Abstract
While a successful HIV vaccine will likely take several more years to become a reality, many anti-retroviral (ARV) drugs are currently available to treat HIV infection, and their efficacious use has improved the quality of life and life expectancy of millions of HIV-infected individuals. A recent addition to these ARVs is a new class of drug that targets the HIV entry process by interfering with the action of the CCR5 coreceptor. The first licensed member of this class is a drug called maraviroc, which is also the first ARV that targets a cellular rather than a viral protein. Several other CCR5 antagonists with varied mechanisms of action are being developed. Key issues with the use of these drugs include determining their potential for use in treatment-naïve versus treatment-experienced patients, the development of sensitive coreceptor phenotyping assays to determine patient eligibility, and finally monitoring the emergence of resistant viruses and their mechanisms of resistance. This review summarizes the preclinical and clinical development of maraviroc as well as studies of HIV resistance to this drug both in vitro and in patients. In addition, a range of diverse CCR5 antagonists currently under development, are also discussed.
Introduction
Twenty-five years after its discovery, the human immunodeficiency virus (HIV) and its ever-burgeoning prevalence continue to represent a growing worldwide public health problem. According to recent estimates, over 33 million people are living with HIV all over the world (CitationWHO/UNAIDS 2007). In addition, acquired immunodeficiency syndrome (AIDS), the disease caused by HIV, is responsible for over 2 million deaths per annum.
The use of drug cocktails combining multiple compounds that comprise highly active anti-retroviral therapy (HAART) has significantly improved the quality-of-life and life expectancy of millions of HIV-infected individuals. The main components of HAART in the past couple decades have been inhibitors of the viral protease and reverse-transcriptase enzymes. However, in most patients HIV eventually develops resistance to all these drugs. The propensity of the virus for acquiring resistance to any given antiviral agent it is faced with, led to the advent of the combination therapies which constitute HAART, with the paradigm of using three anti-retrovirals in combination, being the current standard of care.
In more recent years, in an effort to target other steps in the virus lifecycle and to develop viable treatments for HAART-resistant HIV patients, entry inhibitors have emerged as a new target for anti-retroviral (ARV) therapy. HIV mediates its entry into target cells using the concerted action of the viral Env protein with a cell surface receptor CD4 and a coreceptor (usually chemokine receptors CCR5 or CXCR4). Viruses that use the CCR5 or the CXCR4 coreceptors alone are called R5 or X4 viruses respectively, while those that can use both are referred to as R5X4 viruses. The Env protein exists as a heterotrimer and is comprised of surface gp120 and transmembrane gp41 subunits. Receptor binding is mediated by gp120 and fusion by gp41. The entry process begins with the engagement of the Env trimer on the surface of virions by CD4 on the target cell surface. This is followed by conformational changes that allow coreceptor binding and insertion of the fusion peptide into the target cell membrane. Finally the gp41 protein undergoes dramatic conformational changes, which serve to bring the viral and cellular membranes in close proximity and facilitate membrane fusion.
Entry inhibitors can target the viral entry process, described above, at several steps. These include receptor binding, coreceptor engagement, and membrane fusion. Amongst these candidates the drugs that are farthest along in clinical development include the fusion inhibitors and the CCR5 coreceptor inhibitors.
Enfuvirtide (Fuzeon®/T-20) is a peptide fusion inhibitor that targets a conformational intermediate of the fusion process. While enfuvirtide was the only entry inhibitor on the market until late 2007, the use of this drug has been complicated by its need for twice daily injection, leading to some significant problems with regards to compliance and injection site reactions. Owing to these issues, the use of enfuvirtide has been restricted to treatment-experienced patients who are failing HAART, and are on salvage therapy.
CCR5 antagonists are another new class of entry inhibitors under development. These inhibitors block the Env: CCR5 interaction leading to their antiviral effects. Genetic evidence provided strong biological rationale for targeting CCR5 in the development of new entry inhibitors. A mutation in the CCR5 open reading frame results in the premature truncation and a consequent 32-bp deletion in the protein (CCR5 Δ32). Although this mutation is relatively common in the Caucasian population, with an allele frequency of 15%–20%, it was found to be significantly underrepresented in the HIV-1 infected groups (CitationDean et al 1996; CitationSamson et al 1996), and individuals homozygous for the mutation are only rarely infected with HIV (CitationBiti et al 1997; CitationO’Brien et al 1997; CitationTheodorou et al 1997; CitationMichael et al 1998; CitationGorry et al 2002). In fact, in a group of people at high risk, two individuals that remained uninfected despite repeated exposure were found to be homozygous for the same Δccr5 mutation (CitationLiu et al 1996). Lymphocytes from these individuals are resistant in vitro to R5-using strains but permissive for X4 strains of HIV-1 (CitationPaxton et al 1996). In addition, HIV-1 infected individuals who are heterozygous for the Δccr5 mutation, have around a 2-year delay in their progression to AIDS compared with wildtype controls (CitationDean et al 1996; CitationHuang et al 1996; CitationMichael et al 1997; CitationZimmerman et al 1997). Moreover, both heterozygous as well as homozygous carriers of the CCR5 Δ32 allele were apparently immunocompetent with no obvious abnormalities, suggesting that the absence of CCR5 function might not be harmful and that a CCR5 antagonist should be well tolerated. It should be noted that more recently, an association between lack of CCR5 and an increased susceptibility to West Nile Virus has been reported (CitationGlass et al 2005, Citation2006), although the mechanistic basis of this observation is not understood.
Most recently in October 2007, maraviroc, the first-in-class CCR5 antagonist, was licensed by the FDA for use in treatment-experienced patients. This review summarizes the recent literature on the use of maraviroc in the treatment of HIV infection as well as the future of CCR5 inhibitors.
