Abstract
The open-label, phase II clinical trial of antituberculosis therapy (ATT) with or without oral immunomodulator Dzherelo (Immunoxel) was conducted in TB/HIV coinfected, antiretroviral therapy naïve patients to evaluate the effect on CD4 T-lymphocyte counts and viral load. The arm A (n = 20) received isoniazid (H); rimfapicin (R); pyrazinamide (Z); streptomycin (S); and ethambutol (E), and arm B (n = 20) received 50 drops of Dzherelo twice per day in addition to HRZSE. After 2 months in 90% of Dzherelo patients the population of absolute CD4 T-cells expanded by an average of 71.2% (from 174 to 283 cells/μl; P = 0.00003), but declined in ATT-alone patients (182 to 174; P = 0.34). The ratio between CD4/CD8 cells deteriorated in 80% of individuals in arm A (1.213 > 0.943; P = 0.002), but improved in the same proportion of patients in arm B (1.244 > 1.536; P = 0.007). The number of total CD3+ lymphocytes rose from 728 to 921 cells in arm B (P = 0.025) whereas it fell from 650 to 585 cells in arm A (P = 0.25). The viral load, as measured by plasma RNA-PCR, decreased in 70% of Dzherelo recipients (2.174 > 1.558 copies/ml; P = 0.002), but increased in 70% of HRZSE only receivers (1.907 > 2.076 copies/ml; P = 0.03). Dzherelo has a favorable effect on the immune status and viral burden in TB/HIV patients when given as an immunomodulating adjunct to ATT.
Introduction
In developing countries, tuberculosis (TB) coinfection is common among human immunodeficiency virus (HIV)-positive individuals with probability of death 2- to 3-fold higher than among acquired immunodeficiency syndrome (AIDS) patients without active TB (CitationGupta et al 2007). The World Health Organization estimates that a person with both HIV and TB infection is thirty times more likely to become ill with TB than a person with Mycobacterium tuberculosis infection alone (CitationReid et al 2006). Ukraine has the highest rate of TB/HIV coinfection in Eastern Europe (Citationvan der Werf et al 2006). It is recognized that the effectiveness of TB therapy is significantly lower among patients with HIV/AIDS. The occurrence of mortality and relapse are consistently higher even when TB/HIV patients are treated with antituberculosis therapy (ATT) under directly observed therapy (DOT) (CitationKhauadamova et al 2001). Drug resistance accompanied by HIV-associated immunodeficiency is the main cause of treatment failure. The recent survey of CitationNikolayevskyy and colleagues (2007) had shown that in Southern Ukraine the multi-drug resistance (MDR) rates were significantly higher among former prison inmates compared with nonprisoners (54.8% vs. 27.3%).
The oral immunomodulator Dzherelo has been successfully used in Ukraine for the management of TB and HIV infections, including patients with dual infection. Published studies by us and others have demonstrated that Dzherelo can significantly shorten the duration of treatment and helps to achieve higher response rate even in those who are HIV coinfected, have MDR or extensively-drug resistant (XDR) forms of TB (CitationChkhetiany et al 2007; CitationPrihoda et al 2007; CitationNikolaeva et al 2008). Dzherelo has also been found to decrease the hepatotoxicity resulting from TB chemotherapy (CitationZaitzeva 2006). Dzherelo was approved in 1997 by the Ministry of Health of Ukraine as an immunomodulating supplement. In 1999, Dzherelo was recommended as an immune adjunct to the conventional therapy of pulmonary tuberculosis (CitationMelnik et al 1999). Dzherelo contains aqueous-alcohol extract from various medicinal plants. So far, Dzherelo has been used by over 150,000 individuals for various indications including chronic bacterial and viral infections such as TB and HIV, autoimmune diseases, and malignancy.
Several studies have shown that, similarly to HIV, the immune control of M. tuberculosis is dependent from CD4+ T-lymphocytes (CitationSerbina et al 2001; CitationMorris et al 2003; CitationManas et al 2004; CitationKalou et al 2005). The M. tuberculosis and HIV infections are associated with pronounced deterioration of the immune system as evidenced by higher depletion rate of helper CD4+ cells and enhanced HIV replication. The immunotherapeutic approaches toward TB began receiving more attention recently (CitationTomioka 2004; CitationKaufmann 2006; CitationAchkar et al 2007). There are many types of immune modulators that have been used clinically for viral infections, but for TB the choice of immune interventions is limited (CitationErshov 2003). Our study was aimed at evaluating the effect of Dzherelo on T-lymphocyte populations and viral load among TB/HIV patients in comparison with control population, which received the ATT only.
