EU funded research initiative is attempting to answer these sensible research and clinical questions.Citation75 Other approaches, again applying a longitudinal perspective, are attempting to disentangle the interaction of several biological components, including the microbiome, on the risk of recurrences in bipolar disorder.Citation76 In conclusion, the potential for precision psychiatry is large, but can be realized only by taking into account the temporal dynamics of mental disorders.
Conclusions
In summary, we have described the intensification of precision approaches in psychiatry. There is a strong expectation that the implementation of precision psychiatry, even if challenging, will ultimately improve the standard of care for patients suffering from mental illness. It remains to be seen whether precision psychiatry will cause the expected paradigm shift, or if it will just prove to be the next in a long line of disillusionments.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgment
The authors wish to thank all patients affected by mental illness who make our research possible and most importantly, meaningful. This paper was partly funded by Fondo Integrativo per la Ricerca (FIR)-2018 granted to AS.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
- Jameson JL, Longo DL. Precision medicine — personalized, problematic, and promising. N Engl J Med. 2015;372(23):2229–2234. doi:10.1056/NEJMsb150310426014593
- Ashley EA. Towards precision medicine. Nat Rev Genet. 2016;17(9):507. doi:10.1038/nrg.2016.8627528417
- Tonelli MR, Shirts BH. Knowledge for precision medicine: mechanistic reasoning and methodological pluralism. JAMA. 2017;318(17):1649–1650. doi:10.1001/jama.2017.1191429052713
- Häfner H. Descriptive psychopathology, phenomenology, and the legacy of Karl Jaspers. Dialogues Clin Neurosci. 2015;17(1):19–29.25987860
- Alda M. Personalized psychiatry: many questions, fewer answers. J Psychiatry Neurosci. 2013;38(6):363–365. doi:10.1503/jpn.13022124148844
- Le Tourneau C, Delord J-P, Gonçalves A, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16(13):1324–1334. doi:10.1016/S1470-2045(15)00188-626342236
- June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. doi:10.1126/science.aar671129567707
- Carroll B. Clinical applications of the dexamethasone suppression test for endogenous depression. Pharmacopsychiatry. 1982;15(01):19–25. doi:10.1055/s-2007-1019504
- Barlow DH. On the relation of clinical research to clinical practice: current issues, new directions. J Consult Clin Psychol. 1981;49(2):147. doi:10.1037/0022-006X.49.2.1477217481
- LeFort SM. The statistical versus clinical significance debate. Image J Nurs Sch. 1993;25(1):57–62. doi:10.1111/j.1547-5069.1993.tb00754.x8240480
- Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98(2):127–134. doi:10.1002/cpt.14725974703
- Hicks J, Sangkuhl K, Swen J, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther. 2017;102(1):37–44. doi:10.1002/cpt.59727997040
- Brown JT, Bishop JR, Sangkuhl K, et al. Clinical pharmacogenetics implementation consortium guideline for cytochrome P450 (CYP)2D6 genotype and atomoxetine therapy. Clin Pharmacol Ther. 2019;106(1):94–102. doi:10.1002/cpt.140930801677
- Phillips EJ, Sukasem C, Whirl-Carrillo M, et al. Clinical pharmacogenetics implementation consortium guideline for HLA genotype and use of carbamazepine and oxcarbazepine: 2017 update. Clin Pharmacol Ther. 2018;103(4):574–581. doi:10.1002/cpt.100429392710
- Caudle K, Klein T, Hoffman J, et al. Incorporation of pharmacogenomics into routine clinical practice: the clinical pharmacogenetics implementation consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15(2):209–217. doi:10.2174/138920021566614013012491024479687
- Lunenburg CATC, van der Wouden CH, Nijenhuis M, et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene–drug interaction of DPYD and fluoropyrimidines. Eur J Hum Genet. 2020;28(4):508–517. doi:10.1038/s41431-019-0540-031745289
- Caudle KE, Sangkuhl K, Whirl-Carrillo M, et al. Standardizing CYP2D6 genotype to phenotype translation: consensus recommendations from the clinical pharmacogenetics implementation consortium and Dutch pharmacogenetics working group. Clin Transl Sci. 2020;13(1):116–124. doi:10.1111/cts.1269231647186