Importance of coreceptor usage analysis
Although HIV can use one of two coreceptors CCR5 or CXCR4 to mediate entry into target cells, upon transmission the majority of newly infected individuals harbor only R5-using viruses. In fact 80% of ART therapy-naïve patients have only R5-viruses, while 20% have R5X4 and very few (<1%) have X4 viruses (CitationBrumme et al 2005; CitationMoyle et al 2005). Due to conflicting results from different studies, it remains unclear if treated patients (with detectable viremia) maintain similar rates of R5X4 viral prevalence as treatment-naïve patients (CitationMoyle et al 2005; CitationHunt et al 2006). However, in highly treatment-experienced patients the prevalence of R5X4 viruses has been shown to approach that of R5 viruses in at least two studies (CitationMelby et al 2006; CitationWilkin et al 2007). The emergence of X4 using viruses, which usually occurs later in disease, has historically been associated with lowered CD4 cell counts and more rapid progression to disease (CitationKoot et al 1993, Citation1999; CitationShankarappa et al 1999). However, it is not clear whether the emergence of X4 using strains is a cause or an effect of the severe immunodeficiency associated with disease progression to AIDS.
It has been anticipated that the use of CCR5 antagonists can lead to the emergence of CXCR4-using viruses. While de novo coreceptor switching is observed less commonly, the selection of pre-existing X4 viruses in association with the emergence of resistance to CCR5 antagonists has been reported in several studies. This highlights the importance of accurate and ultrasensitive detection of minority X4-using viruses in a patient’s viral quasispecies while determining eligibility for CCR5 antagonist therapy.
Interactions between the HIV Env protein and CD4 lead to the exposure of the coreceptor-binding site, which includes the third variable loop (V3) of gp120 as well as the bridging sheet, a discontinuous epitope that is formed only after CD4 engagement. The bridging sheet interacts with the N-terminus of CCR5 or CXCR4. The V3 loop, on the other hand, mediates interactions with the second extracellular loop (ECL2) of CCR5 or CXCR4 and is the principle determinant of coreceptor specificity. In fact specific mutations affecting the charge in the V3 region have been shown to correlate with coreceptor selectivity. For instance, X4 using viruses usually have higher net positive V3 charge than R5 viruses, which is consistent with the fact that CXCR4 has a lower net positive charge than CCR5. Therefore in principle, env genotypic information should be able to determine the coreceptor usage phenotype.
Several methods have been developed for coreceptor usage prediction based on V3 region sequence. The simplest of these approaches is the 11/25 rule (CitationDe Jong et al 1992; CitationFouchier et al 1992; CitationKorber et al 1993; CitationFouchier et al 1995), which predicts that a virus is X4 using if there are basic amino acids present at positions 11 and 25 of the V3 loop, and R5 using if no basic amino acids present at these positions. While this rule is quite accurate for R5 viruses, it tends to misclassify many X4 using viruses (CitationJensen et al 2003). Other more sophisticated methods for coreceptor usage prediction, including Webcat, WebPSSM, and geno2-pheno[coreceptor], have recently been reviewed in detail elsewhere (CitationSierra et al 2007). However, while improvements are being made in this area, the bottomline is that none of these methods have a high enough sensitivity and/or specificity to accurately and consistently predict the tropism of a given Env from its V3 genotype.
Due to the lack of better genotypic predictors, at the moment phenotypic assays based on cell culture experiments, despite their higher cost and slower turnaround time, appear to be the most reliable predictors of coreceptor tropism. Several such approaches have been developed. These include the Trofile assay (Monogram Biosciences), Tropism Recombinant Test (VIRalliance), HIV Phenoscript assay (BioAlliance Pharma) and deCIPhR (inPheno Molecular Diagnostics) among others. However, the most popular one in use at least in the US appears to be the Trofile assay and recently a more sensitive version of this assay called the enhanced Trofile assay has been developed and is being used for detection of X4-using viruses at low levels (CitationReeves et al 2007).
Maraviroc: preclinical development
Developed by Pfizer Global Research and Development, maraviroc is the first CCR5 antagonist approved by the FDA for use in treatment-experienced patients harboring only R5 viruses. The drug was identified in a high-throughput screen designed to select compounds that prevented the binding of radiolabeled macrophage inflammatory factor (MIP)-1β, an endogenous chemokine, to the CCR5 receptor. The details of the discovery of maraviroc have been reviewed elsewhere (CitationWood and Armour 2005; CitationMeanwell and Kadow 2007). Briefly, lead optimization efforts focused on improving the binding efficiency of the screening hits and reducing their persistent type 1 CYP2D6 inhibition as well as the potent hERG cardiac potassium channel inhibition liabilities. Eventually, after a lengthy optimization process that entailed the synthesis of almost 1000 compounds, maraviroc was developed. Maraviroc is a potent inhibitor of MIP-1β binding to the CCR5 receptor (IC50 = 2 nM) and a potent antiviral agent (EC90 = 1 nM for inhibition of HIVBAL replication in PM1 cells), while its inhibition of the hERG potassium channel is modest (CitationWood and Armour 2005).
Similar to other small molecular CCR5 inhibitors (discussed below), the mechanism of action of maraviroc is one of allosteric modification. Insertion of these small molecules into a cavity located within the transmembrane helices disrupts the geometry of a multi-point interaction between CCR5 and HIV-1 gp120 (CitationDragic et al 2000; CitationTsamis et al 2003; CitationWatson et al 2005; CitationSeibert et al 2006). This multi-point interaction is formed by binding of the second extracellular loop (ECL-2) to elements of the gp120 V3 region and the tyrosine-sulfated N-terminus (Tyr-Nt) of CCR5 binding the more conserved bridging sheet that forms between the C1, C2, and C4 domains upon CD4 binding (CitationCormier and Dragic 2002; CitationHuang et al 2007).
In vitro resistance to maraviroc
Antiretroviral drug resistance is a major hurdle in the success of long-term HIV therapy. HIV has a propensity for acquiring mutations and developing resistance to antiviral drugs owing to its high replication rate coupled with the low fidelity and lack of RT proof-reading resulting in a high error rate. Resistance to entry inhibitors has been a particularly difficult problem since these drugs target the env gene, which is the most variable of all the HIV genes. Specifically, this diversity in env can lead to variable baseline susceptibilities and the pre-existence of resistance mutations in patients naïve to these drugs.