Materials and methods
Patients
Male patients, aged 19–42 years, have been randomized into arms A and B, each consisting of 20 patients. The majority of patients in our study were correctional facility inmates in advanced clinical stage III of HIV-1 infection with average baseline CD4+ T-cell counts below 200 cells/microliter (cells/μl). The diagnosis of HIV infection was established by standard ELISA test and further confirmed by Western blot. Active pulmonary tuberculosis was certified by a medical history and clinical findings compatible with pulmonary tuberculosis, a chest X-ray showing lung involvement, and positive sputum smear for acid-fast bacilli or the culture of M. tuberculosis. The conduct of the trial was approved by the State Department of the Penitentiary of Kharkov region, Ukraine. The participation in this trial was voluntary and patients were eligible to enroll only after signing the written consent.
Treatment regimens
None of the patients received antiretroviral therapy prior to and during 2-months of follow-up. All patients received standard antituberculosis therapy administered under DOT schedule which consisted of once daily dose of isoniazid (H) 300 mg; rimfapicin (R) 600 mg; pyrazinamide (Z) 2,000 mg; streptomycin (S) 1,000 mg; and ethambutol (E) 1,200 mg. The arm B received, in addition to HRZSE, twice per day dose of Dzherelo which was given as 50 drops diluted in a half-glass of water. Dzherelo contains concentrated aqueous-alcohol extract from medicinal plants such as Aloe (Aloe arborescens), Common knotgrass (Polygonum aviculare), Yarrow (Achillea millefolium), Purple coneflower (Echinacea purpurea), St. John’s Wort (Hypericum perforatum), Centaury (Centaurium erythraea), Snowball tree berries (Viburnum opulus), Nettle (Urtica dioica), Dandelion (Taraxacum officinale), Sweet-sedge (Acorus calamus), Oregano (Oreganum majorana), Marigold (Calendula officinalis), Seabuckthorn berries (Hippophae rhamnoides), Elecampane (Inula helenium), Tormentil (Potentilla erecta), Greater plantain (Plantago major), Wormwood (Artemisia sp.), Siberian golden root (Rhodiola rosea), Cudweed (Gnaphalium uliginosum), Licorice (Glycyrrhiza glabra), Fennel (Foeniculum vulgare), Chaga (Inonotus obliquus), Thyme (Thymus vulgaris), Three-lobe Beggarticks (Bidens tripartite), Sage (Salvia officinalis), Dog rose (Rosa canina), and Juniper berries (Juniperus communis). The over-the-counter phytoconcentrate Dzherelo was generously supplied by Ekomed company (Kiev, Ukraine).
Immunophenotyping of lymphocyte subsets
The peripheral blood of patients with TB/HIV was analyzed with Clonospectr panel of monoclonal antibodies against surface antigens of T-lymphocytes (MedBioSpectr, Moscow, Russia). The absolute and relative (%) values of total CD3+ lymphocytes, helper T lymphocytes (CD3+CD4+) and cytotoxic T lymphocytes (CD3+CD8+) were assessed in a blinded fashion by fluorescent microscopy at baseline and after 1 and 2 months on the therapy. In addition the changes in the ratio between CD4 and CD8 cells were evaluated as a part of assessment of the immune status of patients. The samples of the blood from 19 healthy blood donors were analyzed as a reference for normal values.
PCR analysis
Stored frozen samples of plasma were processed in bulk with commercially available PCR kit (AmpliSense HIV-1, Central Research Institute of Epidemiology, Moscow, Russia) designed for quantitative analysis of HIV-RNA copies. Tests were carried out at baseline and after two months of the therapy.