- Liu Y, Chen PHC, Krause J, Peng L. How to read articles that use machine learning: users’ guides to the medical literature. JAMA. 2019;322(18):1806–1816. doi:10.1001/jama.2019.1648931714992
- Tarca AL, Carey VJ, Chen XW, Romero R, Drǎghici S. Machine learning and its applications to biology. PLoS Comput Biol. 2007;3(6):e116. doi:10.1371/journal.pcbi.003011617604446
- Beam AL, Manrai AK, Ghassemi M. Challenges to the reproducibility of machine learning models in health care. JAMA. 2020;323(4):305. doi:10.1001/jama.2019.20866
- Doshi-Velez F, Perlis RH. Evaluating machine learning articles. JAMA. 2019;322(18):1777–1779. doi:10.1001/jama.2019.1730431714974
- Kim TT, Dufour S, Xu C, et al. Predictive modeling for response to lithium and quetiapine in bipolar disorder. Bipolar Disord. 2019;21(5):428–436. doi:10.1111/bdi.1275230729637
- Nunes A, Ardau R, Berghöfer A, et al. Prediction of lithium response using clinical data. Acta Psychiatr Scand. 2020;141(2):131–141. doi:10.1111/acps.1312231667829
- Fleck DE, Ernest N, Adler CM, et al. Prediction of lithium response in first-episode mania using the LITHium Intelligent Agent (LITHIA): pilot data and proof-of-concept. Bipolar Disord. 2017;19(4):259–272. doi:10.1111/bdi.1250728574156
- Perlis RH. A clinical risk stratification tool for predicting treatment resistance in major depressive disorder. Biol Psychiatry. 2013;74(1):7–14. doi:10.1016/j.biopsych.2012.12.00723380715
- Nie Z, Vairavan S, Narayan VA, Ye J, Li QS. Predictive modeling of treatment resistant depression using data from STAR*D and an independent clinical study. PLoS One. 2018;13(6):e0197268. doi:10.1371/journal.pone.019726829879133
- Talpalaru A, Bhagwat N, Devenyi GA, Lepage M, Chakravarty MM. Identifying schizophrenia subgroups using clustering and supervised learning. Schizophr Res. 2019;214:51–59. doi:10.1016/j.schres.2019.05.04431455518
- Brodey BB, Girgis RR, Favorov OV, et al. The Early Psychosis Screener for Internet (EPSI)-SR: predicting 12 month psychotic conversion using machine learning. Schizophr Res. 2019;208:390–396. doi:10.1016/j.schres.2019.01.01530777603
- Fond G, Bulzacka E, Boucekine M, et al. Machine learning for predicting psychotic relapse at 2 years in schizophrenia in the national FACE-SZ cohort. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:8–18. doi:10.1016/j.pnpbp.2018.12.00530552914
- Koutsouleris N, Kahn RS, Chekroud AM, et al. Multisite prediction of 4-week and 52-week treatment outcomes in patients with first-episode psychosis: a machine learning approach. Lancet Psychiatry. 2016;3(10):935–946. doi:10.1016/S2215-0366(16)30171-727569526
- Alda M. Who are excellent lithium responders and why do they matter? World Psychiatry. 2017;16(3):319–320. doi:10.1002/wps.2046228941103
- Alda M. Lithium in the treatment of bipolar disorder: pharmacology and pharmacogenetics. Mol Psychiatry. 2015;20(6):661–670. doi:10.1038/mp.2015.425687772
- Kleindienst N, Engel R, Greil W. Which clinical factors predict response to prophylactic lithium? A systematic review for bipolar disorders. Bipolar Disord. 2005;7(5):404–417. doi:10.1111/j.1399-5618.2005.00244.x16176433
- Hui TP, Kandola A, Shen L, et al. A systematic review and meta analysis of clinical predictors of lithium response in bipolar disorder. Acta Psychiatr Scand. 2019;140(2):94–115. doi:10.1111/acps.1306231218667
- Grof P, Duffy A, Cavazzoni P, et al. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63(10):942–947. doi:10.4088/JCP.v63n101312416605
- Thase ME. Treatment-resistant depression. J Clin Psychiatry. 2011;72(05):e18. doi:10.4088/JCP.8133tx4c21658343
- Manchia M, Cullis J, Turecki G, Rouleau GA, Uher R, Alda M. The impact of phenotypic and genetic heterogeneity on results of genome wide association studies of complex diseases. PLoS One. 2013;8(10):e76295. doi:10.1371/journal.pone.007629524146854
- Lee Y, Ragguett R-M, Mansur RB, et al. Applications of machine learning algorithms to predict therapeutic outcomes in depression: a meta-analysis and systematic review. J Affect Disord. 2018;241:519–532. doi:10.1016/j.jad.2018.08.07330153635
- Serretti A, Smeraldi E. Neural network analysis in pharmacogenetics of mood disorders. BMC Med Genet. 2004;5(1):27. doi:10.1186/1471-2350-5-2715588300
