?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The systemic bioavailability of free fatty acid (FFA) forms of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) compared with ethyl ester (EE) forms is dependent on the presence of intestinal lipases and is highest during consumption of high-fat meals. Given that patients with cardiovascular disease are advised to reduce dietary fat intake, potentially lowering the bioavailability and therapeutic benefit, the hypothesis that FFA forms provide for higher bioavailability compared with EE forms under low-fat diet conditions was tested where the pharmacokinetics of the FFA form (Epanova™) were compared with those of an ethyl ester form (Lovaza®) following repeat dosing. Fifty-two healthy male and female subjects were equally allocated to one of two open-label, parallel-group cohorts. Following a Therapeutic Lifestyle Changes diet for a minimum of 7 days, blood samples were drawn for endogenous values for EPA and DHA over a 24-hour period. Subjects were then administered 4 × 1 g capsules of either Epanova (OM3 FFA) or Lovaza (OM3 EE) once daily for 14 days, following which serial blood samples were drawn over a 24-hour period to characterize the bioavailability of EPA and DHA from the respective formulations. In addition, changes from baseline in lipid profile were explored. Systemic bioavailability, as measured by area under the curve from time zero to 24 hours (AUC0-τ) and the maximum measured plasma concentrations during the 0–24 hour dosing interval (Cmax,ss) of unadjusted total plasma EPA + DHA were approximately 3-fold and 3.9-fold higher, respectively, for Epanova relative to Lovaza. Following baseline adjustment, the magnitude of difference in bioavailability was approximately 5.8-fold and 6.5-fold higher in AUC0-τ and Cmax, respectively, for Epanova relative to Lovaza. Serum triglycerides were reduced by a significantly greater extent (P = 0.013) for Epanova relative to Lovaza (21% versus 8%). The bioavailability of the FFA forms of EPA and DHA in Epanova are significantly greater than the bioavailability from the EE forms present in Lovaza under low-fat dietary conditions normally recommended for patients with cardiovascular disease. This increased bioavailability may lead to improved triglyceride-lowering in patients with hypertriglyceridemia.
Introduction
Hypertriglyceridemia is increasing in the US and throughout the world with an estimated prevalence of four million Americans meeting the definition of severe hypertriglyceridemia (≥500 mg/dL).Citation1 The risk of pancreatitis is clinically significant in severe hypertriglyceridemia, and actions to prevent acute pancreatitis are warranted.Citation2 Clinical guidelines for management of severe hypertriglyceridemia recommend that the patient be admitted to hospital, if symptomatic, to secure a careful follow-up of the patient’s triglyceride values. Restriction of overall energy and energy derived from fat (10%–15% recommended) as well as alcohol abstinence are encouraged. Therapeutic options include fibrate therapy, prescription omega-3 fatty acids, and nicotinic acid.Citation3 Challenging aspects of current omega-3 therapy for this indication include the dose required (4 g/day), and that optimal absorption of an ethyl ester formulation requires the medication be taken with a high-fat meal, which is contraindicated in patients with severe hypertriglyceridemia due to impaired lipoprotein lipase activity and the resulting postprandial hypertriglyceridemia.Citation4
In order for omega-3 fatty acids to be absorbed when provided as ethyl esters (OM3 EE), the ethyl ester bond must undergo hydrolysis by pancreatic lipase enzymes to be converted into free fatty acids for intestinal absorption. In contrast with OM3 EE forms that require this digestive step, the free fatty acid forms of omega-3 are not dependent on pancreatic enzyme activityCitation5–Citation12 and are readily absorbed, so have improved bioavailability that is less dependent on meal fat content.Citation13 A novel free fatty acid formulation of omega-3 (OM3 FFA, Epanova™, Omthera Pharmaceuticals Inc, Princeton, NJ, USA) was developed to maximize the bioavailability of the omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic (DHA), in patients with hypertriglyceridemia who are advised to restrict fat intake.Citation3 A previous study (ECLIPSE I [Epanova® Compared to Lovaza® In a Pharmacokinetic, Single-dose, Evaluation])Citation4 compared bioavailability only after a single dose; the present study (ECLIPSE II) was designed to characterize how the OM3 FFA and OM3 EE formulations would compare in terms of plasma levels of EPA and DHA at steady state over a 2-week period under low-fat dietary intake conditions. The hypothesis being tested was that the EPA and DHA delivered in the OM3 FFA product (Epanova) would produce higher blood levels of EPA, DHA, and EPA + DHA than those from the comparator OM3 EE product (Lovaza® GlaxoSmithKline, Research Triangle Park, NC, USA), which would provide evidence to support greater EPA and DHA bioavailability from the free fatty acid formulation.