Statistical analysis
The obtained results were analyzed with the aid of statistical software STATMOST (Datamost, South Sandy, UT). The baseline cell numbers relative to 1st and 2nd months post-therapy were evaluated by paired Student t-test. Unpaired t-test was used to compare data from TB/HIV patients to normal blood donors. The nonparametric values of viral load were evaluated by Wilcoxon signed-rank test. Spearman rank-order test was used for correlation analysis, which was carried out on absolute and relative numbers of T-cells and PCR results. The correlation analysis was also conducted on differential values of these parameters, ie, differences between baseline and 2-month results. All statistical calculations were per intent-to-treat basis or the total number of patients without subgrouping them into responders and nonresponders. The probability values were considered as significant at P ≤ 0.05.
Results
After one month of therapy there was a clear distinction in measured T-cell counts between recipients of ATT-alone and those who received ATT with Dzherelo. This disparity became even more evident at the end of the second month of therapy (). The changes in viral load among TB/HIV patients of both groups have also reached statistical significance even though none of the patients have ever received the antiretroviral therapy (). These findings are described in detail as follows.
Table 1 Effect of 2-month HRZSE therapy without or with Dzherelo on HIV-RNA plasma levels
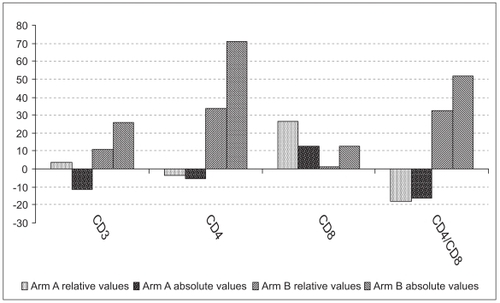
Figure 1 Changes in T-lymphocyte relative and absolute numbers at 2 months post-therapy as expressed in percentage values relative to their respective baseline levels.
CD3+ total T-lymphocytes
After one month on ATT-alone arm, the absolute and percent (%) values of total CD3+ lymphocytes per microliter of blood have not changed appreciably, ie, 650 (36.8%) vs. 634 (37.2%); P = 0.38 (P = 0.23), as analyzed by paired Student t-test. However, in the HRZSE group, which received Dzherelo, there was a significant difference in total CD3+ values as early as one month post-therapy, ie, 728 (37.5%) vs. 902 (40.4%); P = 0.015 (P = 0.04). After 2 months, the number of total CD3+ lymphocytes increased further to 921 in arm B (P = 0.025) whereas in the control arm it decreased to 585 cells (P = 0.25). The difference in treatment outcomes between two groups, ie, 921 vs. 585 was significant (P = 0.004), while baseline values were not statistically different (P = 0.15). Compared with baseline there was a 26% increase and 11.2% decrease in absolute number of total lymphocytes in arms B and A respectively (). In arm B, 15 out of 20 (80%) patients were responders displaying the increase in absolute CD3 lymphocytes, while in arm A, less than half of patients (45%) had a positive response.
CD4+ T-lymphocytes
No significant changes were seen after one month in ATT-alone arm, ie, from 182 (24.2%) to 203 (24.5%) cells/μl; P = 0.063 (P = 0.28), whereas in the Dzherelo arm, the number of helper cells have risen significantly from 174 (23.3%) to 257 (27.3%) cells; P = 0.00003 (P = 0.0004). At the end of the second month, CD4 lymphocytes in arm B have risen further to 283 (31%) P = 0.0000004. However, the changes in arm A were insignificant 174 (25.3%) P = 0.13. When study completion results of Dzherelo recipients were compared in terms of accrual of CD4+ lymphocytes with the entry levels, there was a highly significant increase of 34% and 71% in relative and absolute values. In arm A, relative and absolute CD4 cell numbers fell by 4.5% and 5.5%, respectively. The absolute and relative CD4 numbers were indistinguishable at baseline with P = 0.4 (P = 0.2), but at study conclusion the differences became highly significant with P = 0.001 and P = 0.003 for absolute and relative values, respectively. Out of 20 patients on Dzherelo 18 (90%) had seen their T-cells rise, while among those who received HRZSE-alone only 40% of patients had shown the increase in absolute CD4 lymphocytes ().