- Bartova L, Dold M, Kautzky A, et al. Results of the European Group for the study of resistant depression (GSRD) - basis for further research and clinical practice. World J Biol Psychiatry. 2019;20(6):427–448. doi:10.1080/15622975.2019.163527031340696
- Kautzky A, Baldinger P, Souery D, et al. The combined effect of genetic polymorphisms and clinical parameters on treatment outcome in treatment-resistant depression. Eur Neuropsychopharmacol. 2015;25(4):441–453. doi:10.1016/j.euroneuro.2015.01.00125769916
- Maciukiewicz M, Marshe VS, Hauschild A-C, et al. GWAS-based machine learning approach to predict duloxetine response in major depressive disorder. J Psychiatr Res. 2018;99:62–68. doi:10.1016/j.jpsychires.2017.12.00929407288
- Guilloux J-P, Bassi S, Ding Y, et al. Testing the predictive value of peripheral gene expression for nonremission following citalopram treatment for major depression. Neuropsychopharmacology. 2015;40(3):701–710. doi:10.1038/npp.2014.22625176167
- Athreya A, Iyer R, Neavin D, et al. Augmentation of physician assessments with multi-omics enhances predictability of drug response: a case study of major depressive disorder. IEEE Comput Intell Mag. 2018;13(3):20–31. doi:10.1109/MCI.2018.284066030467458
- Pei C, Sun Y, Zhu J, et al. Ensemble learning for early-response prediction of antidepressant treatment in major depressive disorder. J Magn Reson Imaging. 2019. doi:10.1002/jmri.27029
- Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. doi:10.1016/S0006-3223(02)01866-812946886
- Chang B, Choi Y, Jeon M, et al. ARPNet: antidepressant response prediction network for major depressive disorder. Genes (Basel). 2019;10(11):907. doi:10.3390/genes10110907
- Pisanu C, Squassina A. Treatment-resistant schizophrenia: insights from genetic studies and machine learning approaches. Front Pharmacol. 2019;10:617. doi:10.3389/fphar.2019.0061731191325
- Vivian-Griffiths T, Baker E, Schmidt KM, et al. Predictive modeling of schizophrenia from genomic data: comparison of polygenic risk score with kernel support vector machines approach. Am J Med Genet B Neuropsychiatr Genet. 2019;180(1):80–85. doi:10.1002/ajmg.b.3270530516002
- Hamshere ML, Walters JTR, Smith R, et al. Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDCCAG8, and extensive replication of associations reported by the Schizophrenia PGC. Mol Psychiatry. 2013;18(6):708–712. doi:10.1038/mp.2012.6722614287
- Schizophrenia Working Group of the Psychiatric Genomic Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi:10.1038/nature1359525056061
- Boloc D, Gortat A, Cheng-Zhang JQ, et al. Improving pharmacogenetic prediction of extrapyramidal symptoms induced by antipsychotics. Transl Psychiatry. 2018;8(1):276. doi:10.1038/s41398-018-0330-430546092
- Mas S, Gassó P, Ritter MA, Malagelada C, Bernardo M, Lafuente A. Pharmacogenetic predictor of extrapyramidal symptoms induced by antipsychotics: multilocus interaction in the mTOR pathway. Eur Neuropsychopharmacol. 2015;25(1):51–59. doi:10.1016/j.euroneuro.2014.11.01125499605
- Martín-Flores N, Fernández-Santiago R, Antonelli F, et al. MTOR pathway-based discovery of genetic susceptibility to L-DOPA-induced dyskinesia in Parkinson’s disease patients. Mol Neurobiol. 2019;56(3):2092–2100. doi:10.1007/s12035-018-1219-129992529
- Juengst E, McGowan ML, Fishman JR, Settersten Jr RA. From “personalized” to “precision” medicine: the ethical and social implications of rhetorical reform in genomic medicine. Hastings Cent Rep. 2016;46(5):21–33. doi:10.1002/hast.61427649826
- Ball TM, Kalinowski A, Williams LM. Ethical implementation of precision psychiatry. Pers Med Psychiatry. 2019;19:100046.
- Carpiniello B, Pinna F. The reciprocal relationship between suicidality and stigma. Front Psychiatry. 2017;8:35. doi:10.3389/fpsyt.2017.0003528337154
- Plumpton CO, Pirmohamed M, Hughes DA. Cost effectiveness of panel tests for multiple pharmacogenes associated with adverse drug reactions: an evaluation framework. Clin Pharmacol Ther. 2019;105(6):1429–1438. doi:10.1002/cpt.131230466189
- Hay AE, Cheung MC. CAR T-cells: costs, comparisons, and commentary. J Med Econ. 2019;22(7):613–615. doi:10.1080/13696998.2019.158205930747012
- Health Quality Ontario. Pharmacogenomic testing for psychotropic medication selection: a systematic review of the assurex genesight psychotropic test. Ont Health Technol Assess Ser. 2017;17(4):1–39.
