Abstract
Everolimus (RAD001, Afinitor® Novartis) is the first oral inhibitor of mTOR (mammalian target of rapamycin) to reach the oncology clinic. Everolimus 10 mg daily achieves complete inhibition of its target at below the maximum tolerable dose for most patients. A phase III randomized placebo-controlled trial has examined the impact of everolimus in patients with clear cell renal cancers and progressive disease on or within 6 months of the VEGFR tyrosine kinase inhibitors sunitinib and/or sorafenib. The primary endpoint of progression-free survival was increased from median 1.9 to 4.9 months (hazard ratio 0.33, P < 0.001) and 25% were still progression-free after 10 months of everolimus therapy. There was a delay in time to decline of performance status and trends to improvement in quality of life, disease-related symptoms, and overall survival despite crossover of the majority of patients assigned to placebo. In 2009, everolimus was approved in the US and Europe as the only validated option for this indication. Toxicities are usually mild to moderate and can be managed with dose reduction or interruption if necessary. Opportunistic infections and non-infectious pneumonitis are seen as a class effect. Management of common practical management issues are discussed. Clinical trials are in progress to examine additional roles for everolimus in renal cancer, alone and in combination with other agents.
Introduction
Kidney cancer, more specifically renal cell cancer (RCC), is a significant cause of premature death and has been resistant to drug therapy until the past 5 years. There will be a projected 4600 new cases and 1600 deaths from kidney cancer in Canada in 2009,Citation1 and 8 times that number in the US.Citation2 About two-thirds of these events will occur in men, partly related to smoking incidence that increases risk substantially.Citation3 There is no proven role for population screening or prevention. An average of 14.2 years of life are lost to each person dying of kidney cancer (based on British Columbia data).Citation4
The extent of disease at the initiation of systemic therapy for advanced RCC is a major predictor of outcome, and patients can be divided into three prognostic groups – good, intermediate, or poor risk – using the Memorial Sloan-Kettering criteriaCitation5 with or without the additional predictive power of the number of involved sites.Citation6 The most common presentation of advanced kidney cancer is now during follow-up after nephrectomy: such patients are often asymptomatic and in better general health than in the past with low metastatic burden and less comorbidity. A further substantial fit group of patients will have metastases diagnosed on screening before nephrectomy. Improving survival figures are in part due to lead-time bias from earlier recognition and therapy of metastases with the advent of more options. For example the median survival of the interferon alfa randomized trial arms in two pivotal studies was 9 months in 1999,Citation7 compared with 20 months in 2008 allowing for crossover to a more active agent.Citation8 Patients are therefore often candidates for multiple sequential lines of systemic therapy that are the focus of this review. Smaller cohorts of patients present with symptoms from metastases, paraneoplastic syndromes, or locally advanced tumors in the kidney, and may require a more palliative approach such as radiation as well as attempted systemic therapies.
There have been four eras of systemic therapy for advanced renal cancer: hormone therapy, chemotherapy, immunotherapy,Citation9 and currently targeted therapy.Citation10 One published randomized controlled trial has used a placebo control demonstrating that the natural history of RCC can sometimes be indolent, with 6.6% spontaneous partial remissions.Citation11 Hormone therapy has been widely used as control therapyCitation7 even recently.Citation12 RCC is strikingly resistant to chemotherapy despite most new agents being tested. In the 1980s, immunotherapy became the dominant drug therapy, based on occasional durable remissions in highly selected patients treated with high dose interleukin-2,Citation13 and small survival gains with the more generally applicable agent interferon alfa.Citation7,Citation14 Interferon became the safe and common standard of care for metastatic RCC;Citation5 however most patients did not benefit and toxicity was substantial, setting the stage for the present era of targeted drugs.Citation9
The first major recent advance was the recognition that renal cell cancer (RCC) includes several diagnostic entities that differ at the molecular level. The most common type of RCC is clear cell (ccRCC), about 75% of kidney cancers.Citation15 Families with the rare von Hippel Lindau syndrome develop vascular tumors including ccRCC, and studies indentified underlying loss of a recessive tumor suppressor gene now known as the VHL gene. Subsequently it was shown that sporadic ccRCC has biallelic deletion, mutation, or methylation of the VHL gene, and this feature has a major role in the pathogenesis of the disease.Citation16 Loss of the normal VHL gene product in ccRCC results in constitutional high expression of the hypoxia response gene HIF-1α (hypoxia-inducible factor 1 alpha) and its many downstream products including angiogenic growth factors like vascular endothelial growth factor (VEGF). Recently introduced drugs that target the VEGF pathway include bevacizumab (Avastin®; Genentech) with or without interferon alfa, and especially the VEGFR TKIs (VEGF receptor small molecule tyrosine kinase inhibitors) sunitinib (Sutent®; Pfizer), sorafenib (Nexavar®; Bayer), and others in various stages of development. Of these agents, only sunitinib has resulted in unequivocally improved overall survival for good and intermediate risk patients compared to first-line interferon alfa,Citation17 and is now approved and widely used for first-line therapy of advanced ccRCC. Sorafenib is the best documented agent for second-line therapy after interferon alfa.Citation18 Therefore there are increasing numbers of good performance status patients in need of further active treatment of metastatic RCC after disease progression on or soon after sunitinib and/or sorafenib. Everolimus (RAD001, Afinitor®; Novartis) has emerged as the leader in that setting, and the first to receive approval in the USA (March 2009) and Europe (August 2009) for use after failure of VEGFR TKI therapy.Citation19 Poor prognostic risk patients treated with the intravenous mTOR inhibitor temsirolimus (CCI-779, Torisel® Wyeth) have improved overall survival,Citation20 but represent a more palliative clinical situation than good-intermediate risk patients suitable for multiple lines of therapy.