CD8+ T-lymphocytes
After one month in the HRZSE-alone group, CD8+ cells increased in a significant manner from 159 (21.7%) to 199 (24.3%); P = 0.01 (P = 0.02), while in the Dzherelo group, the changes were insignificant, ie, from 159 (20.8%) to 190.2 (19.8%); P = 0.21 (P = 0.05). At the end of the second month, the CTL population in the ATT-alone group was still above baseline, ie, 180 cells (27.7%), an accrual that was statistically significant in percent terms (P = 0.000009), but not significant when calculated in absolute numbers (P = 0.17). On the contrary, in the second month, numbers of CD8 cells among Dzherelo recipients had not increased in any significant manner, from 159 (20.8%) to 183 (20.7%), with P values being 0.13 and 0.47 for absolute and percent figures, respectively (). The absolute and relative CD8 numbers were almost identical between two arms at baseline 159 (21.7%) vs. 159 (20.8%) at P = 0.5 (P = 0.3), but at study conclusion they reached P = 0.4 and P = 0.00002 for absolute and relative values, respectively. In the HRZSE arm, 70% and 85% of patients had shown an increase in absolute and relative numbers of CD8 lymphocytes, while in the Dzherelo group, 65% and 50% of patients had a similar response.
CD4/CD8 ratio
The differential changes in CD4 and CD8 lymphocyte numbers had affected the CD4/CD8 ratio in patients on the HRZSE-alone regimen as early as one month after treatment initiation. Their ratio had declined from 1.213 baseline value to 1.06 (P = 0.009). In contrast, the CD4/CD8 ratio among Dzherelo recipients increased from 1.244 to 1.416, which was, however, slightly above the cut-off value (P = 0.06). The disparity between CD4 and CD8 lymphocytes had progressed even further by the end of the second month. Among HRZSE-alone patients the ratio had declined to 0.943 (P = 0.002), while in the Dzherelo group the ratio had risen to a level that is commonly considered to be as a normal, ie, 1.536 (P = 0.007). This ratio was statistically indistinguishable from the ratio of normal blood donors, ie, 1.54 vs. 1.76 (P = 0.09). The proportion of patients who experienced increase in their CD4/CD8 ratio was 80% and 20% for arms B and A, respectively.
Lymphocyte subsets in normal blood donors
Samples of the peripheral blood of 19 healthy individuals were analyzed to obtain the normal distribution values of lymphocyte subsets. The average number of absolute and relative (%) CD3 lymphocytes were 1,370 ± 169 cells/μl (52.9 ± 6.8). The values of CD4 and CD8 lymphocytes were 622 ± 89 (35.9 ± 4.3) and 349 ± 42 (19.9 ± 2.1) respectively, with ratio being 1.76 ± 0.19. The absolute numbers of CD3 and CD8 cells were approximately two-fold and CD4 cells three-fold higher than in patients with TB/HIV.
Viral load
The viral load, as measured by plasma RNA-PCR at baseline and at the end of the second month, increased in the ATT group (1907 to 2076 copies/ml; P = 0.025, by Wilcoxon signed rank test), but decreased in the Dzherelo group (2174 to 1558 copies; P = 0.002). About two-thirds of the patients (14/20) on HRZSE-alone had shown the increase in viral load, while the same proportion of patients on Dzherelo (70%) had a reduction in their number of viral copies ().
Correlation of viral load with T-cells
The Spearman rank-order analysis has revealed that at study conclusion the differential accrual in HIV copies in arm A patients seem to be inversely dependent on differential of absolute CD4 numbers (r = −0.4124; P = 0.07) but directly correlated with relative CD8 counts at two months (r = 0.42; P = 0.07). However, the corresponding correlation values in Dzherelo recipients were r = −0.01 (P = 0.96) and r = −0.03 (P = 0.91) for CD4 and CD8 cells, respectively. The only significant correlation found in the Dzherelo arm was an inverse relation between differentials of relative CD8 counts and viral load (r = −0.46; P = 0.04). The viral load was inversely dependent from CD4/CD8 ratio in arm A (r = −0.49; P = 0.03), but not in the Dzherelo arm (r = 0.24; P = 0.3). No correlation was found between relative and absolute CD3 cell numbers and HIV RNA levels in arm A, but in arm B higher differential counts were significantly correlated with lower viral load (r = −0.49; P = 0.03).
Discussion
Our results indicate that when Dzherelo is added to ATT, it can produce a significant increase in CD3+ and CD4+ lymphocytes, and a better CD4/CD8 ratio, but no significant change in CD8-bearing lymphocytes. Furthermore, Dzherelo produces statistically significant lower viral load; an effect that we have shown in HIV-infected individuals without concomitant TB (CitationNikolaeva et al 2008).