For CCR5 inhibitors such as maraviroc, several possible mechanisms of resistance can be expected (). A coreceptor-switching event could occur with R5-using viruses switching to using CXCR4 or an alternative coreceptor, or the emergence of pre-existing X4 viruses. Alternatively, viruses could acquire the ability to bind and enter using a drug-bound coreceptor. Resistance to CCR5 inhibitors could also result from viruses that bind coreceptor with higher affinity (and can therefore compete out bound drug), or are able to enter by scavenging low levels of coreceptor either due to higher affinity or a greater proclivity for Env protein triggering.
Figure 1 Potential mechanisms of resistance of HIV to CCR5 antagonists. HIV can become resistant to CCR5 inhibitors in a number of ways. The virus can adapt to scavenge low levels of unbound coreceptors more efficiently either by binding coreceptors with higher affinity or triggering fusion more quickly (1). HIV could also become resistant by competing off drug from coreceptors (2) or by using a drug-bound conformation of the coreceptor (3). Alternatively, the virus could switch to using CXCR4, either via a de novo switch or due to emergence of a pre-existing X4 virus (4), or it could switch to using an alternative coreceptor (5).
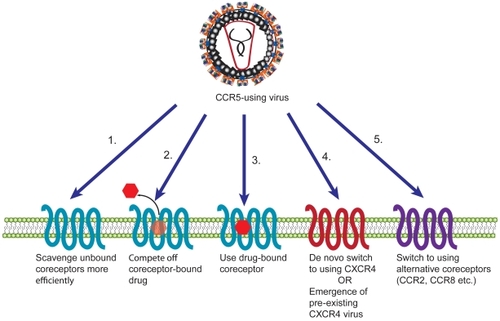
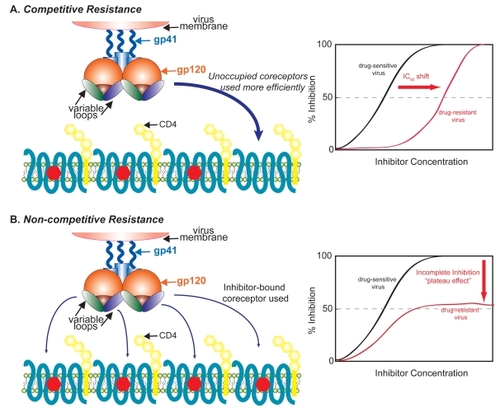
An in vitro study has shown that maraviroc does not lead to a de novo switch to X4-using viruses during serial passaging of laboratory-adapted and three of the six CCR5-tropic primary isolates studied (CitationWestby et al 2007). However, in the case of one virus (SF162) the emergence of pre-existing X4-using viruses was reported in this study. For two of the passaged primary isolates, maraviroc resistance arose with the mutant Envs acquiring an ability to use a drug-bound form of CCR5. This leads to a characteristic “plateau” of maximal inhibition in a dose-response curve; in other words, beyond a certain maraviroc concentration, increasing drug levels did not inhibit virus infection. These plateaus in dose-response have been observed for viruses resistant to other coreceptor antagonists, suggesting that the recognition of an altered conformation of the coreceptor is a common mechanism for escape from non-competitive inhibitors of entry (). This is in contrast to a competitive mode of resistance that typically leads to clear IC50shifts in dose-response curves ().
Figure 2 Competitive and non-competitive mechanisms of resistance to coreceptor inhibitors. In a competitive mechanism of resistance (A), HIV acquires the ability to bind unoccupied coreceptors more efficiently, and in this case there is an IC50 shift in a dose response curve relative to a sensitive virus. Alternatively, in a non-competitive mechanism of resistance (B), the virus adapts to enter using a drug-bound conformation of the coreceptor, resulting in a dose-response curve where no further inhibition of virus entry occurs beyond a certain drug concentration (“plateau effect”) and no shift in IC50 is observed.
Genotypic changes in resistant viruses are a common hallmark of all classes of anti-retrovirals. Typically mutations accumulate in a step-wise manner in the gene targeted by a drug and either confer resistance or compensate for impaired activity resulting from resistance mutations at the drug target site. For several antiviral agents, the viral genotype is a good measure of phenotype, or the sensitivity of a virus to any given drug. Charts containing mutations that are known to impart resistance to particular drugs are available to physicians, who can create a tailored regimen for each patient based on their viral genotype. In this way molecular information can be used to guide clinical decisions.
In the case of coreceptor antagonists, the V3 loop of gp120 is the expected site for resistance mutations since this region of the protein is important in mediating the specificity of Env-coreceptor interactions. However, while genotypic changes are found in the V3 region of some coreceptor inhibitor-resistant viruses, in other cases mutations are also observed in other regions of both gp120 and gp41 (CitationTrkola et al 2002; CitationMarozsan et al 2005; CitationBaba et al 2007; CitationPugach et al 2007; CitationWestby et al 2007; CitationOgert et al 2008). Moreover, for each parental strain passaged in the presence of coreceptor inhibitors, different resistance-associated changes are observed. Therefore the use of viral env genotype as a predictor of resistance to coreceptor inhibitors such as maraviroc, might be more complicated than for other entry inhibitors such as ENF, where resistance mutations usually map to the HR1 region of gp41 which is the targeted binding site of ENF.
Maraviroc use in the clinic
Phase 1 and 2 clinical trials
Several phase 1/2a clinical studies have been conducted with maraviroc, which all showed significant reductions in HIV viral load in HIV-infected individuals (CitationFätkenheuer et al 2005). In a study that reviewed data from 5 multiple-dose, phase 1/2a double-blinded, placebo-controlled studies of maraviroc, the drug appeared to be well-tolerated in 10-day monotherapy (CitationFätkenheuer et al 2004). The most adverse effects, which included nausea, rhinitis, dizziness, and headache, were moderate. Some subjects on maraviroc showed elevations in levels of transaminases or occasional elevation in creatinine; however, these effects were not considered to be severe. Postural hypotension was the only dose-limiting complication that occurred at higher rates than placebo in patients receiving a maraviroc dose of 600 mg or higher. Encouragingly, 10-day monotherapy studies with maraviroc resulted in mean viral load reductions of 1.6 log10 copies/mL with a dose of 300 mg once daily and 1.84 log10 copies/mL with a dose of 300 mg twice daily. Based on these results, phase 2b/3 studies of maraviroc were initiated in treatment-experienced and treatment-naïve patients.
