Abstract
Aim: The aim of this study was to determine the predictive value of clinical presentations on functional ambulation following thrombolytic therapy. Materials & methods: Logistic regression analysis was used to determine associations between functional ambulation and thrombolytic therapy. Results & conclusion: In the results, Hispanic ethnicity (odds ratio (OR): 2.808; p = 0.034; 95% CI: 1.08–7.30), high National Institute of Health Stroke Scale (NIHSS) (OR: 1.112; p ≤ 0.001; 95% CI: 1.06–1.17), weakness/paresis (OR: 1.796; p = 0.005; 95% CI: 1.19–2.71), Broca’s aphasia (OR: 1.571; p = 0.003; 95% CI = 1.16–2.12) and antihypertensive medication (OR: 1.530; p = 0.034; 95% CI: 1.03–2.26) were associated with an improved ambulation in patients without thrombolytic therapy. In thrombolytic treated patients, Broca’s aphasia was associated with improved functional outcome.
Predictive models for the risk of stroke are essential tools to guide evaluations and develop interventions in the event of an ischemic stroke [Citation1]. Since the heterogeneity of stroke manifestations provide a source of variability that should be amenable to clinical and motor parsing, the accurate prediction of motor recovery following an ischemic stroke represents a strong tool to evaluate the functional outcome. Motor recovery would represent a suitable cut-off assessment based on direct evaluation of ambulation status before and after treatment and recovery.
Primarily to assess recovery following thrombolytic therapy, recent guidelines from the American Heart Association (AHA; TX, USA) suggested the importance of considering outcome variables that capture the wide variation in treatment outcomes [Citation2]. Such a variable is expected to reflect the wide variety of patients’ baseline functional status pre- and poststroke, as well as provide useful information regarding the state of a patient’s recovery [Citation2,Citation3]. Since stroke is a major reason for physical immobility [Citation4], a suitable cut-off assessment based on direct evaluation of daily motoric recovery may help identify patients who would benefit from physical rehabilitation even before they begin. The accurate prediction of motor recovery after stroke could represent a strong tool that could help predict patients’ ability to carry out activities of daily living. For example, the recovery of motor impairments after recombinant tissue plasminogen activator (rtPA) therapy may predict the degree of recovery in the affected corticospinal tract or other motor pathways after stroke [Citation5–7]. Moreover, it will allow clinicians to plan tailored rehabilitation programs and provide realistic goals for their patients.
Although there are studies on outcome variables [Citation8,Citation9], the prognostic variables in the ambulatory recovery of stroke patients following thrombolytic therapy are not well understood. The interaction of specific clinical risk factors with thrombolytic therapy may affect ambulatory functions. Knowledge of these clinical risk factors may contribute to determining treatment and predicting the outcome of ischemic stroke patients who are being considered for thrombolytic therapy. This is particularly true for ambulatory recovery; however, accurately predicting the degree of ambulation recovery following thrombolytic therapy for ischemic events is still challenging [Citation10,Citation11]. Living an independent life after stroke depends largely on the recovery of motor functions. To date, no precise or generally accepted motor assessment tool is available [Citation12–14]. Existing studies on poststroke motor recovery are based on different evaluation tools, including the Barthel Index score and the functional independence measure motor score, among others [Citation15,Citation16].
The purpose of this study is to determine prognostic variables associated with the improvement of ambulatory function in ischemic stroke patients following thrombolytic therapy. We hypothesize that our predictive model will identify baseline clinical factors associated with improvement or nonimprovement of ambulatory outcome following thrombolytic therapy. First, we evaluated the demographic and clinical variables associated with inclusion for thrombolytic therapy in an ischemic stroke population. This allowed us to develop a model to predict ambulatory function in the whole stroke population, rtPA-treated stroke patients and in ischemic stroke patients with the odds of an increase or decrease in improvement in ambulation following thrombolytic therapy.