Materials and methods
Study design and procedures
This was an open-label, single-site investigation (Celerion Inc, Tempe, AZ, USA) to compare the relative oral bioavailability of unadjusted and baseline-adjusted total EPA, total DHA, and total EPA + DHA following repeated dose administration of Epanova compared with repeated dose administration of Lovaza. Additionally, comparisons of changes in lipids between Epanova and Lovaza, and their relationship to plasma EPA + DHA, were evaluated in an exploratory manner. Another objective of this study was to assess the effects of repeated-dose Epanova on the pharmacokinetics and pharmacodynamics of a single 25 mg dose of warfarin, the results of which will be reported in a separate publication.
Prior to the first procedure conducted on any subject, the ECLIPSE II protocol underwent review by the Celerion Institutional Review Board in compliance with US Code of Federal Regulations (21 CFR 56) and International Conference on Harmonization guidelines, (www.ich.org; ICHE6 Good Clinical Practice), and a signed informed consent form was obtained from all subjects, who were informed of their right to withdraw from the study at any time. The study was conducted according to Good Clinical Practice and in accordance with the ethical requirements referred to in the European Union directive 2001/20/EC and the principles enunciated in the Declaration of Helsinki (Seoul, 2008).
Within 28 days prior to receiving the first dose of investigational product, medical history and demographic data (including name, sex, age, race, and tobacco use) were recorded. Each of the 52 subjects enrolled received a physical examination, including complete vital signs and a 12-lead electrocardiogram. Laboratory screening tests included hematology, coagulation, serology, urinalysis, and clinical chemistry tests, which included a lipid panel for total cholesterol, low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), and triglycerides.
The study employed an open-label, two-cohort, parallel design. In both cohorts (n = 26 per cohort), subjects received either 4 g (4 × 1 g) of Epanova or 4 g (4 × 1 g) of Lovaza once daily for 14 consecutive days. In terms of free fatty acid composition per 1 g capsule, the Epanova formulation was assayed as containing 550 mg EPA and 200 mg DHA, whereas the Lovaza was assayed to contain 465 mg EPA and 375 mg DHA. The ECLIPSE II study design is presented in .
Subjects in both cohorts were maintained on a low-fat diet throughout the entire study (from check-in [9 days prior to the first dose of Epanova or Lovaza] until release from confinement) consistent with the recommendations of the Therapeutic Lifestyle Changes (TLC, National Cholesterol Education Program [NCEP] Adult Treatment Panel III) diet.Citation3 They were also educated on, and requested to maintain, a constant diet with respect to vitamin K intake through study completion (vitamin K instruction started 2 weeks prior to the first dose). Subjects were confined in the clinical research unit from the evening before the first dose through to the final blood draw for pharmacokinetic and/or pharmacodynamic assessment and any end of study procedures. All food consumed after check-in was prepared by study staff under the supervision of a registered dietitian to ensure consistency with the TLC diet recommendations. The subjects received the same breakfast on all days where a breakfast was served; lunch, dinner, and snacks differed each day, but all meals were consistent between cohorts. Beginning approximately 30 minutes prior to each dose, a breakfast containing less than 10% of energy as fat was consumed daily. The breakfast consisted of one cup of oatmeal, one cup of fat-free milk, one quarter of a cup of raisins, one cup of honeydew melon, and one cup of calcium-fortified orange juice. Subjects were required to fast for a minimum of 10 hours overnight prior to the breakfast and continue to fast for at least 4 hours thereafter. The proportion of each consumed meal was recorded in the subject’s case report form.
Inclusion/exclusion criteria
Non-tobacco-using males and/or females aged 18–55 years and with a body mass index of 18–29.9 kg/m2 were allowed to participate if they were considered medically healthy; defined as having no clinically significant abnormalities in terms of clinical chemistry laboratory values, electrocardiography, and physical examination results. Female patients assigned to the Epanova cohort were to be of non-childbearing potential due to the exposure to warfarin in this cohort. Non-childbearing potentia was defined as: having undergone a sterilization procedure at least 6 months prior to check-in, ie, basal tubal ligation, hysterectomy, hysterectomy with unilateral or bilateral oophorectomy, bilateral oophorectomy; or having been postmenopausal for at least 24 consecutive months prior to check-in, with a screening follicle-stimulating hormone level >40 mIU/dL. Female subjects assigned to the Lovaza cohort could be of childbearing potential, but had to remain sexually inactive or be using an appropriate method of contraception for the entire duration of the study. In addition to a willingness to comply with the prescribed TLC diet, subjects were not to have consumed fish oil or other EPA and/or DHA containing supplements within 2 months prior to study start.