Discovery and development of rapamycin and rapalogs
Rapamycin (sirolimus) is a macrolide antibiotic named for the remote Pacific island of Rapa Nui (formerly Easter Island), the origin of a soil sample obtained in 1965 () which yielded a new streptomycete, Streptomyces hygroscopicus, from which rapamycin was later derived at Ayerst Research Labs in Montreal.Citation21 This discovery deserves major recognition because extended investigations of rapamycin and its derivatives successively demonstrated a unique combination of antifungal, immunosuppressive,Citation22 antineoplastic,Citation23 and even anti-aging properties.Citation24 In 1999, rapamycin was approved in the USA for immunosuppression after organ transplantation and marketed as Rapamune® (Wyeth-Ayerst). Its target is a highly conserved kinase known in mammals as mTOR (mammalian target of rapamycin). Rapamycin has no other target so the inhibition of mTOR is one of the most specific targeted drug actions known.
Figure 1 A plaque commemorating the discovery of rapamycin (sirolimus) on Rapa Nui (Easter Island), near Rano Kau. The plaque is written in Brazilian Portuguese, and reads: In this location were obtained, in January 1965, soil samples that led to the discovery of rapamycin, a substance that inaugurated a new era for organ transplant patients. An homage from the Brazilian investigators, November 2000. Photo credit: Anypodetos, Wikipedia Commons.

The original compound rapamycin was too insoluble and unstable for parenteral use as an antineoplastic agent.Citation25 Subsequently rapamycin ester analogs have been developed, known as rapamycins,Citation25 or rapalogs.Citation26 Current rapalogs include temsirolimus, everolimus (RAD001, Afinitor®), and agents still in development such as ridaforolimus (deforolimus, AP23473 Ariad®; Ariad Pharmaceuticals). These rapalogs have similar and highly selective action discussed below such that at least some overlap of clinical efficacy, toxicity, and predictors of benefit might be expected.
Mechanism of action of rapalogs
mTOR has a role in cell growth, proliferation, cell survival, and angiogenesis. Unravelling mTOR function is still incomplete but much progress has been made and a number of excellent reviews are available.Citation23,Citation25–Citation30 Originally identified from rapamycin-resistant yeast strains, TOR and its homologues are highly conserved in evolution, and centrally located in molecular pathways involved in cell proliferative responses to external factors including insulin-like growth factors and availability of oxygen and nutrients. mTOR is a large single chain polypeptide with 2549 amino acids and at least 5 binding domains ().
Figure 2 mTORC1, mammalian target of rapamycin complex 1, consists of mTOR, raptor (regulatory protein of mTOR), and mLST8 (mammalian lethal with SEC 13). An additional component, PRAS40, has been omitted (see text). mTOR domainsCitation27 are shown in italics. The serine/threonine kinase catalytic activity is inhibited by the binding to FRB (FKBP12-rapamycin binding protein) of the rapalog-FKBP12 complex (rapamycin analogs complexed to the cytophilin FK-506 binding protein 12 kD).
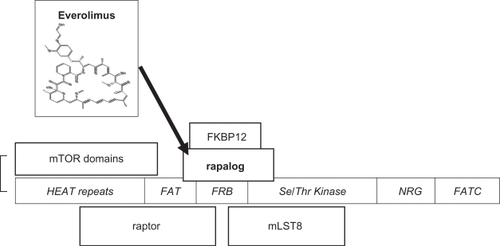
mTOR forms two different multi-protein complexes called mTORC1 and mTORC2. mTORC1 is formed with raptor (regulatory protein of mTOR) and mLST8, and upon activation via the upstream PI3K-Akt pathway, has Ser/Thr kinase activity for its main downstream targets S6K1 (ribosomal p70S6 kinase 1), and 4E-BP1 (4E binding protein 1) resulting in disinhibition of the eukaryotic initiation factor 4E (eIF4E) and translation of multiple cell cycle regulating proteins (). Recently an additional component of mTORC1, PRAS40 (proline rich Akt substrate 40 kDa), has been described that may allow Akt to directly inhibit TORC1 in energy-deprived conditions.Citation23 mTORC1, but not mTORC2, is directly inhibited by rapamycin complexed to a cytophilin FKBP12 (originally identified as the binding protein for the immunosuppressive agent tacrolimus, FK-506). Rapamycin binds with high affinity (Kd ∼0.3 nM) and specificity by binding into a hydrophobic cleft between mTOR and FKBP12.Citation31
Figure 3 Major mTOR pathways.