Our 2-month DOT study conducted in a population consisting mostly of incarcerated individuals reveals that when Dzherelo is added to ATT the absolute and relative numbers of CD4+ T-cells increase by 71% and 34%, respectively. Thus, in our hands Dzherelo produced the same outcome as reported earlier (CitationChkhetiany et al 2007; CitationPrihoda et al 2007; CitationNikolaeva et al 2008). For example, AIDS patients who received Dzherelo with or without standard antiretroviral therapy gained between 38%–93.5% more CD4 lymphocytes (CitationChkhetiany et al 2007; CitationNikolaeva et al 2008). It is well established that elevated CD3 and CD4 counts and higher CD4/CD8 ratio are associated with better prognosis in patients with HIV as well as TB (CitationSerbina et al 2001; CitationMorris et al 2003; CitationManas et al 2004; CitationKalou et al 2005). Dzherelo appears to normalize the lymphocyte homeostasis since at the end of study the CD4/CD8 ratio of treated patients became indiscernible from the ratio of normal healthy individuals, ie, 1.54 vs. 1.76 (P = 0.09). Thus, Dzherelo is likely to influence positively the outcome of treatment and disease progression in our study population.
Treatment of TB in HIV-infected individuals is a daunting task when compared with TB in HIV-negative persons (CitationKhauadamova et al 2001; CitationReid et al 2006; CitationGupta et al 2007). This task is particularly challenging when one has to treat inmate populations, which as a rule have higher prevalence of drug-resistant TB and more advanced HIV disease (CitationNikolayevskyy et al 2007). Due to frequent failure rate of TB therapy in this particular population of patients the immune intervention with Dzherelo has been sought to evaluate its benefit. In the prior studies Dzherelo has been shown to increase by two- to three-fold the success rate of ATT and shorten significantly the duration of treatment even among those who had MDR-TB or XDR-TB (CitationPrihoda et al 2007). It had also reduced the toxic side effects of ATT, the hepatotoxicity in particular. Elevated liver aminotransferase ALT and AST levels caused by ATT have been shown to return to normal levels after addition of Dzherelo (Zaitzeva 2003). Furthermore, cachexic patients who received Dzherelo were shown to gain weight (CitationChkhetiany et al 2007; CitationPrihoda et al 2007). However, these studies have not dealt with the effect of Dzherelo on viral load among dually infected TB/HIV patients.
The viral load is a predictor of HIV disease progression, its persistent elevation in TB/HIV coinfected patients is indicative of poor prognosis (CitationMorris et al 2003; CitationKalou et al 2005). While there were earlier indications that Dzherelo reduces viral burden (CitationChkhetiany et al 2007; CitationNikolaeva et al 2008), our study is the first to report this phenomenon in TB/HIV patients. Despite the fact that the HIV RNA levels had decreased by less than a log the difference between baseline and outcome levels was highly significant. 70% of patients in arm A experienced the increase in viral load, while the same proportion of patients in arm B had reduced viral burden. This observation is encouraging since successful ATT regimens, some which reported to restore the immunity in TB/HIV patients, were not affecting the viral load (CitationNewton et al 2000; CitationMorris et al 2003; CitationKizza et al 2005). Thus, our observation is quite unique. Except, perhaps, thalidomide, prednisone, and likopid, we are not aware of any other immunity-restoring preparation that would affect both the TB and HIV (CitationBekker et al 2000; CitationSvistunova et al 2002; CitationMayanja-Kizza et al 2005). However, several experimental immunotherapeutic regimens were tried with various degrees of success in TB patients including for example interferon, tumor necrosis factor antagonists, cytokines, thymus-derived Russian immunomodulators such as thymalin, thymogen, T-activin, and vilosen (CitationKhudzik et al 1998; CitationWallis 2005).
Many studies have been conducted aimed at identifying the immune cells controlling TB and TB/HIV coinfections. While there is a consensus that cellular immune responses play a critical role in disease progression, much more has to be learned in order to understand the relationship. The correlation analysis has revealed a certain degree of relation between viral load and CD4 or CD8 cells in arm A, but they were not statistically significant except CD4/CD8 ratio. Dzherelo does not influence directly HIV replication and it is likely that the observed effect on viral load is mediated via immune cells (CitationMelnik et al 1999).