Compliance and exposure
Epanova 1 g capsules containing a mixture of polyunsaturated free fatty acids were provided by Omthera Pharmaceuticals Inc. Lovaza 1 g capsules were sourced from a pharmacy wholesaler. All expiry dates were confirmed to be within the appropriate shelf-life throughout the entire study period. All investigational products were administered under the supervision of the principal investigator and clinical research staff to ensure compliance. All doses were administered with 240 mL of water, following which clinical staff performed hand and mouth checks of subjects to ensure the dose was completely swallowed. Subjects remained ambulatory or seated upright for the first 4 hours following dosing and were to avoid unusual, unaccustomed, or strenuous physical activity (eg, weightlifting, running, bicycling) from prestudy (screening) through study completion.
Pharmacokinetic blood samples
For both the Epanova and Lovaza cohorts, samples (n = 7) for analysis of total EPA and DHA in plasma were collected for 24-hour baseline assessment prior to the first dose. In addition, fasting predose trough samples were drawn prior to the 11th, 12th, 13th, and 14th doses for assessment of steady-state, as well as 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, and 24 hours following the 14th dose to determine plasma concentrations of total EPA, total DHA, and total EPA + DHA. Lipoprotein lipid parameters were collected at screening, check-in, prior to the initial dose, and at the end of the dosing period or prior to release from the clinic or upon early withdrawal.
For both cohorts, the blood sample for total EPA and DHA analysis was drawn into 4 mL chilled evacuated tubes containing K2EDTA and stored at room temperature for up to 60 minutes until centrifugation. Plasma aliquots were placed in storage at −80°C within 60 minutes of collection. Sample analysis for total EPA and total DHA in human plasma was performed at Cetero Research (Toronto, ON, Canada). Briefly, the liquid chromatography-tandem mass spectrometry method employed deuterated forms of EPA and DHA as internal standards with linear ranges of 1–200 μg/mL and 2–400 μg/mL for EPA and DHA, respectively. Percent coefficients of variation and accuracy for all calibration and quality control standards were <5% and >95% for both total EPA and total DHA.
The biochemical analysis of serum lipids was performed using the photometric measuring unit of a Cobas® 6000 analyzer (Roche Diagnostics Ltd Indianapolis, IN, USA). Serum lipoprotein lipid parameters reported included triglycerides, LDL-c, HDL-c, and non-HDL-c. Non-HDL-c was calculated as the difference between measured concentrations of total and HDL-c. LDL-c was calculated as non-HDL-c minus one fifth of the measured triglyceride concentration.
Pharmacokinetic analysis
Total EPA and DHA
Using noncompartmental pharmacokinetic analysis of the actual sample collection time, parameters were derived for the unadjusted and baseline-adjusted plasma total EPA, total DHA, and total EPA + DHA concentration-time data using WinNonlin® version 5.2 (Pharsight Corp, Mountainview, CA, USA), and SAS® version 9.1.3 (SAS® Drug Development Platform, version 3.4; SAS Institute Inc., Cary, NC, USA), as appropriate. Pharmacokinetic parameters calculated are presented in .
Table 1 Pharmacokinetic parameters calculated for baseline-adjusted total EPA + DHA, total EPA and total DHA following repeated dosing
Since the human body is capable of converting dietary α-linolenic acid to EPA and DHA, albeit in limited quantities, such that these latter two fatty acids might be present endogenously in measurable quantities in the subjects in this study, unadjusted plasma total EPA and total DHA concentrations that were below the limit of bioanalytical quantitation were set to half of the lower limit of quantitation. Molar ratios were then used to calculate unadjusted total EPA + DHA concentrations in SAS® version 9.1.3 (SAS® Drug Development Platform, version 3.4). Total EPA + DHA concentration was calculated by the following equation and expressed in nmol/mL:
where molecular weights (MW) of 302.451 g/mol and 328.48828 g/mol were applied for EPA and DHA, respectively. For each subject, baseline adjustment was performed by subtracting the mean predose baseline (mean of seven predose plasma concentrations) prior to calculation of the pharmacokinetic parameters. The adjustment was subject-specific. All negative values were set to zero.