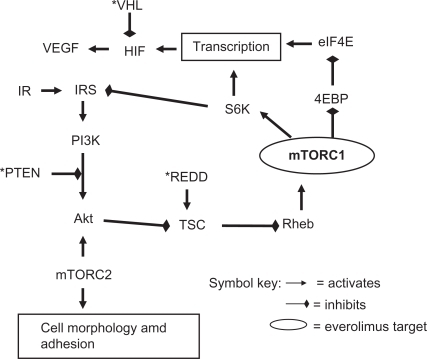
mTORC1 is located on the PI3K-Akt-mTOR-p70S6K pathway, upregulated in many malignancies, that increases transcription of protein RNAs including HIF-1 (hypoxia inducible factor 1). HIF-1 lies at the crossroads for agents that target the mTOR pathway and those that target the VEGF pathway discussed earlier, thereby providing the basis for consecutive or concurrent use of agents that target the two pathways. It should however be noted that the site of action for VEGF targeted agents is thought to be vascular endothelial cells, whereas mTOR inhibitors act directly on tumor cells, as well as support tissues such as vascular endothelium where there are differences from VEGFR inhibitors.Citation32 The combined pathway actions of everolimus provide a rationale for radiosensitization especially observed against vascular endothelium.Citation33 Strategies for overcoming resistance may be novel for targeted agents and different for the VEGF and mTOR pathways.Citation34
The role of a second complex of mTOR, mTORC2, is becoming clearer and requires revision of the simplistic cascade referred to previously. Inhibition of mTOR disrupts S6K negative feedback on the insulin receptor substrate (IRS) precursor of the Akt pathway resulting in undesirable upregulation and a potential mechanism of resistance.Citation35 However in a minority of cell lines, prolonged rapamycin exposure also inhibits mTORC2 that is a key activator of Akt and therefore could mitigate or even block the Akt pathway.Citation28,Citation29 The mechanism of rapamycin inhibition of mTORC2 is not fully elucidated but could be by intracellular scavenging of mTOR. Another reason to block mTORC2 is that it appears to control the expression of HIF-2α that may be more important than HIF-1α in RCC.Citation36 The complex pathway feedback loops are challenging for disease control but also provide numerous opportunities for the combination of everolimus with agents that block other targets,Citation23 but whether these combinations will prove to have a higher therapeutic index than their consecutive use remains to be seen.
Clinical pharmacology
The approach to determining the standard dose of a chemotherapy agent in phase I trial is escalation to the maximum tolerated dose (MTD). However, targeted agents may fully block their target(s) below the MTD and this dose may be directly assessed especially where there is a single target as with mTOR inhibitors.Citation37 Such an approach was used in the clinical development of temsirolimus and subsequently with everolimus. The dose-dependant antitumor efficacy of everolimus was shown in a rat pancreatic tumor model to correlate with prolonged inactivation of ribosomal protein S6 kinase 1.Citation38 This relationship was examined in detail in phase I human studies with similar effects also seen for the other mTOR downstream effector eukaryotic initiation factor p4E-BP1.Citation39,Citation40 Modelling suggested that a daily schedule would exert a greater effect than weekly administration.Citation41 Everolimus 10 mg by mouth daily was recommended for phase II studies.Citation42
At least five metabolites of everolimus are known, all with low mTOR inhibitory effect.Citation43 Unlike temsirolimus, everolimus is not a prodrug of rapamycin. The majority of metabolite excretion is hepatic – biliary – fecal. The peak concentration is reduced by a high fat diet, but the AUC is not; the AUC is proportional to dose. The terminal half-life is approximately 30 hours.Citation44
Efficacy of everolimus: phase II
A phase II study of everolimus 10 mg daily for ccRCC has been completed and fully published.Citation45 Forty-one patients were enrolled and were generally good performance status and minimally treated: 17% had no prior systemic therapy, 62% had one prior immunotherapy, and 22% had a VEGF inhibitor or other therapy. 37 were assessable for efficacy, with 14% objective partial remissions lasting 8 to 37 months and another 57% with stable disease for more than 6 months (the majority with at least minor tumor shrinkage). The safety analysis observed grade 1–2 anorexia, nausea, diarrhea, rash, and stomatitis in >10% of treated patients, grade 1–3 pneumonitis in 49%, and a variety of laboratory changes. However only 13/39 patients required a dose reduction to 5 mg, and no patient withdrew because of drug toxicity. These findings were confirmed in an additional cohort of patients previously treated with sunitinib or sorafenib,Citation46 paving the way to the subsequent phase III study.
Second-line phase III study: RECORD-1; NCT00410124 (clinicaltrials.gov)
RECORD-1 (REnal Cell cancer treatment with Oral RAD001 given Daily)
A pivotal trial of second-line everolimus for advanced clear cell RCC has been fully reported.Citation47,Citation48 All patients had progressive disease on (75%) or within 6 months (25%) after prior treatment with sunitinib, sorafenib, or both agents. Patient eligibility also required measurable disease, as well as adequate organ function and Karnofsky performance status (minimum KPS 70%, capable of self-care). The investigators used exemplary design and methodology, with central randomization, placebo control, independent blind radiologic review, and intent-to-treat analysis. It was powered to detect a 50% improvement in the primary endpoint of progression-free survival (PFS) allowing for two interim analyses. Patients were stratified by risk category,Citation49 and by number of VEGFR TKIs (1 vs 2). All 410 enrolled patients received best supportive care; additionally two thirds of patients were randomly assigned to receive everolimus and one third received identically appearing placebo tablets. The patients, investigators, and independent assessment reviewers were all unaware of the random assignment. Everolimus 10 mg was taken daily by mouth unless protocol-specified adverse events (AEs) required a delay and/or dose reduction to 5 mg daily. Over three-quarters of enrolled patients were KPS 90% to 100% despite being heavily pretreated – over half had received immunotherapy, chemotherapy, or radiation in addition to sunitinib and/or sorafenib. The trial was closed early after the second interim analysis (first efficacy analysis) showed that a pre-specified degree of benefit had been surpassed and the criterion for a positive study met. The risk for disease progression at study closure on the everolimus arm was reduced by 70% compared to placebo () with similar reduction on more mature analysis (hazard ratio [HR] 0.33, 95% confidence interval [CI] 0.25 to 0.43, P < 0.001).Citation48 All pre-specified and exploratory subgroups appeared to show improvement in the primary outcome of delayed progression resulting from disease stabilization and minor tumor shrinkage: 67% of everolimus vs 32% placebo-treated patients had stable disease for at least 8 weeks. Updated median PFS was 4.9 vs 1.9 months and, more importantly, the probability of remaining progression-free for at least 10 months was 25% on everolimus vs <2% on placebo.Citation48 However remissions as conventionally defined by RECIST criteriaCitation50 occurred in only 2% of patients on the active treatment arm, and overall survival was similar for patients receiving everolimus or placebo. There was no difference in the time to deterioration of global quality of life (QOL) in the initial report,Citation47 but subsequent analyses of performance status and disease-related symptoms did suggest a benefit.Citation48 The time to a decline in performance status was longer on everolimus than placebo (5.8 vs 3.8 months, HR 0.66, P = 0.004). A summary of efficacy measures from the RECORD-1 trial is presented in .