Maraviroc use in treatment-experienced patients
MOTIVATE-1 and MOTIVATE-2 were randomized, double-blind placebo-controlled phase 2b/3 clinical trials assessing the safety and efficiacy of maraviroc in heavily-treatment experienced patients with triple-class ARV resistance. MOTIVATE-1 included 601 participants from the US and Canada, whereas MOTIVATE-2 included 475 subjects from Europe, Australia and USA. In both studies, subjects were randomly assigned to receive maraviroc at doses of 300 mg once-daily (qd) or twice-daily (bid), or else placebo, in combination with an optimized background therapy (OBT) regimen.
Results from 24 weeks of follow-up in both studies indicated significantly better efficacy in patients treated with maraviroc (qd or bid) + OBT versus placebo + OBT, with the virologic response rates being about twice as high in the maraviroc arms compared to the placebo arm (CitationLalezari et al 2007; CitationNelson et al 2007). Specifically, mean decreases in viral load from baseline were 1.95–1.97 log10 copies/mL in the maraviroc bid arms, 1.82–1.95 log10 copies/mL in the maraviroc qd arms, and 0.93–1.03 log10 copies/mL in the placebo arms. 45.6%–48.5% of patients in the maraviroc bid arms, and 40.8%–42.2% in the qd arms achieved viral loads below 50 copies/mL, compared with 20.9%–24.6% in the placebo arms. Similar trends were observed for viral loads below 400 copies/mL, indicating that a higher proportion of patients achieved viral loads <50 and <400 copies/mL when maraviroc was administered twice instead of once daily. CD4 cell counts increased from baseline by 102–111 and 107–112 cells/mm3 in the bid and qd arms, respectively, compared with 52–64 cells/mm3 in the placebo arms. While fewer patients in the maraviroc arms experienced treatment failure compared with placebo, more patients in the maraviroc arm experienced a shift in HIV coreceptor usage from CCR5-tropic to CXCR4-tropic or dual/mixed tropism. Adverse event profiles were similar in both maraviroc arms and the placebo arm. Based on these 24-week results, in August 2007 the Food and Drug Administration (FDA) approved maraviroc for use in treatment-experienced patients.
More recently, data from a 48-week combined analysis of the MOTIVATE 1 and 2 studies were presented (CitationHardy et al 2008). As seen in the 24-week analysis, maraviroc produced greater virological response compared with placebo. CD4 cell benefits were greater in the maraviroc arms (124 cells/mm3 bid, 116 cells/mm3 qd) compared with the placebo arm (61 cells/mm3). The pooled analysis revealed no new or unique safety findings beyond the 24-week analysis. Discontinuations due to adverse events, serious adverse events, and laboratory abnormalities occurred with similar frequency in the maraviroc and placebo arms. Therefore, treatment with maraviroc appears to provide sustained ARV efficacy and tolerability in treatment-experienced patients.
Maraviroc use in treatment-naïve patients
Maraviroc in Treatment-naïve Patients (MERIT) is an ongoing phase 3 clinical trial designed to compare the efficacy and safety of maraviroc 300 mg twice daily versus efavirenz 600 mg once daily, each administered in combination with fixed-dose zidovudine/lamivudine (Combivir®). The study is continuing for 96 weeks; however, preliminary analysis from 48-week data were presented recently (CitationSaag et al 2007; CitationHeera et al 2008). Twice-daily maraviroc was non-inferior to efavirenz in patients with vRNA <400 copies/mL (70.6% vs 73.1%) but not in the <50 copies/mL analysis (65.3% vs 69.3%). In subjects with high viral loads (vRNA > 100,000 copies/mL) the difference was even more pronounced in favor of efavirenz, with the proportion of subjects with <50 copies/mL on efavirenz 66.6% and on maraviroc 59.6%. However, maraviroc had a superior safety profile and a more benign lipid profile than efavirenz and an overall higher CD4 benefit.
In another study aimed at analyzing the virological correlates of treatment failure in the MERIT trial, it was found that of 721 patients, 24 (3.3%) changed from R5 at screening to dual/mixed at baseline (CitationHeera et al 2008). The virologic response in this group of patients was lower both for the efavirenz as well as maraviroc groups, with the proportion of subjects with <50 copies/mL being only 54.6% and 7.1%, respectively. Moreover, X4 using viruses were detected at failure in 10/32 (31.3%) maraviroc patients with R5 virus at baseline. Therefore the conclusions from this study were that the presence of X4 using viruses at baseline is an important predictor of virologic failure on maraviroc. Also, similar to treatment-experienced patients, failure due to the emergence of X4-using virus is an important, albeit infrequently observed, mechanism associated with maraviroc failure.
Future of CCR5 inhibition
Besides maraviroc, several promising strategies for CCR5 blockade are currently being employed in the development of CCR5 antagonists. These include other small-molecule inhibitors, monoclonal antibodies, and genetically derived molecules. Modified chemokine derivates (analogs of the CCR5 ligand RANTES) are also being developed for potential use as microbicides (CitationKish-Catalone et al 2006; CitationLusso 2006). In general, CCR5 antagonists can be classified into three broad categories (). First, small molecule inhibitors cause allosteric modifications in CCR5, leading to the induction or stabilization of a conformation of CCR5 that can not be bound by gp120, thereby preventing virus entry. Second, monoclonal antibodies sterically block access of virus to CCR5. Finally, genetically derived molecules (zinc finger nucleases) as well as chemokine analogs induce the intracellular trapping of CCR5 to the endoplasmic reticulum, preventing CCR5 expression on the surface of lymphocytes.