Statistical analysis
The relative systemic exposure to total EPA, total DHA, and total EPA + DHA following repeat-dose administration of Epanova versus Lovaza was assessed by analysis of variance on the natural log-transformed pharmacokinetic parameters AUC0-τ and Cmax,ss of baseline-adjusted (primary analysis) and unadjusted (secondary analysis) values.
The results were then used for the calculation of geometric least-squares means and the differences between geometric least-squares means of the natural log-transformed pharmacokinetic parameters following repeat-dose administration of Epanova versus repeat-dose administration of Lovaza. The 90% confidence intervals for the geometric least-squares means ratios of the back-transformed pharmacokinetic parameters AUC0-τ and Cmax,ss for Epanova versus Lovaza were presented. The 90% confidence intervals from the primary analysis on baseline-adjusted values were expected to fall entirely above the 80%–125% range.
Analyses of changes in serum lipoprotein parameters were performed for exploratory purposes. All analyses of lipid parameters were performed using SAS® version 9.3. Percent change from baseline in lipid parameter levels after 2 weeks of dosing was summarized for each treatment cohort using all subjects. For each treatment cohort, baseline lipid levels were defined as the average of the values from 7 days prior to the day 1 dose and the day 1 predose value. For each lipid parameter, comparisons of percent change from baseline in lipid levels between the two treatment cohorts were performed using analysis of covariance with treatment cohort as a factor and baseline lipid value as a covariate in the model. Unadjusted P-values from the equal slope analysis of covariance model were calculated for testing the difference in geometric least-squares means between treatment cohorts.
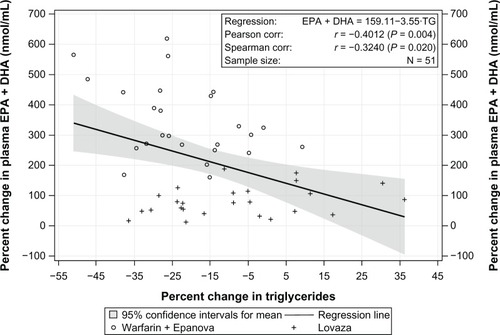
Additional exploratory analyses examining the relationship between the percent change in triglyceride levels and percent change in unadjusted plasma total EPA + DHA levels were performed for all subjects combined using the Pearson product-moment correlation coefficient (r). The Spearman rank-order correlation coefficient (rs) was used to evaluate the robustness of, or replace, Pearson’s r, when assumptions for application of parametric methods were questionable. Baseline total EPA + DHA levels were defined as stated above for pharmacokinetic parameter calculations. End-of-treatment total EPA + DHA levels were defined as the average of three predose values from the 11th, 12th, and 13th day of dosing for both cohorts. For these exploratory analyses of lipids, two-sided P-values < 0.05 were considered to be statistically significant. No adjustment for multiplicity was applied.
Results
Study population
Subject demographics are presented in . Fifty-two subjects entered the study and were assigned to one of the two study cohorts. Fifty-one subjects completed the study, with one subject having withdrawn for personal reasons following the fifth dose of Epanova. Baseline DHA levels of the women in both cohorts in this study were comparable (45–50 μg/mL [Epanova cohort] and 47–53 μg/mL [Lovaza cohort]). The average daily energy intake during the study was approximately 2,410 kcal, with approximately 16.6% (approximately 399 kcal) coming from protein, 61.7% (about 1,490 kcal) coming from carbohydrates, and 21.7% (about 523 kcal) coming from fat.
Table 2 Subject demographics and disposition
Pharmacokinetic and statistics
Mean unadjusted plasma total EPA + DHA concentrations were similar prior to the first dose and markedly greater following repeat dosing with Epanova than with Lovaza from day 11 through to 24 hours following day 14 dosing ().
Figure 2 Mean unadjusted plasma total EPA + DHA concentrations.
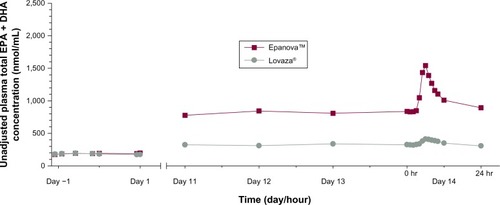
After 7 days following a TLC diet, subjects in both cohorts exhibited relatively similar baseline concentrations of total EPA + DHA () with arithmetic mean concentrations ranging from 175–197 nmol/mL and 172–193 nmol/mL for Epanova and Lovaza, respectively, over a 24-hour interval. Following single daily doses of either Epanova or Lovaza for 14 days, the unadjusted total EPA + DHA Cavg,ss in the Epanova cohort was approximately three-fold that of the Lovaza cohort, suggesting a substantially higher relative bio-availability of Epanova. When EPA, DHA, and EPA + DHA were corrected for endogenous EPA and DHA from dietary sources, the magnitude of the difference was even more apparent, with the greatest difference being evident in plasma EPA concentrations (–).