Figure 4 Kaplan–Meier estimates of progression-free survival. Reprinted from The Lancet. 372:449–456. Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Copyright © 2008, with permission from Elsevier.
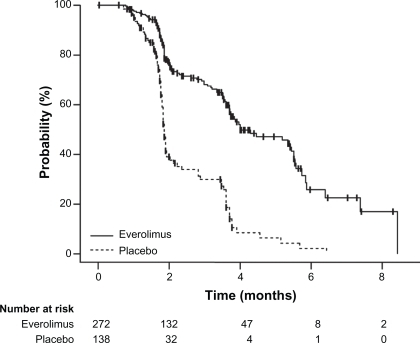
Table 1 Efficacy measures from RECORD-1 trial
Therefore although a robust biological effect was clearly demonstrated, the clinical utility of this effect is less obvious and interpretation is required. For example, it is possible that there were beneficial effects on QOL resulting from stability and minor remissions that were offset by adverse effects of everolimus, a balance that might be quite sensitive to the details of toxicity management. PFS benefit may be a predictor of overall survival impact for this disease,Citation51,Citation52 and a beneficial impact on survival might be obscured by the protocol requirement to permit crossover of placebo-treated patients to receive everolimus after investigator-assessed disease progression, justified by ethical and recruitment considerations;Citation47 112/139 (81%) patients initially assigned to placebo did cross to everolimus and enjoyed a median PFS of 5.1 months, ie, very similar duration to those initially assigned to the everolimus arm. In the absence of standardized ways to examine such possibilities, further analyses by post-hoc crossover censoring techniques must be regarded as hypothesis generating but are consistent with an improvement in overall survival ().Citation53,Citation54
Safety and tolerability of everolimus
Everolimus has turned out to be relatively safe and well tolerated, considering that mTOR blockade might cause disruption of diverse molecular pathways or serious consequences of immunosuppression. In the pivotal phase III study,Citation47 safety was evaluated every 2 weeks for 6 weeks and then monthly, using the US National Cancer Institute Criteria v3.0.Citation55 Due to adverse events, 39% of patients required a dose reduction from everolimus 10 mg to 5 mg by mouth daily, with or without temporary interruption of therapy, compared to 15% on placebo; 13% discontinued active treatment because of toxicity compared with 2% on placebo, usually because of lung disorder or fatigue.Citation47,Citation48 The subjective toxicities that were seen at least 10% more often in patients assigned to everolimus compared to placebo were stomatitis or mucosal inflammation, rash, asthenia, diarrhea, and nausea, mostly grade 1 or 2. Anemia and fatigue were common in both study arms. The following events occurred to grade 3 or 4 significantly more often in patients receiving everolimus than placebo: stomatitis, infections, pneumonitis, elevated cholesterol, hyperglycemia, lymphopenia, and hypophosphatemia. Four deaths were attributed to everolimus, one each due to pneumonitis, sepsis, candidiasis, and aspergillosis ().
Table 2 Serious adverse events in RECORD-1 trialCitation47,Citation48
A serious class toxicity for rapalogs including everolimus is drug-induced (non-infectious) pneumonitis,Citation56 and this may be substantially more common than clinically apparent. For example, a blinded retrospective review of a phase III trial of temsirolimus found that 29% of patients had radiologic pneumonitis, compared to 9% clinical pneumonitis with dyspnea or cough and only 1% discontinued therapy.Citation57 Likewise, a single institution subset of the RECORD-1 pivotal study of everolimus observed CT radiologic changes in 46% but only 7% clinical drug-induced pneumonitis.Citation58 Only grade 3 or clinical pneumonitis requires drug interruption or discontinuation, others may be treated symptomatically and monitored for deterioration or resolution; grade 4 life-threatening pneumonitis was not seen. The average time to development of pneumonitis was 4 months and half were treated with steroids.Citation48 A more detailed analysis and recommendations for pneumonitis management arising from this study is pending.Citation47
Expanded access experience of everolimus
Following presentation of the early RECORD-1 study results,Citation59 in July 2008 an expanded access program that made everolimus available to patients following therapy with sunitinib or sorafenib had enrolled 342 patients in 22 countries as of April 2009,Citation60 and is ongoing in some locations pending regulatory approval.Citation61 Eligibility permitted non-measurable disease. The safety profile is similar to the phase III study with no new toxicities recognized. Seventeen percent discontinued due to AE, similar to the phase III experience. However, of the first 168 patients for whom data are available, 71% experienced at least one grade 3 or 4 toxicity, suggesting that close monitoring and patient education are required to more clearly define the indications for dose reduction or interruption for evolving toxicity.
