Abstract
Objective:
To report side effects seen in a clinical cohort of patients aged >65 years with rheumatoid arthritis (RA) treated with the tumor necrosis factor-α TNF-α blocker etanercept and to compare the side effects rate with patients aged ≤65 years.
Methods:
All patients with RA that started etanercept and who were referred to our rheumatology unit from November 2005 to March 2009 were included in this study and prospectively followed to collect side effects related to therapy.
Results:
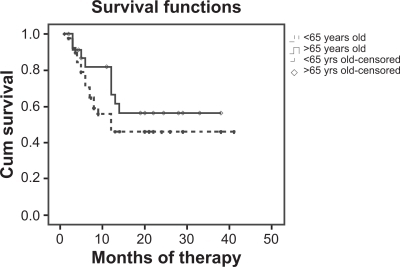
One hundred three patients were enrolled: 41 (37 females, 4 males) aged >65 years and 62 (40 females, 22 males) aged <65 years. In the patients aged >65 years, the safety profile (defined as rate of side effects) of etanercept was similar to that in patients aged ≤65 years (P > 0.05) and the survival curves between the groups were similar (P > 0.05).
Conclusions:
In our three-year experience, the anti-TNFα agent etanercept has been well tolerated and safe in elderly patients. The risk of side effects in these patients was no greater than in subjects aged ≤65 years. However, such inhibitors are associated with various and numerous side effects and elderly patients with RA should be carefully monitored to limit the risk of side effects during anti-TNFα therapy as much as possible.
Introduction
Rheumatoid arthritis (RA) is an erosive disease that predominantly involves the synovial tissue of joints and is characterized by variable disease onset and clinical course, potentially resulting in structural joint destruction and subsequent permanent disability.Citation1
Epidemiological studies have shown that RA is most prevalent in those who are aged 65 years or older.Citation2,Citation3 The disease is commonly diagnosed between the ages of 30 and 50 years, but up to 33% of subjects may be diagnosed after the age of 60 years. Despite the high prevalence of RA in the elderly, and the likelihood that older patients have adverse reactions to medications as a result of age-related changes in drug metabolism and the presence of comorbid illnesses requiring concomitant medications, patients older than 65 years tend to be inadequately represented in RA clinical trials.Citation5,Citation6 Also, the tolerability of and response to therapy in these patients as a group has not been studied in detail. In one retrospective analysis of disease-modifying antirheumatic drugs (DMARDs) including gold, D-penicillamine, azathioprine, and methotrexate (MTX), a substantially higher withdrawal rate due to toxicity was observed in patients older than 65 years compared to those younger than 65 years.Citation5
Etanercept is a recombinant human fusion protein that inhibits the activity of tumor necrosis factor (TNF) and lymphotoxin-α. In clinical trials of RA, etanercept alone or in combination with MTX was well tolerated and produced significant sustained improvement in disease activity in patients with long-standing RA who failed multiple DMARDs and in patients with early aggressive disease who never received MTX.Citation6–Citation10 Etanercept is effective in inhibiting radiographic progression in patients with RA; 72% of patients with early aggressive RA who received 25 mg etanercept monotherapy had no new radiographic erosions during one year of treatment.Citation10 In these clinical trials, the response to etanercept therapy in elderly patients was not described in detail.
The aim of our study was to assess whether the use of etanercept is associated with a different rate of adverse events in elderly versus younger RA subjects and whether the drug survival curves are different in the two groups.
Materials and methods
Subjects with RA (American College of Rheumatology criteriaCitation11) that started etanercept from November 2005 to March 2009 and referring to our rheumatology unit were included in this study and prospectively followed. The etanercept dose was 50 mg subcutaneously once a week. Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids, if needed, MTX, or other previous DMARDs, if tolerated, were continued. A complete physical examination, standard laboratory tests, were assessed at baseline and either every two months thereafter. At the screening visit a chest X-ray, an electrocardiogram (EKG), a tuberculin skin test were performed and a complete hematic biometry analysis (complete blood count, C-reactive protein, erythrocyte sedimentation rate, creatinine, prostate-specific antigen total and free, aspartate aminotransferase and alanine aminotransferase, antinuclear antibodies, urinalysis). Patients aged >65 years also received an abdominal ecography, a mammography, three serial stool blood tests, and serum electrophoresis. A previous malignancy, history of cardiac failure with New York Heart Association (NYHA) class ≥3, or clinical findings suspect for malignancies at the study screening were used as exclusion criteria. Safety profile points included incidence rates of all side effects (SEs) defined as: adverse events (AE, with a temporary therapy suspension), serious AE (SAE, defined as an AE that required the definitive discontinuation of the therapy and hospitalization, malignancies, and deaths), infectious diseases (IAE, with temporary or definitive therapy suspension associated with intravenous antibiotic treatment).
Statistical analysis
Pairwise comparisons were based on the Wilcoxon matched pairs signed-ranks test. The safety profile in the two groups was estimated by survival analysis, taking any AE as the event of interest using the Kaplan–Meier method. Comparisons between the resultant curves were made by log rank test. All values of <0.05 were considered to indicate statistical significance (two-tailed test).