Figure 3 Mechanisms of action of different types of CCR5 inhibitors. Several CCR5 antagonists with diverse modes of action are being developed. In general, there are three categories of these inhibitors: those that allosterically modify CCR5 (such as small molecules), those that sterically block viral access to CCR5 (such as monoclonal antibodies) and finally those that lead to decreased expression of CCR5 on the cell surface (eg, RANTES analogs and genetic therapies such as ZFNs).
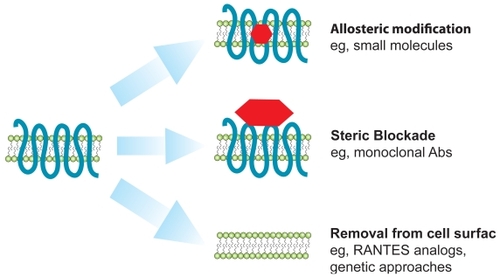
Different types of CCR5 inhibitors have their advantages and disadvantages. For instance, small-molecule inhibitors are orally bioavailable, while monoclonal antibodies need to be injected intravenously. On the other hand, monoclonal antibodies usually have prolonged serum half-lives allowing for relatively infrequent administrations, whereas orally administered small molecules need more frequent dosing to maintain adequate concentrations in the face of serum protein binding and active catabolic mechanisms. The therapies discussed below are not intended as an exhaustive summary of all CCR5 inhibitors under development, but rather a sampling of the same to give the reader a flavor for the diversity of potential future options in this drug class.
Allosteric modulation: small molecule inhibitors
Vicriviroc
Vicriviroc is the second most advanced agent in the CCR5 antagonist class of ARV drugs. Forty-eight week data from the Phase II VICTOR-E1 study (Vicriviroc in Combination Treatment with Optimized ART Regimen in Experienced Subjects) that examined the safety and efficacy of vicriviroc (30 mg or 20 mg qd) in combination with an optimized ritonavir-boosted, protease inhibitor-containing ART regimen, were recently presented (CitationZingman et al 2008). Potent and sustained viral suppression was achieved in the vicriviroc arms with 1.77 and 1.75 log10 copies/mL mean decreases from baseline viral load compared with 0.79 log10 copies/mL in the control group. Significantly more patients (56%) who added vicriviroc 30 mg once daily to a new OBT had fully suppressed HIV-RNA down to <50 copies/mL compared with patients on OBT alone (14%). Also mean increases from baseline in CD4 cell counts in the vicriviroc groups were + 102 and + 134 cells/mm3, respectively, compared with + 65 in the control group. Moreover, there were no significant differences in the safety profile between the vicriviroc and control arms with respect to liver toxicity, opportunistic infections, malignancies or other conditions. Based on the VICTOR-E1 results, 2 large global phase III trials (VICTOR-E3 and VICTOR-E4) are currently enrolling approximately 375 treatment-experienced subjects (at more than 160 sites worldwide) who will receive 30 mg vicriviroc qd in combination with OBT or OBT alone.
Second-generation maraviroc
Following the successful licensing of maraviroc, Pfizer is developing a second-generation CCR5 inhibitor. Their lead candidate PF-232798 is an imidazopiperidine CCR5 inhibitor that was identified in a medicinal chemistry synthetic campaign guided by a biological screening cascade after the identification of the triazole inhibitor maraviroc (CitationDorr et al 2008). PF-232798 is a potent oral CCR5 antagonist with a primary and selectivity/safety pharmacological profile similar to maraviroc. It also has broad-spectrum anti-HIV-1 activity similar to maraviroc. PF-232798 is also active against lab-generated maraviroc-resistant CCR5 tropic HIV-1. The drug binds to the same pocket as maraviroc within the transmembrane region of CCR5, but also shows interactions with the ECL2 hinge region. PF-232798 was well tolerated in normal volunteers and exhibited more favorable pharmacokinetic profile than maraviroc, highlighting its potential for once-daily dosing.
Steric blockade: monoclonal antibodies
Several antibody-based agents targeting the HIV entry process are being developed. For example, TNX-355 targets CD4, PRO 542 is aimed at gp120, and PRO 140 and HGS004 bind CCR5.
PRO 140
PRO 140 is a humanized monoclonal antibody that binds to CCR5 without altering its structure or normal function; it sterically blocks binding of the coreceptor to virus. Interestingly, viral mutants resistant to small-molecule inhibitors (derived by in vitro passage), appear to remain susceptible to inhibition by PRO 140 (CitationKuhmann et al 2004; CitationMarozsan et al 2005). PRO 140 can also act synergistically with maraviroc and vicriviroc to block membrane fusion mediated by a primary R5 virus (CitationMurga et al 2006).
PRO 14 was humanized from the original mouse monoclonal antibody PA14 and entered phase 1b clinical trials in 2007. In this single-dose study, PRO 140 was well tolerated at all doses and produced significant reductions in viral load and suppressed viral replication for 2–3 weeks (Progenics). PRO 140 has been granted Fast Track designation by the FDA.
HGS004 (CCR5mAb004)
HGS004 is a fully human IgG4 monoclonal antibody against CCR5 with robust in vitro activity against several HIV-1 isolates (CitationLalezari et al 2008). A phase I clinical trial examined the safety and preliminary antiviral activity of this antibody. This was a single-blind, placebo-controlled study that enrolled 63 subjects randomized into 5 dosage cohorts (0.4, 2, 8, 20, and 40 mg/kg) who received a single intravenous dose of HGS004 or placebo. HGS004 was well tolerated even at the highest dose and high-levels of receptor occupancy were observed for up to 28 days in the higher dose cohorts. On day 14, 54% of subjects in the 8-, 20- and 40-mg/kg groups had plasma HIV-1 RNA reductions of > 1 log10 copies/mL. In the 40 mg/kg group, 4 of 10 subjects had a > 1 log10 copies/mL plasma RNA reduction at day 28.