Figure 3 Mean (SD) baseline-adjusted plasma total EPA + DHA concentrations.
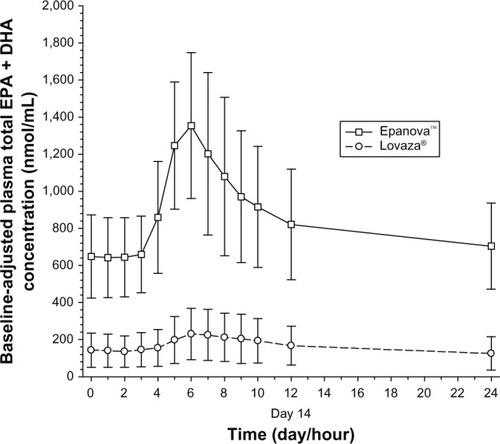
Figure 4 Mean (SD) baseline-adjusted plasma total EPA concentrations.
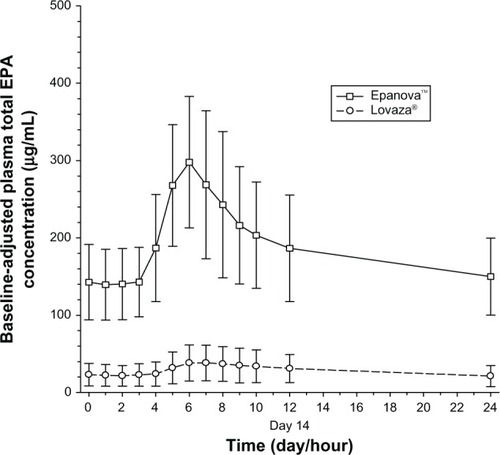
Figure 5 Mean (SD) baseline-adjusted plasma total DHA concentrations.
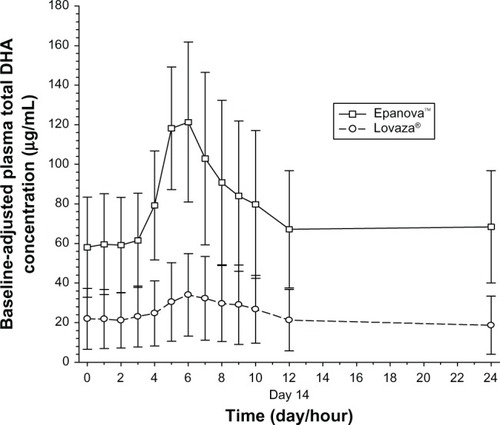
Based on the coefficient of variation for the geometric mean pharmacokinetic parameters, when adjusting for baseline EPA, DHA, and EPA + DHA, the variability of Epanova was approximately half of that observed for Lovaza (), suggesting that Epanova provides more consistent delivery of omega-3 fatty acids under low-fat dietary conditions. In terms of relative bioavailability (as measured by the geometric least-squares means for AUC0-τ), Epanova resulted in approximately 733%, 309%, and 576% of the values for total EPA, DHA and EPA + DHA, respectively, compared with Lovaza (). The magnitude of the difference can be better appreciated by considering the 90% confidence intervals of the geometric least-squares means ratios for the main measures of oral bioavailability (ie, AUC0-τ and Cmax,ss), where, in all cases, the lower tail exceeded 200% and the upper tail ranged from 390% to greater than 1,000% (for total EPA Cmax,ss). Consistent with the unadjusted Cavg,ss, adjustment for baseline endogenous omega-3 resulted in an approximate six-fold difference in total EPA + DHA Cavg,ss (), most of which was due to the substantial difference (seven-fold) in total EPA between the two treatments ().
Figure 6 Mean baseline-adjusted plasma total EPA + DHA − Cavg.ss
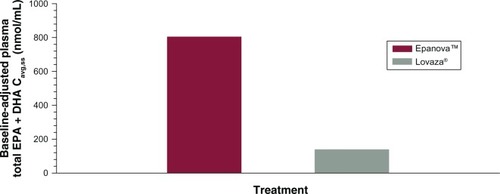
Figure 7 Mean baseline-adjusted plasma total EPA + DHA − Cavg,ss.