Surprisingly, in arm B, the viral load was not dependent on changes in CD4 cells although significant inverse correlation was found with CD3 and CD8 cell numbers. However, this arm is characterized by a significant gain in total and CD4 T-lymphocytes but not changes in CD8-bearing population. Thus, we do not know how and which cells are involved in Dzherelo-influenced viral control. It is possible that the viral replication is regulated by a subpopulation of T lymphocytes which cannot be reliably identified by surface markers we have used. The protective immune response also needs to be separated from undesired inflammatory response (CitationAchkar et al 2007). The possibility that CD8 T-cells are involved in upregulation instead of suppression of viral replication is suggested by correlation analysis in arm A. On the other hand, in arm B the inverse relation between differentials of relative CD8 counts and viral load suggests the opposite effect. The full significance of elevated CD3, CD4, CD4/CD8, but unchanged CD8 cells resulting from Dzherelo administration is yet to be understood. It is clear that the understanding of the immune mechanism controlling M. tuberculosis may aid in design of better vaccines and immunotherapies. Dzherelo confers cellular responses suggestive of immunity-restoring action, which perhaps explains the higher success rate of the therapy when it is combined with ATT.
Currently available chemotherapy for the treatment of TB are not perfect, they require multiple anti-TB drugs to be taken in combination for long periods of time (CitationGupta et al 2007; CitationReid et al 2006). This can cause side effects, poor drug adherence, treatment failure, and the emergence of drug resistance with major social and economic consequences, especially in low-income countries. It is agreed that novel immune-based therapies are urgently needed to complement antitubercular drug discovery (CitationKhudzik et al 1998; CitationTomioka 2004; CitationWallis 2005; CitationKaufmann 2006; CitationAchkar et al 2007). We also believe that the immunotherapy is the indispensable part of therapeutic strategies against TB (CitationPylypchuk 2003). Many effective immunomodulators are available against bacteria, protozoa, fungi and viruses (CitationErshov 2003). While clinically effective their mechanism is not well understood in most cases. This drawback should be balanced against therapeutic benefits. Some medicinal herbs were shown to modulate the immune response to TB (CitationTomioka 2004), while others exerted direct antimycobacterial activity (CitationNewton et al 2000). But it is unlikely that Dzherelo is tuberculostatic since it does not affect mycobacterial growth in vitro and diseases or pathogens etiologically unrelated to M. tuberculosis were responsive to the therapy (CitationMelnik et al 1999; CitationPylypchuk 2003).
Our study provides preliminary evidence into the putative mechanism of action of Dzherelo, which has been recommended in Ukraine as an immune adjunct to TB therapy. Further studies are required to develop better understanding of the potential of Dzherelo and to enlarge the arsenal of tuberculosis drugs.
Acknowledgments
We thank all participants who volunteered in this study. The generosity of Ekomed in supplying Dzherelo is appreciated. The enthusiastic support of clinical and technical staff who contributed to this study has been critical to successfully conclude this study. The discussions with other investigators of Dzherelo who shared their insight and provided helpful suggestions have guided our study and we are thankful to all of them. This work was presented at the Keystone Symposia on HIV Pathogenesis and HIV Vaccines, March 27 – Apr 1, 2008, Fairmont Banff Springs, Banff, Alberta, Canada, with support from Bill and Melinda Gates Foundation’s Global Health Travel Award, which is gratefully acknowledged.