Removal of CCR5 from cell surfaces
Chemokine analogs
The natural ligands of CCR5 have been shown to possess anti-HIV activity in vitro (CitationCocchi et al 1995). Moreover, increased expression of the CCR5 ligand, MIP-1a/CCL3, in humans (as a result of gene duplication) provides protection from HIV acquisition (CitationGonzalez et al 2005). Chemokine analogs can be engineered to enhance their anti-HIV activity (reviewed in (CitationHartley and Offord 2005)). Their mechanism of action involves binding of the chemokine receptor (eg. CCR5), followed by agonist-induced receptor internalization, leading to intracellular sequestration (). While N-terminally modified chemokine analogs such as AOP-RANTES (CitationSimmons et al 1997) and PSC-RANTES (CitationHartley et al 2004) have shown prolonged receptor sequestration and potent anti-HIV activity, these protein molecules are not orally bioavailable and are likely to have poor pharmacokinetics after injection due to aggregation on cell surface proteoglycans. However, these factors do not preclude the development of these chemokine analogs as components of topical microbicides in the prevention of HIV transmission (CitationMoore 2005).
Genetic therapies
Several anti-HIV gene therapy approaches, that target HIV genes or their products, have also been investigated in the past several years (reviewed in [CitationRossi et al 2007]). These genetic-based approaches, such as intrakines (CitationYang et al 1997), degrakines (CitationCoffield et al 2003), and zinc-finger nucleases (ZFNs), aim to create phenotypic knockouts of CCR5.
ZFN proteins can be engineered to bind with a high degree of specificity to particular sequence motifs in the genome, and the associated nuclease cleaves the bound DNA (CitationMani et al 2005). Repair of these double-stranded breaks is associated with the introduction of high frequency deletions and insertions at the cleavage site. CCR5-ZFN proteins have been developed and their administration to T-cell lines and primary human CD4+ T-cells resulted in a population of CCR5-modified HIV-resistant cells (CitationPerez et al 2008). ZFN-modified T-cell lines expanded in culture in the presence of HIV, and comprised the majority of cells in the population after 70 days. Genetic disruption of CCR5 imparted robust, stable, and heritable protection against HIV-1 in a NOG/SCID mouse model of infection (CitationPerez et al 2008). The eventual clinical goal is to use these CCR5-ZFNs to perform ex vivo gene therapy on T cells from HIV-infected individuals and generate a reservoir of permanently HIV-resistant T cells that can be engrafted back into patients by reinfusion. Therefore, the fact that HIV-infected mice engrafted with ZFN-modified CD4+ T cells had lower viral loads and higher CD4+ T cell count than mice engrafted with wild-type CD4+ T cells, in this study, holds promise for the potential to reconstitute immune function in individuals with HIV/AIDS by maintenance of an HIV-resistant CD4+ T-cell population.
Conclusion
Maraviroc is a valuable addition to the arsenal of ARV drugs available in the combat against HIV and AIDS. It heralds a new class of ARV drugs, namely CCR5 inhibitors, with several other second-generation candidates following in the clinical pipeline. While they comprise a promising new approach in controlling HIV infection, CCR5 inhibitors come with their share of problems and important caveats that should be kept in mind for ensuring their successful use. One of the greatest hurdles in the success of this class of drugs is the need to phenotype the patients’ viral quasispecies and sensitive detection of very low level CXCR4 usage, to prevent treatment of ineligible patients. In the coming years, more sophisticated coreceptor usage prediction programs as well as phenotypic assays with higher sensitivities are required to overcome this problem.
Resistance is another hurdle that inevitably rears its ugly head in the use of any ARV drug. Coreceptor inhibitors are no exception to this rule. One of the most feared predicted mechanism of resistance to CCR5 inhibitors was a de novo switch to X4-using viruses, which are associated with late-stage disease. However, results from most resistance studies, thus far, suggest that this switch to CXCR4 usage is in fact not observed frequently and instead viruses usually acquire resistance to these drugs by using a drug-bound conformation of the CCR5 coreceptor.
Another key issue in the optimal administration of CCR5 antagonists, such as maraviroc, is which patient should receive these drugs: treatment-experienced or treatment-naïve patients? On the one hand, the use of new ARV drugs finds favor with physicians in a salvage regimen administered to multi-class resistant treatment-experienced patients. In fact this is the current recommendation for the use of maraviroc. However, in the case of CCR5 inhibitors the percentage of patients harboring R5 only using viruses is much higher in treatment-naïve patients (about 80% in naïve versus 48%–60% in experienced patients), arguing for a more favorable target population in these rather than more experienced subjects. Moreover, maraviroc’s new mechanism of action may result in a reduction in the number of latently infected cells, and add to its promise as a candidate for first line therapy. While there is no doubt in the benefit that treatment-experienced patients (with R5 viruses) can derive from using maraviroc (MOTIVATE trial results), recent clinical data from treatment-naïve patients (MERIT trial) can potentially revitalize interest in CCR5 antagonists as a treatment option for treatment-naïve patients especially since despite its lack of non-inferiority in patients reaching viral loads below 50 copies/mL, maraviroc had a superior safety profile and greater CD4 benefit compared to efavirenz.
Since CCR5 antagonists such as maraviroc target a cellular instead of viral protein (as do all other ARVs), another point to consider in the prescription of these drugs is whether they should be used in addition to triple therapy or as a replacement for one drug of triple therapy. Further studies into the different combinations of maraviroc with existing ARV regimens are required to address this question.
In conclusion, with the advent of maraviroc, an exciting new class of drugs has entered the arena of ARV therapy. Several key issues and questions regarding its optimal use and application remain unanswered, and should be addressed in upcoming clinical trials. Moreover, in the years to come, we can look forward to several other CCR5 antagonists with diverse mechanisms of action becoming available to patients.
Disclosures
The author reports no conflicts of interest.