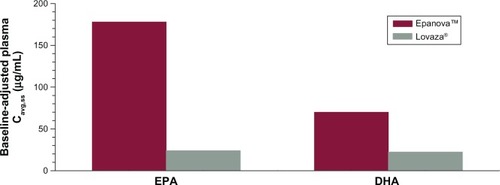
Table 3 Summary of the baseline-adjusted pharmacokinetic parameters of total EPA + DHA, total EPA and total DHA in plasma
Table 4 Summary of the statistical comparisons of the pharmacokinetic parameters of baseline-adjusted total EPA + DHA, total EPA, and total DHA in plasma
Lipoprotein lipids
Baseline and percent change from baseline in lipid values are presented in . Mean baseline triglycerides were higher in the Epanova cohort (185.8 mg/dL) relative to the Lovaza cohort (145.3 mg/dL). After 2 weeks of dosing, there was a significantly larger percent reduction in triglycerides observed in the Epanova cohort compared with the Lovaza cohort (geometric least-squares means 21% versus 8%, P = 0.013). There were no statistically significant differences observed in percent change from baseline in HDL-c, LDL-c, or non-HDL-c between the two cohorts.
Table 5 Summary of the lipid parameters at baseline (mg/dL) and percent change from baseline after 2 weeks of dosing
Correlations between percent change in triglyceride levels and percent change in unadjusted plasma total EPA + DHA over combined cohorts are presented in . The data exhibited a potential departure from Pearson’s r assumptions; however, the direction and magnitude of the trend was supported by Spearman’s r. There was a statistically significant inverse relationship observed between percent change in triglycerides and percent change in total EPA + DHA (r = −0.4012, P = 0.004; r = −0.3240, P = 0.020).
Safety and tolerability
Throughout the study, both treatments were well tolerated. One subject (Epanova cohort) experienced an increase in bilirubin and a decrease in neutrophil count, which were both considered unrelated to the study drug.
Discussion
Previous studies have demonstrated that the marine EPA and DHA in triglyceride form are more resistant to pancreatic lipase hydrolysis compared with other polyunsaturated fatty acids.Citation5–Citation7 Further, OM3 EEs are up to 50 times more resistant than the natural triglyceride form to pancreatic lipase hydrolysis and require an additional hydrolysis step that utilizes carboxyl ester lipase to yield EPA and DHA free fatty acids, with ethanol as a byproduct.Citation8–Citation12 Several studies have compared human intestinal absorption of fish oil fatty acids in the form of triglycerides, ethyl esters, and free fatty acids, and found that free fatty acids have up to five times more bioavailability than the ethyl ester form as determined by the plasma AUC.Citation9,Citation11 Because Epanova is a free fatty acid formulation, it has less dependence on meal fat content and has significantly greater bioavailability than Lovaza, which contains an ethyl ester formulation.Citation4 The NCEP ATP III has recommended that patients with severe hypertriglyceridemia consume very low-fat diets, which results in less release of pancreatic lipase into the intestinal lumen, and could lead to reduced absorption of omega-3 in ethyl ester formulations.Citation3 In this study, we compared the plasma levels of total EPA + DHA after repeat-dose administration of Epanova or Lovaza under low-fat diet conditions. Although no formal assessment of dietary compliance was completed prior to the confinement, at baseline both treatment arms had similar levels of plasma total EPA + DHA. However, at steady-state after 14 days of repeat dosing, subjects receiving Epanova had substantially higher and less variable trough levels of unadjusted total EPA + DHA compared with Lovaza. These observations support the view that after repeated administration in conjunction with a low-fat diet, the absorption of OM3 EE is significantly lower than that of OM3 FFA. This is likely attributable to the reduced amount of pancreatic lipase released in response to a low-fat diet. In addition, after 14 days of repeat dosing in conjunction with a low-fat diet, the rate and extent of systemic exposure over the normal dosing interval was also significantly improved with Epanova compared with Lovaza ().