- Tanner J-A, Brown LC, Yu K, Li J, Dechairo BM. Canadian medication cost savings associated with combinatorial pharmacogenomic guidance for psychiatric medications. Clinicoecon Outcomes Res. 2019;11:779–787. doi:10.2147/CEOR.S22427731849503
- Brown LC, Lorenz RA, Li J, Dechairo BM. Economic utility: combinatorial pharmacogenomics and medication cost savings for mental health care in a primary care setting. Clin Ther. 2017;39(3):592–602.e1. doi:10.1016/j.clinthera.2017.01.02228238356
- Jablonski MR, Lorenz R, Li J, Dechairo BM. Economic outcomes following combinatorial pharmacogenomic testing for elderly psychiatric patients. J Geriatr Psychiatry Neurol. 2019;891988719892341.31842673
- Squassina A, Manchia M, Manolopoulos VG, et al. Realities and expectations of pharmacogenomics and personalized medicine: impact of translating genetic knowledge into clinical practice. Pharmacogenomics. 2010;11(8):1149–1167. doi:10.2217/pgs.10.9720712531
- Anaya J-M, Duarte-Rey C, Sarmiento-Monroy JC, Bardey D, Castiblanco J, Rojas-Villarraga A. Personalized medicine. Closing the gap between knowledge and clinical practice. Autoimmun Rev. 2016;15(8):833–842. doi:10.1016/j.autrev.2016.06.00527302209
- Ta R, Cayabyab MAS, Coloso R. Precision medicine: a call for increased pharmacogenomic education. Per Med. 2019;16(3):233–245. doi:10.2217/pme-2018-010731025601
- Kohane IS. Ten things we have to do to achieve precision medicine. Science. 2015;349(6243):37–38. doi:10.1126/science.aab132826138968
- Rohrer Vitek CR, Abul-Husn NS, Connolly JJ, et al. Healthcare provider education to support integration of pharmacogenomics in practice: the eMERGE network experience. Pharmacogenomics. 2017;18(10):1013–1025.28639489
- Zavorotnyy M, Ehrlich F, Nenadic I. Health-related Internet use and treatment adherence: a transdiagnostic comparison of outpatients with major depressive disorder and schizophrenia. Psych J. 2020. doi:10.1002/pchj.355
- Arandjelovic K, Eyre HA, Lenze E, Singh AB, Berk M, Bousman C. The role of depression pharmacogenetic decision support tools in shared decision making. J Neural Transm. 2019;126(1):87–94. doi:10.1007/s00702-017-1806-829082439
- Hillyer GC, Schmitt KM, Reyes A, et al. Community education to enhance the more equitable use of precision medicine in Northern Manhattan. J Genet Couns. 2020;29(2):247–258. doi:10.1002/jgc4.124432157769
- Sandstrom A, Sahiti Q, Pavlova B, Uher R. Offspring of parents with schizophrenia, bipolar disorder, and depression. Psychiatr Genet. 2019;29(5):160–169. doi:10.1097/YPG.000000000000024031464997
- Oliver D, Radua J, Reichenberg A, Uher R, Fusar-Poli P. Psychosis polyrisk score (PPS) for the detection of individuals at-risk and the prediction of their outcomes. Front Psychiatry. 2019;10:174. doi:10.3389/fpsyt.2019.0017431057431
- Iorfino F, Scott EM, Carpenter JS, et al. Clinical stage transitions in persons aged 12 to 25 years presenting to early intervention mental health services with anxiety, mood, and psychotic disorders. JAMA Psychiatry. 2019;76(11):1167. doi:10.1001/jamapsychiatry.2019.2360
- Scott J, Hidalgo-Mazzei D, Strawbridge R, et al. Prospective cohort study of early biosignatures of response to lithium in bipolar-I-disorders: overview of the H2020-funded R-LiNK initiative. Int J Bipolar Disord. 2019;7(1):20. doi:10.1186/s40345-019-0156-x31552554
- Manchia M, Squassina A, Pisanu C, et al. Investigating the relationship between melatonin levels, melatonin system, microbiota composition and bipolar disorder psychopathology across the different phases of the disease. Int J Bipolar Disord. 2019;7(1):27. doi:10.1186/s40345-019-0163-y31814040