References
- BabaMMiyakeHWangX2007Isolation and characterization of human immunodeficiency virus type 1 resistant to the small-molecule CCR5 antagonist TAK-652Antimicrob Agents Chemother517071517116673
- BitiRFfrenchRYoungJ1997HIV-1 infection in an individual homozygous for the CCR5 deletion alleleNat Med325239055842
- BrummeZLGoodrichJMayerHB2005Molecular and clinical epidemiology of CXCR4-using HIV-1 in a large population of antiretroviral-naive individualsJ Infect Dis1924667415995960
- CocchiFDeVicoALGarzino-DemoA1995Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cellsScience270181158525373
- CoffieldVMJiangQSuL2003A genetic approach to inactivating chemokine receptors using a modified viral proteinNat Biotechnol211321714555957
- CormierEGDragicT2002The crown and stem of the V3 loop play distinct roles in human immunodeficiency virus type 1 envelope glycoprotein interactions with the CCR5 coreceptorJ Virol768953712163614
- De JongJJDe RondeAKeulenW1992Minimal requirements for the human immunodeficiency virus type 1 V3 domain to support the syncytium-inducing phenotype: analysis by single amino acid substitutionJ Virol666777801404617
- DeanMCarringtonMWinklerC1996Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE StudyScience2731856628791590
- DorrPWestbyMMcFadyenL2008PF-232798, a second generation oral CCR5 antagonist [abstract]15th Conference on Retroviruses and Opportunistic InfectionsBoston, MA Abstract 737
- DragicTTrkolaAThompsonDA2000A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5Proc Natl Acad Sci USA9756394410779565
- FätkenheuerGPozniakAJohnsonM2004Evaluation of dosing frequency and food effect on viral load reduction during short-term monotherapy with UK-427, 857 a novel CCR5 antagonist [abstract]The XV International AIDS ConferenceBangkok, Thailand Abstract no. TuPeB4489
- FätkenheuerGPozniakALJohnsonMA2005Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1Nat Med111170216205738
- FouchierRABrouwerMBroersenSM1995Simple determination of human immunodeficiency virus type 1 syncytium-inducing V3 genotype by PCRJ Clin Microbiol33906117790458
- FouchierRAGroeninkMKootstraNA1992Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 moleculeJ Virol66318371560543
- GlassWGLimJKCholeraR2005Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infectionJ Exp Med20210879816230476
- GlassWGMcDermottDHLimJK2006CCR5 deficiency increases risk of symptomatic West Nile virus infectionJ Exp Med203354016418398
- GonzalezEKulkarniHBolivarH2005The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibilityScience30714344015637236
- GorryPRZhangCWuS2002Persistence of dual-tropic HIV-1 in an individual homozygous for the CCR5 Delta 32 alleleLancet3591832412044382
- HardyDReynesJKonourinaI2008Efficacy and safety of maraviroc plus optimized background therapy in treatment-experienced patients infected with CCR5-Tropic HIV-1: 48-week combined analysis of the MOTIVATE studies [abstract]15th Conference on Retroviruses and Opportunistic InfectionsBoston, MA Abstract 792
- HartleyOGaertnerHWilkenJ2004Medicinal chemistry applied to a synthetic protein: development of highly potent HIV entry inhibitorsProc Natl Acad Sci USA10116460515545608
- HartleyOOffordRE2005Engineering chemokines to develop optimized HIV inhibitorsCurr Protein Pept Sci62071915974948
- HeeraJSaagMSIveP2008Virological correlates associated with treatment failure at week 48 in the phase 3 study of maraviroc in treatment-naive patients [abstract]15th Conference on Retroviruses and Opportunistic InfectionsBoston, MA Abstract 40LB
- HuangCCLamSNAcharyaP2007Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4Science3171930417901336
- HuangYPaxtonWAWolinskySM1996The role of a mutant CCR5 allele in HIV-1 transmission and disease progressionNat Med2124038898752
- HuntPWHarriganPRHuangW2006Prevalence of CXCR4 tropism among antiretroviral-treated HIV-1-infected patients with detectable viremiaJ Infect Dis1949263016960780
- JensenMALiFSvan’t WoutAB2003Improved coreceptor usage prediction and genotypic monitoring of R5-to-X4 transition by motif analysis of human immunodeficiency virus type 1 env V3 loop sequencesJ Virol77133768814645592
- Kish-CataloneTMLuWGalloRC2006Preclinical evaluation of synthetic -2 RANTES as a candidate vaginal microbicide to target CCR5Antimicrob Agents Chemother50149750916569870
- KootMKeetIPVosAH1993Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDSAnn Intern Med11868188096374
- KootMvan LeeuwenRde GoedeRE1999Conversion rate towards a syncytium-inducing (SI) phenotype during different stages of human immunodeficiency virus type 1 infection and prognostic value of SI phenotype for survival after AIDS diagnosisJ Infect Dis17925489841850
- KorberBTFarberRMWolpertDH1993Covariation of mutations in the V3 loop of human immunodeficiency virus type 1 envelope protein: an information theoretic analysisProc Natl Acad Sci USA907176808346232
- KuhmannSEPugachPKunstmanKJ2004Genetic and phenotypic analyses of human immunodeficiency virus type 1 escape from a small-molecule CCR5 inhibitorJ Virol78279080714990699
- LalezariJYadavalliGKParaM2008Safety, pharmacokinetics, and antiviral activity of HGS004, a novel fully human IgG4 monoclonal antibody against CCR5, in HIV-1-infected patientsJ Infect Dis197721718266604
- LalezariJPGoodrichJDeJesusE2007Efficacy and safety of maraviroc plus optimized background therapy in viremic ART-experienced patients infected with CCR5-tropic HIV-1: 24 week results from a phase 2b/3 study in the US and Canada [abstract]14th Conference on Retroviruses and Opportunistic InfectionsLos Angeles Abstract 104bLB
- LiuRPaxtonWAChoeS1996Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infectionCell86367778756719
- LussoP2006HIV and the chemokine system: 10 years laterEmbo J254475616437164