In this 2-week study, the percent increases from baseline in plasma total EPA (253%) and DHA (41%) in the Lovaza cohort were consistent with the plasma changes in previous clinical trials with Lovaza that measured steady levels of omega-3 fatty acids. In nine previous Lovaza clinical studies in patients with hypertriglyceridemia, the range of percent change for EPA was 139%–361% with a mean of 236% and for DHA the range was 10%–77% with a mean of 36%. Therefore, the healthy volunteers on a low-fat diet in this trial in the Lovaza cohort had similar changes in omega-3 levels compared with patients with hypertriglyceridemia treated with Lovaza.Citation14
Omega-3 fatty acids produce their triglyceride-lowering properties, at least in part, by acting on nuclear receptors in the liver.Citation15,Citation16 Delivery of omega-3 fatty acids to the liver is dependent on their absorption through the intestines and subsequent incorporation into chylomicron particles. Chylomicron remnants are ultimately removed from the circulation by the liver, delivering EPA and DHA into hepatic tissues in addition to those that are taken up by the liver as free fatty acids released during triglyceride hydrolysis of lipoprotein and adipose triglycerides.Citation17 In healthy subjects, plasma levels of total EPA + DHA are reliable markers of intestinal absorption and liver exposure, and higher total plasma levels of EPA and DHA are consistent with increased bioavailability, although the authors acknowledge that other methods such as tracer studies would be needed to directly demonstrate enhanced bioavailability. ECLIPSE II evaluated a 4 g/day dosage of an OM3 FFA and OM3 EE formulation. After 2 weeks of treatment, Epanova reduced triglycerides by 21%, which was significantly greater than the 8% reduction achieved with Lovaza (P = 0.013). This suggests that the increase in bioavailability associated with Epanova may lead to better triglyceride reductions when administered at equivalent dosages. This is also supported by the significant correlation (r = −0.3240, P = 0.020) between the percent change in serum triglycerides and percent change in plasma total EPA + DHA.
The study population was mostly Hispanics who had low baseline levels of omega-3 fatty acid compared with those observed in other studies. Hispanics may have reduced levels of omega-3 due to a low fish intake, and a high prevalence of fatty acid desaturase gene polymorphism, which is associated with decreased conversion of short-chain to long-chain fatty acids.Citation18 Approximately two thirds of Hispanics have the fatty acid desaturase polymorphism associated with decreased conversion of alpha-linolenic acid to EPA.Citation18,Citation19 However, fatty acid desaturase gene polymorphisms are unlikely to affect EPA and DHA absorption, and steady-state plasma levels of total EPA and DHA in the present study were similar to those observed in other studies where Epanova was used as a treatment for hypertriglyceridemia, suggesting that the present results are generalizable to other ethnic groups and to hypertriglyceridemic patients.Citation20,Citation21 A potential limitation of this study was the use of a nonrandomized, open-label design, which was necessary due to the warfarin component of the study, ie, subjects were recruited to specific cohorts based on the inclusion/exclusion criteria of the respective cohorts. However, as the main endpoints were pharmacokinetic in nature, the risk of bias is considered very low by the authors.
JELIS (the Japan Eicosapentaenoic Acid Lipid Intervention Study) demonstrated that, in Japanese subjects, 2 g of EPA/day decreased cardiovascular events by 19% and that the on-treatment E PA levels correlated with the event reduction (levels greater than 100 μg/mL were associated with a clinical benefit).Citation22 The baseline levels of EPA in this study population were approximately one ninth the on-treatment level of the JELIS study population. The steady-state levels of unadjusted total EPA of 33–35 μg/mL in the Lovaza cohort versus 145–156 μg/mL in the Epanova cohort prior to the 11th, 12th, 13th, and 14th doses suggest that in order to achieve the omega-3 levels associated with cardiovascular benefits in the JELIS trial, in a US population, either much higher doses of OM3 EE are required, or a free fatty acid formulation is needed, to maximize the potential cardiovascular benefits associated with omega-3 fatty acids.
Conclusion
Free fatty acid formulations of omega-3 fatty acids provide significantly higher plasma levels of total EPA + DHA compared with ethyl ester formulations after 14 days of repeat dosing in subjects on a low-fat diet. The higher apparent bioavailability of free fatty acid forms suggests that triglyceride-lowering efficacy with free fatty acid formulations may be higher than with ethyl ester formulations at equivalent daily dosages.
Disclosure
EO and TM are paid employees of Celerion Inc. JJ, DK, DC, and MD are paid employees of Omthera Pharmaceuticals Inc. SF reports no conflicts of interest in this work.