Results
One hundred three caucasian patients out of 254 were eligible: 41 (37 females, 4 males) aged >65 years and 62 (40 females, 22 males) aged <65 years. Mean disease onset age was 57.4 ± 1.7 years in younger and 60.4 ± 3.2 years in older patients (P > 0.05). Mean age at beginning of TNF blocker was 57.8 ± 6.9 years in younger and 68.2 ± 3.2 years in older patients (P < 0.05). The duration of the anti-TNF treatment was 29.4 ± 11.2 and 27.9 ± 3.3 months, respectively (P > 0.05). In the whole population, 35 SEs (33.98% of cases) that led to temporary/permanent withdrawal were observed (9 AEs, 20 IAEs, 6 SAEs). The rate of patients presenting SEs was 41.4% of patients aged >65 years versus 30.6% of patients aged ≤65 years (P = 0.318; ). The survival curves of these two groups were no significantly different (Mantel–Cox log rank test, P = 0.267; ).
Table 1 Number of adverse events in the groups
Discussion
Rheumatoid arthritis is a chronic illness characterized by inflammation of the synovial tissue in joints, which can lead to joint destruction. RA affects around 0.5%–1% of the population, three times as many women as men, and has a peak age of onset between the ages of 40 and 70 years. The prevalence of the disease at the age of 65 years is six times the prevalence at the age of 25 years. RA is a major cause of disability and institutionalization in an aging population.Citation12 The joint pain and swelling that are characteristic of RA often impairs mobility and the performance of activities of daily living, resulting in difficulties in self-care, diminished independence, and reduced involvement in family, work, and social roles.Citation13,Citation14 The influence on functional status is especially critical in older patients with RA, who also tend to have a higher frequency of acute onset and weight loss at presentation and are more likely than younger patients to have large joint involvement.Citation15 Some studies have suggested that RA is more aggressive in the elderly, is associated with more radiographic damage, and may result in more rapid functional decline than in younger patients.Citation16
Special issues confound the choice of therapy for elderly patients. In general, older patients are more likely to have concomitant illness including malignancies and cardiovascular disease. Additionally, they are more susceptible to serious infections and mortality from infections. Medication compliance, drug interactions, and decreased tolerability complicate the management of older patients. The older RA patient is likely to require multiple medications and have difficulties with drug compliance, and may be prone to commit dosage errors. Physiological factors in the elderly may modify pharmacokinetics, tissue responsiveness, and homeostatic mechanisms.Citation17–Citation19 These factors may contribute to differences in toxicity and efficacy among different age groups in patients with RA. We observed no consistent differences in the rates or types of adverse events between the age groups in this study, which suggests that treatment with etanercept does not increase the risk of these events in the elderly population beyond the increases inherent with advanced age and comorbidities.
Disclosures
The authors report no conflicts of interest in this work.
References
- HochbergMCPredicting the prognosis of patients with rheumatoid arthritis: is there a crystal ball?J Rheumatol199320126512678230003
- MitchellDEpidemiologyUtsingerPDZvaiflerNJErlichGERheumatoid Arthritis: Etiology, diagnosis, managementPhiladelphia, PALippincott198513331373
- RobinsonHMultidisciplinary ambulatory care for the elderly arthritis patientMoskowitzRWHaugMRArthritis and the ElderlyNew York, NYSpringer Publishing1986146149
- FleischmannRMBaumgartnerSWTindallEAResponse to etanercept (Enbrel) in elderly patients with rheumatoid arthritis: a retrospective analysis of clinical trial resultsJ Rheumatol20033069169612672185
- DahlSLSamuelsonCOWilliamsHJWardJRKargMSecond-line antirheumatic drugs in the elderly with rheumatoid arthritis: a post hoc analysis of three controlled trialsPharmacotherapy19901079842112243
- van SchaardenburgDBreedveldFCElderly-onset rheumatoid arthritisSemin Arthritis Rheum1994233673787939723
- MorelandLWBaumgartnerSWSchiffMHTreatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion proteinN Engl J Med19973371411479219699
- WeinblattMEKremerJMBankhurstADA trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexateN Engl J Med19993402532599920948
- MorelandLWSchiffMHBaumgartnerSWEtanercept therapy in rheumatoid arthritis. A randomized, controlled trialAnn Intern Med199913047848610075615
- BathonJMMartinRWFleischmannRMA comparison of etanercept and methotrexate in patients with early rheumatoid arthritisN Engl J Med20003431586159311096165
- ArnettFCEdworthySMBlochDAMcShaneDJFriesJFCooperNSThe American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritisArthritis Rheum1988313153243358796
- US Senate Special Committee on Aging, the American Association of Retired Persons, the Federal Council on the Aging, the US Administration on AgingAging America: Trends and projectionsWashington, DCUS Government Publication1991
- CohenFReeseLKaplanGRiggioRCoping with the stress of arthritisMoskowitzRVHaugMRArthritis in the ElderlyLondon, UKSaunders19864755
- Downe-WamboldtBLMelansonPMEmotions, coping, and psychological well-being in elderly people with arthritisWest J Nurs Res1995172502657778308
- van SchaardenburgDBreedveldFCElderly-onset rheumatoid arthritisSemin Arthritis Rheum1994233673787939723
- van der HeijdeDMvan RielPLvan LeeuwenMAvan ‘t HofMAvan RijswijkMHvan de PutteLBOlder versus younger onset rheumatoid arthritis: results at onset and after 2 years of a prospective follow-up study of early rheumatoid arthritisJ Rheumatol199118128512891757926
- O’CallaghanJWBrooksPMDisease-modifying agents and immunosuppressive drugs in the elderlyClin Rheum Dis1986122752892872995
- GardnerGFurstDEDisease-modifying antirheumatic drugs. Potential effects on older patientsDrugs Aging199574204378601050
- FleischmannRIqbalIRisk:benefit profile of etanercept in elderly patients with rheumatoid arthritis, ankylosing spondylitis, or psoriatic arthritisDrugs Aging200724323925417362051