References
- AchkarJMCasadevallAGlatman-FreedmanA2007Immunological options for the treatment of tuberculosis: evaluation of novel therapeutic approachesExpert Rev Anti Infect Ther54617417547510
- BekkerLGHaslettPMaartensG2000Thalidomide-induced antigen-specific immune stimulation in patients with human immunodeficiency virus type 1 and tuberculosisJ Infect Dis1819546510720518
- ChkhetianyRPylypchukVArjanovaO2007Comparative effect of an immunomodulator Immunoxel (Dzherelo) when used alone or in combination with antiretroviral therapy in drug-naïve HIV infected individualsInt J Biotechnol926776
- ErshovFI2003Use of immunomodulators in viral infectionsAntibiot Khimioter48273214558416
- GuptaANayakURamMByramjee Jeejeebhoy Medical College-Johns Hopkins University Study Group2007Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005Clin Infect Dis45241917578786
- KalouMSassan-MorokroMAbouyaL2005Changes in HIV RNA viral load, CD4+ T-cell counts, and levels of immune activation markers associated with anti-tuberculosis therapy and cotrimoxazole prophylaxis among HIV-infected tuberculosis patients in Abidjan, Cote d'IvoireJ Med Virol75202815602734
- KaufmannSH2006Tuberculosis: back on the immunologists’ agendaImmunity24351716618591
- KhauadamovaGTAruinovaBKBidaibaevNS2001Special features of the course of tuberculosis in HIV-infected patientsProbl Tuberk534611588958
- KhudzikLBSalinaTLParolinaLE1998Immunotherapy of pulmonary tuberculosisProbl Tuberk623610067345
- KizzaHMRodriguezBQuinones-MateuM2005Persistent replication of human immunodeficiency virus type 1 despite treatment of pulmonary tuberculosis in dually infected subjectsClin Diagn Lab Immunol12129830416275944
- ManasEPulidoFPenaJM2004Impact of tuberculosis on the course of HIV-infected patients with a high initial CD4 lymphocyte countInt J Tuberc Lung Dis8451715141738
- Mayanja-KizzaHJones-LopezEOkweraAUganda-Case Western Research Collaboration2005Immunoadjuvant prednisolone therapy for HIV-associated tuberculosis: a phase 2 clinical trial in UgandaJ Infect Dis1918566515717259
- Melnik VP, Panasyuk OV, Pylypchuk VS, et al. 1999. Deployment of herbal preparations Dzherelo and Svitanok for combination therapy of pulmonary tuberculosis. Medical Institute of Ukrainian Association of People’s Medicine. Information Bulletin of the Ministry of Health. UDK:616.24-002.5-085-038:615.017. Kiev, Ukraine.
- MorrisLMartinDJBredellH2003Human immunodeficiency virus-1 RNA levels and CD4 lymphocyte counts, during treatment for active tuberculosis, in South African patientsJ Infect Dis18719677112792875
- NewtonSMLauCWrightCW2000A review of antimycobacterial natural productsPhytother Res143032210925394
- NikolaevaLGPylypchukVSVolyanskiiYL2008Effect of immunomodulator Dzherelo on CD4+ T-lymphocyte counts and viral load in HIV infected patients receiving anti-retroviral therapyRes J Pharmacol2812
- NikolayevskyyVVBrownTJBazhoraYI2007Molecular epidemiology and prevalence of mutations conferring rifampicin and isoniazid resistance in Mycobacterium tuberculosis strains from the southern UkraineClin Microbiol Infect131293817328724
- PrihodaNDArjanovaOVYurchenkoLV2007Open label trial of adjuvant immunotherapy with Dzherelo, Svitanok and Lizorm, in MDR-TB, XDR-TB and TB/HIV co-infected patients receiving anti-tuberculosis therapy under DOTJ Med Plant Res111722
- PylypchukVS2003Clinical and experimental aspects rationalizing the need for immunotherapy in the treatment of patients with tuberculosisProbl Ecol Med Gen Clin Immunol707584
- ReidAScanoFGetahunH2006Towards universal access to HIV prevention, treatment, care, and support: the role of tuberculosis/HIV collaborationLancet Infect Dis64839516870527
- SerbinaNVLazarevicVFlynnJL2001CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infectionJ Immunol1676991700011739519
- SvistunovaASPineginBVSelitskaiaRP2002The use of immunomodulator likopid in the combined treatment pulmonary tuberculosisProbl Tuberk321512066527
- TomiokaH2004Adjunctive immunotherapy of mycobacterial infectionsCurr Pharm Des10329731215544517
- van der WerfMJYegorovaOBChentsovaN2006Tuberculosis-HIV co-infection in Kiev City, UkraineEmerg Infect Dis12766816704834
- WallisRS2005Reconsidering adjuvant immunotherapy for tuberculosisClin Infect Dis41201815983916
- ZaitzevaSI2006Clinical efficacy of phytopreparation Dzherelo and its influence on the functional status of liver in patients with destructive forms of tuberculosisProbl Ecol Med Gen Clin Immunol71–7213240