The question arises as to the use of everolimus following disease progression with new VEGFR tyrosine kinase inhibitors as these become available for study. The most recent additions to this drug family are axitinib (AG13736, Pfizer) and pazopanib (GW786034, GlaxoSmithKline), oral multikinase inhibitors with demonstrated activity in phase II studies and now in phase III trials. In phase II, pazopanib achieved a 35% objective response rate in patients who had not received prior VEGFR TKI therapy.Citation62 Axitinib achieved a response rate of 23% in patients who had all received prior sorafenib.Citation63 Such patients would appear to be eligible for inclusion in the expanded access study of everolimus,Citation61 and the differential efficacy of everolimus after different VEGFR TKI sequences may become available in due course. In the meantime it is reasonable to use everolimus in this setting based on generally similar experience after sunitinib or sorafenib.
Although we do not yet have patient satisfaction data, it is likely that this treatment will be well received since it is very convenient as a daily oral pill, has proven efficacy in stabilizing the disease temporarily in the majority of patients, and is the only proven effective option after failure of VEGR TKIs. Significant toxicity is seen but, in most patients, is readily manageable with symptomatic care and dose modification or temporary interruption.
Practical management of safety issues
The integration of everolimus into clinical practice is at a preliminary stage. It appears that the safety profile of oral everolimus is similar to its analog temsirolimus, available since 2007 as a weekly intravenous treatment and for which practical management is well established.Citation64–Citation66 The principles are: safety monitoring, early detection of toxicities or disease progression, and action appropriate to a palliative therapy. Serious toxicity is to be minimized by early treatment interruption and restart at a reduced dose of everolimus 5 mg/day if tolerated.
Patient selection
Approved patient selection is for tyrosine kinase refractory disease (progression on or immediately after), ambulatory performance status, adequate bone marrow and hepatic function, and clear cell predominant histology.
Table 3 Practical recommendations for everolimus therapyCitation44
Where permitted, off-label use in non-clear cell RCC may be considered since temsirolimus has some evidence to support its use in that context.Citation69 Everolimus is in phase II for patients with papillary renal cancers,Citation70,Citation71 and prior to nephrectomy for patients with metastases at diagnosis.Citation72
Prediction of benefit of mTOR inhibitors
Much needed for targeted agents in general are biomarkers to predict patients who are more likely or very unlikely to benefit. A substantial study of 375 patients investigated the prognostic significance of mTOR pathway components in tumors obtained at nephrectomy, and observed adverse prognostic impact on disease-specific survival of pS6K, PTEN and Akt independent of stage.Citation73 A small study further suggested that pS6K and possibly Akt were predictors of response to temsirolimus for advanced RCC.Citation67,Citation74 However an extension of the studyCitation20 of temsirolimus for poor prognosis advanced RCC did not find a correlation between PTEN and HIF-1αwith outcome.Citation75 Work on biomarkers to predict benefit of everolimus is underway.Citation76 Once treatment is initiated, it may be possible to assess pharmacokinetic effects on glucose metabolism in as little as a week by PET scanning.Citation77
Ongoing clinical research
Everolimus has an established place as the preferred single agent for second-line treatment of ccRCC after sunitinib and/or sorafenib. Research is actively examining additional scenarios for its use. Many combinations of everolimus with other classes of targeted drugs or chemotherapy are being tested in phase I/II trials. The relevant combinations for ccRCC combine everolimus with another agent that targets the same PI3K/Akt/mTOR pathway at another level (vertical blockade), or a different pathway (horizontal blockade).Citation78 Vertical blockade with everolimus and imatinib was toxic, gave PFS similar to everolimus alone, and was not recommended for further development.Citation79 Horizontal blockade combining everolimus with a VEGF pathway inhibitor is proceeding actively eg, sunitinib,Citation80 sorafenib,Citation81 or bevacizumab. Of these, the everolimus – bevacizumab combination is the only one where both drugs are tolerated at full dose,Citation82 has promising activity,Citation83 and has now reached randomized phase II testing compared to the established IFN-bevacizumab regimen in the first-line setting (RECORD-2 study).Citation84 Agents that block mTOR complex 2 or the S6K feedback loop () would be of special interest.Citation26
Now that multiple oral targeted agents are available and adequately well tolerated for chronic use, trials are starting to examine whether there might be a preferred sequence eg, for inhibition of the mTOR and VEGF pathways. The phase III RECORD-3 studyCitation85 will randomize patients to everolimus or sunitinib first-line, and cross-over to the other agent at disease progression.
Conclusions
Consequent to a large well performed phase III trial, everolimus has become the standard second-line agent after the approved first-line drugs sunitinib and/or sorafenib for patients with advanced clear cell renal cancer. The majority of patients with advanced disease are now diagnosed early at presentation or on surveillance after nephrectomy and remain in good performance status for an extended time and so are eligible for everolimus. Everolimus has been recently approved by the FDA in the USA, and by European Medicines Agency in Europe. Guidelines in developed countries for the treatment of RCC are reasonably concordant,Citation86 and now include everolimus as the preferred treatment for VEGFR inhibitor resistant disease in the USA,Citation87 Europe,Citation88 and Canada.Citation89 Future randomized trials in the second-line setting after VEGF pathway inhibitors will need to use everolimus as comparator in the control arm. The benefits of second-line everolimus are modest with temporary stablization the most common advantage over placebo. Everolimus drug combinations and/or use earlier in the disease may confer greater benefits. The impact on overall survival has become difficult to demonstrate because of crossover of patients to the alternate arm. Validated and agreed statistical methods are needed and appear feasible for the use of censoring techniques to determine survival benefit in a crossover setting.Citation53,Citation54
Disclosure
The author declares no conflicts of interest.