References
- ChristianJBBourgeoisNSnipesRLoweKAPrevalence of severe (500 to 2,000 mg/dL) hypertriglyceridemia in United States adultsAm J Cardiol201110789189721247544
- ReinerZCatapanoALDe BackerGEuropean Association for Cardiovascular Prevention and Rehabilitation. ESC Committee for Practice Guidelines (CPG) 2008–2010 and 2010–2012 Committees ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS)Eur Heart J2011321769181821712404
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III)Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final ReportCirculation20021063143342112485966
- DavidsonMHJohnsonJRooneyMWKyleMLKlingDFA novel omega-3 free fatty acid formulation has dramatically improved bioavailability during a low-fat diet compared with omega-3-acid ethyl esters: The ECLIPSE (Epanova compared to Lovaza in a pharmacokinetic single-dose evaluation) studyJ Clin Lipidol2012657358423312053
- IkedaISasakiEYasunamiHDigestion and lymphatic transport of eicosapentaenoic and docosahexaenoic acids given in the form of triacylglycerol, free acid and ethyl ester in ratsBiochim Biophys Acta199512592973048541338
- SmallDMThe effects of glyceride structure on absorption and metabolismAnnu Rev Nutr1991114134341892708
- El BoustaniSColetteCMonnierLDescompsBCrastes de PauletAMendyFEnteral absorption in man of eicosapentaenoic acid in different chemical formsLipids1987227117142828810
- HazraATripathiSKGhoshAPharmacology and therapeutic potential of the n-3 polyunsaturated fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in fish oilsIndian J Pharmacol199931247264
- LawsonLDHughesBGHuman absorption of fish oil fatty acids as triacylglycerols, free acids, or ethyl estersBiochem Biophys Res Commun19881523283353358766
- LawsonLDHughesBGAbsorption of eicosapentaenoic acid and docosahexaenoic acid from fish oil triacylglycerols or fish oil ethyl esters co-ingested with a high-fat mealBiochem Biophys Res Commun19881569609632847723
- BeckermannBBenekeMSeitzIComparative bioavailability of eicosapentaenoic acid and docosahexaenoic acid from triglycerides, free fatty acids and ethyl esters in volunteersArzneimittelforschung1990407007042144420
- HansenJBGrimsgaardSNilsenHNordøyABønaaKHEffects of highly purified eicosapentaenoic acid and docosahexaenoic acid on fatty acid absorption, incorporation into serum phospholipids and postprandial triglyceridemiaLipids1998331311389507234
- GuraKStrijboschRArnoldSMcPhersonCPuderMThe role of an intravenous fat emulsion composed of fish oil in a parenteral nutrition-dependent patient with hypertriglyceridemiaNutr Clin Pract20072266467218042955
- Australian Government: Department of Health and AgeingTherapeutic Goods Administration. Australian Public Assessment Report (AusPAR) for omega-3-acid ethyl esters 902010 Available from: http://www.tga.gov.au/pdf/auspar/auspar-omacor.pdfAccessed March 22, 2013
- ChanDCWattsGFMoriTABarrettPHRedgraveTGBeilinLJRandomized controlled trial of the effect of n-3 fatty acid supplementation on the metabolism of apolipoprotein B-100 and chylomicron remnants in men with visceral obesityAm J Clin Nutr20037730030712540386
- YahagiNShimanoHHastyAHA crucial role of sterol regulatory element-binding protein-1 in the regulation of lipogenic gene expression by polyunsaturated fatty acidsJ Biol Chem1999274358403584410585468
- DavidsonMHMechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acidsAm J Cardiol20069827i33i
- SteffenBTSteffenLMTracyREthnicity, plasma phospholipid fatty acid composition and inflammatory/endothelial activation bio-markers in the Multi-Ethnic Study of Atherosclerosis (MESA)Eur J Clin Nutr20126660060522215136
- LemaitreRNTanakaTTangWGenetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE ConsortiumPLoS Genet20117e100219321829377
- KasteleinJJPMakiKCSusekovADose response of a novel free-fatty acid formulation of omega-3 for the management of dyslipidemia in patients with severe hypertriglyceridemia EpanoVa fOr Lowering Very High TriglycErides (The EVOLVE Trial)Abstract APS20204 presented at the American Heart Association Scientific SessionsLos Angeles, CANovember 6, 2012
- MakiKCOrloffDGNichollsSJA highly bioavailable omega-3 free fatty acid formulation improves the cardiovascular risk profile in high-risk statin-treated patients with residual hypertriglyceridemia (the ESPRIT Trial)Clin Ther2013 2013 Aug 30. pii:S0149-2918(13)00807-210.1016/j.clinthera.2013.07.420 [Epub ahead of print]
- YokoyamaMOrigasaHMatsuzakiMEffects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysisLancet20073691090109817398308