- ManiMKandavelouKDyFJ2005Design, engineering, and characterization of zinc finger nucleasesBiochem Biophys Res Commun3354475716084494
- MarozsanAJKuhmannSEMorganT2005Generation and properties of a human immunodeficiency virus type 1 isolate resistant to the small molecule CCR5 inhibitor, SCH-417690 (SCH-D)Virology3381829915935415
- MeanwellNAKadowJF2007Maraviroc, a chemokine CCR5 receptor antagonist for the treatment of HIV infection and AIDSCurr Opin Investig Drugs866981
- MelbyTDespiritoMDemasiR2006HIV-1 coreceptor use in triple-class treatment-experienced patients: baseline prevalence, correlates, and relationship to enfuvirtide responseJ Infect Dis1942384616779731
- MichaelNLChangGLouieLG1997The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progressionNat Med3338409055864
- MichaelNLNelsonJAKewalRamaniVN1998Exclusive and persistent use of the entry coreceptor CXCR4 by human immunodeficiency virus type 1 from a subject homozygous for CCR5 delta 32J Virol72604079621067
- MooreJP2005Topical microbicides become topicalN Engl J Med35229830015659734
- MoyleGJWildfireAMandaliaS2005Epidemiology and predictive factors for chemokine receptor use in HIV-1 infectionJ Infect Dis1918667215717260
- MurgaJDFrantiMPevearDC2006Potent antiviral synergy between monoclonal antibody and small-molecule CCR5 inhibitors of human immunodeficiency virus type 1Antimicrob Agents Chemother5032899617005807
- NelsonMFatkenheuerGKonourinaI2007Efficacy and safety of maraviroc plus optimized background therapy in viremic ART-experienced patients infected with CCR5-tropic HIV-1 in Europe, Australia and North America: 24 week results [abstract]14th Conference on Retroviruses and Opportunistic InfectionsLos Angeles Abstract 104aLB
- O’BrienTRWinklerCDeanM1997HIV-1 infection in a man homozygous for CCR5 delta 32Lancet3491219
- OgertRAWojcikLBuontempoC2008Mapping resistance to the CCR5 co-receptor antagonist vicriviroc using heterologous chimeric HIV-1 envelope genes reveals key determinants in the C2-V5 domain of gp120Virology3733879918190945
- PaxtonWAMartinSRTseD1996Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposureNat Med241278597950
- PerezEWangJMillerJ2008Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleasesNat Biotechnol268081618587387
- Progenics PRO 140Accessed 17 July 2008 URL: http://www.progenics.com/pro140.cfm
- PugachPMarozsanAJKetasTJ2007HIV-1 clones resistant to a small molecule CCR5 inhibitor use the inhibitor-bound form of CCR5 for entryVirology3612122817166540
- ReevesJDHanDHuntP2007Enhancements to the Trofile HIV coreceptor tropism assay enable improved detection of CXCR4-using subpopulations and earlier detection of CXCR4-using viruses in sequential patient samples [abstract]3rd International Workshop on Targeting HIV EntryWashington, DC Abstract 11
- RossiJJJuneCHKohnDB2007Genetic therapies against HIVNat Biotechnol2514445418066041
- SaagMSIvePHeeraJ2007A multicenter, randomized, double-blind, comparative trial of a novel CCR5 antagonist, maraviroc versus efavirenz, both in combination with Combivir (zidovudine [ZDV]/lamivudine [3TC]), for the treatment of antiretroviral naive patients infected with R5 HIV 1: Week 48 results of the MERIT study4th International AIDS Society ConferenceSydney, Australia
- SamsonMLibertFDoranzBJ1996Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor geneNature38272258751444
- SeibertCYingWGavrilovS2006Interaction of small molecule inhibitors of HIV-1 entry with CCR5Virology349415416494916
- ShankarappaRMargolickJBGangeSJ1999Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infectionJ Virol731048950210559367
- SierraSKaiserRThielenA2007Genotypic coreceptor analysisEur J Med Res124536217933727
- SimmonsGClaphamPRPicardL1997Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonistScience27627699092481
- TheodorouIMeyerLMagierowskaM1997HIV-1 infection in an individual homozygous for CCR5 delta 32. Seroco Study GroupLancet3491219209130946
- TrkolaAKuhmannSEStrizkiJM2002HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 useProc Natl Acad Sci USA9939540011782552
- TsamisFGavrilovSKajumoF2003Analysis of the mechanism by which the small-molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entryJ Virol775201812692222
- WatsonCJenkinsonSKazmierskiW2005The CCR5 receptor-based mechanism of action of 873140, a potent allosteric noncompetitive HIV entry inhibitorMol Pharmacol6712688215644495
- WestbyMSmith-BurchnellCMoriJ2007Reduced maximal inhibition in phenotypic susceptibility assays indicates that viral strains resistant to the CCR5 antagonist maraviroc utilize inhibitor-bound receptor for entryJ Virol8123597117182681
- WHO/UNAIDS2007AIDS epidemic updateAccessed date: 17 July 2008 URL: http://www.unaids.org:80/en/KnowledgeCentre/HIVData/Epi-Update/EpiUpdArchive/2007/
- WilkinTJSuZKuritzkesDR2007HIV type 1 chemokine coreceptor use among antiretroviral-experienced patients screened for a clinical trial of a CCR5 inhibitor: AIDS Clinical Trial Group A5211Clin Infect Dis44591517243065
- WoodAArmourD2005The discovery of the CCR5 receptor antagonist, UK-427,857, a new agent for the treatment of HIV infection and AIDSProg Med Chem432397115850827
- YangAGBaiXHuangXF1997Phenotypic knockout of HIV type 1 chemokine coreceptor CCR-5 by intrakines as potential therapeutic approach for HIV-1 infectionProc Natl Acad Sci USA9411567729326650
- ZimmermanPABuckler-WhiteAAlkhatibG1997Inherited resistance to HIV-1 conferred by an inactivating mutation in CC chemokine receptor 5: studies in populations with contrasting clinical phenotypes, defined racial background, and quantified riskMol Med323369132277
- ZingmanBSuleimanJDeJesusE2008Vicriviroc, a next generation CCCR5 antagonist, exhibits potent, sustained suppression of viral replication in treatment-experienced adults: VICTOR-E1 48-week results [abstract]15th Conference on Retroviruses and Opportunistic InfectionsBoston, MA Abstract 39LB