References
- Canadian Cancer Society’s Steering CommitteeCanadian Cancer Statistics 2009TorontoCanadian Cancer Society2009 www.cancer.ca/statistics Accessed Nov 30, 2009.
- JemalASiegalRWardEHaoYXuJThunMJCancer statistics, 2009Ca Cancer J Clin20085922524919474385
- MooreLEWilsonRTLifestyle factors, exposures, genetic susceptibility, and renal cell cancer risk: a reviewCancer Invest20052324025515945510
- British Columbia Cancer Registry Potential years of life lost. BC Cancer Agency, Vancouver BC, Canada. www.bccancer.bc.ca/HPI/CancerStatistics/FF/LifeLost.htm Accessed Nov 30, 2009.
- MotzerRJBacikJMurphyBARussoPMazumdarMInterferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinomaJ Clin Oncol20022028929611773181
- MekhailTMAbou-JawdeRMBouMerhiGValidation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinomaJ Clin Oncol20052383284115681528
- Medical Council Renal Cancer CollaboratorsInterferon-α and survival in metastatic renal carcinoma: early results of a randomized controlled trialLancet1999353141710023944
- FiglinRAHutsonTETomczakPOverall survival with sunitinib versus interferon-alfa as first-line treatment of metastatic renal cell carcinomaJ Clin Oncol200826suppl:abstr 5024. Abstract and associated presentation available from http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_meeting_categories_view&confID=55 Accessed Nov 30, 2009.
- CoppinCPorzsoltFAutenreithMKumpfJColdmanAWiltTImmunotherapy for advanced renal cell cancerCochrane Database Syst Rev20043CD001425, updated 18 May, 2006.
- CoppinCLeLPorzsoltFWiltTTargeted therapy for advanced renal cell cancerCochrane Database Syst Rev20082CD00601718425931
- GleaveMEElhilaliMFradetYInterferon gamma-1b compared with placebo in metastatic renal-cell carcinomaN Engl J Med1998338126512719562580
- RavaudAHawkinsRGardnerJLapatinib versus hormone therapy in patients with advanced renal cell carcinoma: a randomized phase III clinical trialJ Clin Oncol2008262285229118467719
- FyfeGFisherRIRosenbergSASznolMParkinsonDRLouieACResults of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapyJ Clin Oncol1995136886967884429
- PyrhonenSSalminenERuutuMProspective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancerJ Clin Oncol1999172859286710561363
- RiniBICambellSCEscudierBRenal cell carcinomaLancet20093731119113219269025
- RiniBIMetastatic renal cell carcinoma: many treatment options, one patientJ Clin Oncol2009273225323419470934
- MotzerRJHutsonTETomczakPOverall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinomaJ Clin Oncol2009273584359019487381
- EscudierBEisenTStadlerWMSorafenib in advanced clear-cell renal-cell carcinomaN Engl J Med200735612513417215530
- BracardaSPatardJRavaudACurrent perspectives in metastatic renal cell carcinoma treatment: the role of mammalian target of rapamycin (mTOR) inhibitionEur Urol Suppl20098785786
- HudesGCarducciMTomczakPTemsirolimus, interferon alfa, or both for advanced renal-cell carcinomaN Engl J Med20073562271228117538086
- VezinaCKudelskiASehgalSNRapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing Streptomycete and isolation of the active principleJ Antibiot (Tokyo)1975287217261102508
- CalneRYLimSSamaanARapamycin for immunosuppression in organ allograftingLancet198922272568561
- StrimpakosASKarapanagiotouEMSaifMWSyrigosKNThe role of mTOR in the management of solid tumors: an overviewCancer Treat Rev20093514815919013721
- HarrisonDEStrongRSharpZDRapamycin fed late in life extends lifespan in genetically heterogeneous miceNature200946039239619587680
- HuangSBjornstiMHoughtonPJRapamycins. Mechanism of action and cellular resistenceCancer Biol Ther2003222223212878853
- HudesGRTargeting mTOR in renal cell carcinomaCancer200911510 Suppl2313232019402072
- HayNSonenbergNUpstream and downstream of mTORGenes Dev2004181926194515314020
- SabatiniDMmTOR and cancer: insights into a complex relationshipNat Rev Cancer2006672973416915295
- AbrahamRTGibbonsJJThe mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapyClin Cancer Res2007133109311417545512
- HannaSCHeathcoteSAKimWYmTOR pathway in renal cell carcinomaExpert Rev Anticancer Ther2008828329218279068
- ChoiJChenJSchreiberSLClardyJStructure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAPScience19962732392428662507
- LaneHAWoodJMMcSheehyPMJmTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitorClin Cancer Res2009151612162219223496
- ManegoldPCParingerCKulkaUAntiangiogenic therapy with mammalian target of rapamycin inhibitor RAD001 (everolimus) increases radiosensitivity in solid cancerClin Cancer Res20081489290018245553
- RiniBIAtkinsMBResistance to targeted therapy in renal-cell carcinomaLancet Oncol200910992100019796751
- O’ReillyKERojoFSheQmTOR inhibition induces upstream receptor kinase signaling and activates AktCancer Res2006661500150816452206
- ToschiALeeEGadirNOhhMFosterDADifferential dependence of hypoxia-inducible factors 1α and 2α on mTORC1 and mTORC2J Biol Chem2008283344953449918945681
- HardingMWImmunophilins, mTOR, and pharmacodynamic strategies for a targeted cancer therapyClin Cancer Res200392882288612912931
- BoulayAZumstein-MeckerSStephanCAntitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in perpheral blood mononuclear cellsCancer Res20046425226114729632
- TaberneroJRojoFCalvoEDose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumorsJ Clin Oncol2008261603161018332469
- O’DonnellAFaivreSBurrisHAPhase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumorsJ Clin Oncol2008261588159518332470
- TanakaCO’ReillyTKovarikJMIdentifying optimal biological doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic dataJ Clin Oncol2008261596160218332467
- TaberneroJRojoFBurrisHA phase I study with tumor molecular pharmacodynamic (MPD) evaluation of dose and schedule of the oral mTOR-inhibitor Everolimus (RAD001) in patients (pts) with advanced solid tumorsJ Clin Oncol200523suppl:abstr 3007.
- StromTHaschkeMZhangYLIdentification of everolimus metabolite patterns in trough blood samples of kidney transplant patientsTher Drug Monit20072959259917898649
- European summary of product characteristics: Afinitor 10 mg. Novartis global. http://www.afinitor.com/global/docs/Afinitor-CD-EN-10mg.pdf Accessed Nov 30, 2009.
- AmatoRJJacJGiessingerSSaxenaSWillisJPA phase 2 study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastatic clear cell renal cell cancerCancer20091152438244619306412
- JacJAmatoRJGiessingerSSaxenaSWillisJPA phase II study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastatic renal cell carcinoma which has progressed on tyrosine kinase inhibition therapyJ Clin Oncol200826suppl;abstr 5113.
- MotzerRJEscudierBOudardSEfficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trialLancet200837244945618653228
- KayAMotzerRFiglinRUpdated data from a phase III randomized trial of everolimus (RAD001) versus PBO in metastatic renal cell carcinomaAmerican Society of Clinical Oncology Genitourinary Cancers Symposium2009;abstr 278. Abstract and associated presentation by RJ Motzer available from http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_meeting_categories_view&confID=64 Accessed Nov 30, 2009.
- MotzerRJBacikJSchwartzLHPrognostic factors for survival in previously treated patients with metastatic renal cell carcinomaJ Clin Oncol20042245446314752067
- TherassePArbuckSGEisenhauerEANew guidelines to evaluate the response to treatment in solid tumorsJ Natl Cancer Inst20009220521610655437
- KnoxJJProgression-free survival as endpoint in metastatic RCC?Lancet200837242742918653227
- DeleaTKhuuAKayAZhengJBaladiJFAssociation between time to disease progression endpoints and overall survival in patients with meta-static renal cell carcinomaEur J Cancer20097S2:abstr 7124.
- WiederkehrDHoweCJCascianoRMotzerRZhengJBaladiJFOverall survival among metastatic renal cell carcinoma patients corrected for crossover using inverse probability of censoring weights: analysis from the RECORD-1 phase 3 trialEur J Cancer20097S2: abstr 7131.
- KorhonenPHaasTZuberEKayALebwohlDMotzerROverall survival among metastatic renal cell carcinoma patients corrected for crossover using a rank preserving structural failure time model: analysis from the everolimus phase 3 trialEur J Cancer20097S2: abstr 7155.
- Common terminology criteria for adverse events v3.0. Cancer Therapy Evaluation Program, US National Cancer Institute. 2006http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_30 Accessed Nov 30, 2009.
- ChhajedPNDickenmannMBubendorfLMayrMSteigerJTammMPatterns of pulmonary complications associated with sirolimusRespiration20067336737416127266
- PabloMHudesGDutcherJRadiographic findings of drug-induced pneumonitis and clinical correlation in patients with advanced renal cell carcinoma treated with temsirolimusEur J Cancer20097S2:abstr 7113.
- AlbigesLCaramellaCFerteCInterstitial pneumonitis during RAD001 treatment: incidence by blinded radiological analysisEur J Cancer20097S2:abstr 7114.
- MotzerRJEscudierBOudardSRAD001 vs placebo in patients with metastatic renal cell carcinoma after progression on VEGFr-TKI therapy: results from a randomized, double-blind, multicenter phase-III studyJ Clin Oncol200826suppl:abstr LBA5026.
- GrunwaldVRhaSYMillerKAn international expanded access program of RAD001(everolimus) in patients with metastatic renal cell carcinoma who fail or become intolerant of a prior vascular endothelial growth factor therapyEur J Cancer20097S2:abstr 7154.
- Expanded access study of RAD001 in metastatic renal cell cancer patients who are intolerant of or who have failed despite prior vascular endothelial growth factor therapy. NCT00655252. www.clinicaltrials.gov/ct2/results?term=NCT00655252 Accessed Nov 30, 2009.
- HutsonTEDavisIDMachielsJ-PHEfficacy and safety of pazopanib in patients with metastatic renal cell carcinomaJ Clin Oncol20102847548020008644
- RiniBIWildingGHudesGPhase II study of axitinib in sorafenib-refractory metastatic renal cell carcinomaJ Clin Oncol2009274462446819652060
- HutsonTEFiglinRAKuhnJGMotzerRJTargeted therapies for metastatic renal cell carcinoma: an overview of toxicity and dosing strategiesOncologist2008131084109618838439
- BellmuntJSzczylikCFeingoldJStrahsABerkenblitATemsirolimus safety profile and management of toxic effects in patients with advanced renal cell carcinoma and poor prognostic featuresAnn Oncol2008191387139218385198
- SerugaBGanHKKnoxJJManaging toxicities and optimal dosing of targeted drugs in advanced kidney cancerCurr Oncol200916S1S52S5919478903
- AtkinsMBHidalgoMStadlerWMRandomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinomaJ Clin Oncol20042290991814990647
- PendergrassKBHudesGRadulovicSCharacterization of hyperglycemia, hypercholesterolemia and hyperlipidemia in patients with advanced renal cell carcinoma treated with temsirolimus or interferon-αASCO Genitourinary Cancers Symposium 2009; abstr 297. Abstract and slides available from http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_meeting_categories_view&confID=64 Accessed Nov 30, 2009.
- DutcherJPde SouzaPMcDermottDFiglinRAEffect of temsirolimus versus interferon-α on outcome of patients with advanced renal cell carcinoma of different tumor histologiesMed Oncol20092620220919229667
- CaglioSSundlovASlimaneKMayCEscudierBOpen-label phase II trial of everolimus monotherapy for patients with advanced papillary renal cell cancer (RAPTOR): rationale and designEu J Cancer20097S2:abstr 7156.
- RAPTOR: RAD001 as monotherapy in the treatment of advanced papillary renal cell tumors program in Europe (RAPTOR/LFR08). NCT00688753. Available from http://www.clinicaltrials.gov/ct2/results?term=NCT00688753 Accessed Nov 30, 2009.
- RAD001 (everolimus) for advanced renal cell carcinoma before kidney removal. NCT00831480. http://www.clinicaltrials.gov/ct2/results?term=NCT00831480 Accessed Nov 30, 2009.
- PantuckAJSeligsonDBKlatteTPrognostic relevance of the mTOR pathway in renal cell carcinomaCancer20071092257226717440983
- ChoDSignorettiDDaboraSPotential histologic and molecular predictors of response to temsirolimus in patients with advanced renal cell carcinomaClin Genitourin Cancer2007537938517956710
- FiglinRAde SouzaPMcDermottDAnalysis of PTEN and HIF-1α and correlation with efficacy in patients with advanced renal cell carcinoma treated with temsirolimus versus interferon-αCancer20091153651366019526589
- Biomarker trial of everolimus in patients with advanced renal cell carcinoma. NCT00827359. http://www.clinicaltrials.gov/ct2/results?term=NCT00827359 Accessed Nov 30, 2009.
- NogovaLZanderTGrossSHPharmacodynamics of RAD001 measured by early FDG PET in patients with recurrent NSCLCJ Clin Oncol200826suppl:abstr 14616.
- SosmanJPuzanovICombination targeted therapy in advanced renal cell carcinomaCancer200911510 suppl2368237519402058
- RyanCWVukyJChanJSBeerTMRothkopfMPhase II study of everolimus with imatinib in patients with previously-treated renal carcinomaJ Clin Oncol200927suppl:abstr e16075.
- KroogGSFeldmanDRKondaguntaGVPhase I trial of RAD001 (everolimus) plus sunitinib in patients with metastatic renal cell carcinomaJ Clin Oncol200927suppl:abstr 5037.
- HarzstarkALRosenbergJEWeinbergVKA phase I study of sorafenib and RAD001 for metastatic clear cell renal cell carcinomaJ Clin Oncol200927suppl:abstr 5104.
- ZafarYBendellJLagerJPreliminary results of a phase I study of bevacizumab in combination with everolimus in patients with advanced solid tumorsJ Clin Oncol200624suppl:abstr 3097.
- WhorfRCHainsworthJDSpigelDRPhase II study of bevacizumab and everolimus (RAD001) in the treatment of advanced renal cell carcinomaJ Clin Oncol200826suppl:abstr 5010. Abstract and associated presentation by JD Hainsworth available from http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_meeting_categories_view&confID=55 Accessed Nov 30, 2009.
- Safety and efficacy of bevacizumab plus RAD001 versus interferon alfa-2a and bevacizumab in adult patients with kidney cancer (L2201). NCT00719264. http://www.clinicaltrials.gov/ct2/results?term=NCT00719264 Accessed Nov 30, 2009.
- Efficacy and safety comparison of RAD001 versus sunitinib in the first-line and second-line treatment of patients with metastatic renal cell carcinoma (RECORD3). NCT00903175. http://www.clinicaltrials.gov/ct2/results?term=NCT00903175 Accessed Nov 30, 2009.
- SoulieresDReview of guidelines on the treatment of metastatic renal cell carcinomaCurr Oncol200916S1S67S7019478902
- Kidney cancer treatment for advanced disease, predominant clear cell histology, subsequent therapy after tyrosine kinase inhibitor therapy. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Kidney Cancer. V.2.2010http://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed Nov 30, 2009.
- EscudierBKatajaVRenal cell carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow-upAnn Oncol200920S4iv81iv82
- Canadian Kidney Cancer Forum 2009Management of kidney cancer: Canadian Kidney Cancer Forum consensus updateCan Urol Assoc J2009320020419543462