Materials & methods
Research design
The retrospective data for this study was obtained from the PRISMA Health Stroke registry. This study was approved by the PRISMA Institutional Health Ethics Committee. The data within the PRISMA Health Stroke Registry was standardized according to the Get With The Guidelines (GWTG). The GWTG program is a hospital-based quality improvement initiative formed by the AHA and American Stroke Association (TX, USA) in an effort to improve the quality of care patients with acute stroke or transient ischemic attack receive [Citation17]. Our analysis was conducted on anonymous subjects from all participants following 14 days of stroke recovery. Early rehabilitation is described as interventions beginning within 2 weeks (14 days) of acute stroke following thrombolytic therapy [Citation18]. It has been shown that by 1–2 weeks post-stroke, neuronal activity (indicating reorganization of sensory inputs or motor activity) shifts back to the injured hemisphere, with the spared perilesional cortex taking on functions of the damaged brain. Functional MRI and PET imaging studies have demonstrated these sequences of events in the brain of stroke patients, especially within the motor function [Citation19] and language domains [Citation20–22]. Therefore, 14 days of recovery was chosen as it provides adequate information for recovery prior to rehabilitation when compared with 90 days. Our data analysis complies with the STROBE guidelines for cohort studies [Citation23]. Eligibility for rtPA was based on the AHA inclusion guidelines, in conjunction with CT scans for the early management of patients with acute ischemic stroke [Citation24–28]. Moreover, a stroke neurologist reviewed all patient data points to make a determination of whether or not the patient met the clinical description of acute stroke. Patient demographics and clinical variables were identified and abstracted by certified stroke nurses.
The baseline clinical variables present prior to patient admission that was included in this study: coronary artery disease, carotid stenosis, diabetes, dyslipidemia, atrial fibrillation/flutter, congestive heart failure, hypertension, previous stroke or transient ischemic attack, smoking history, peripheral vascular disease and neurological status at presentation. Ambulation was evaluated and used to develop a model for functional outcomes. Patient ambulation status was based on scoring prior to admission, during admission and the following discharge. These scores for each patient were retrieved from the stroke registry database. The scoring was done using functional ambulatory classification scores, which is dependable and sensitive to changes in stroke patients [Citation29,Citation30]. The validity of the scoring method has been described in a previous study [Citation31]. On admission, patients were assigned a score 0–2 based on the individual outcomes: unable to ambulate (0), ambulate with assistance (1) or able to ambulate independently (2). Analysis of the ambulation data allowed us to determine whether or not there was a significant change in ambulation status among patients receiving rtPA and patients who did not receive rtPA from hospital admission to discharge.
Data analysis
The SPSS Statistics Software version 15.0 (IL, USA) was used for all statistical analyses, and p < 0.05 was used to establish statistical significance in all comparisons. Patients were divided into groups based on whether or not they received rtPA – ‘rtPA group’ and ‘no rtPA group’, respectively. Descriptive statistics, which included measures of frequency, central tendency and variation were calculated using demographic and clinical characteristics of patients in the rtPA group and the no rtPA groups. Significant differences between the rtPA and no rtPA groups were determined using the Student’s t-test for independent samples for normally distributed variables. The Mann–Whitney U-test or χ2 test, where appropriate, was used for nominal qualitative variables. The mean, standard deviation and range were calculated for all continuous variables; the number and percentage of patients within each group were calculated for all discrete variables.
The logistic regression model was developed to identify predictive factors associated with functional outcome based on ambulation in rtPA and no rtPA groups. The model was developed for the whole ischemic stroke population (rtPA and no rtPA group), the no rtPA and rtPA subgroups. In general, we used the same selection method of significant predictors to assess the fitness of the regression model. First, we performed univariate analysis for each variable to initiate the selection process. Second, we considered a variable with a p-value of less than 0.01 for inclusion in our regression model [Citation32]. Third, we entered the selected variables into our logistic regression model. For variables that showed high collinearity, we used variance inflation factors to examine interactions between independent variables. A variable was excluded from the multivariate model if its p-value was greater than 0.01. We adjusted for confounding factors to control the masking effects of specific demographic and clinical variables. Therefore, we used forward selection which is a very attractive approach due to its tractability and it gives a good sequence of models, known to be similar to the c-statistic [Citation33]. This approach allowed us to select the significance of variables at p < 0.01. Our resulting model(s) had no outliers or data values with negative impacts on the model. As a result, we were able to identify the prognostic factors in the population of stroke patients, which were further divided into two groups: one group with rtPA and improvement and another rtPA group with a nonimprovement outcome.
After establishing the main effect of our model, we assessed the validity of our model using a Hosmer–Lemeshow test. Predicted parameters were estimated using standard errors and odds ratios (OR); the α was set to 5% and all variables were analyzed using a 95% CI. Moreover, we calculated the overall correct classification percentage and the area under the Receiver Operating Characteristic (ROC) curve for score prediction. The use of ROC allowed us to determine the sensitivity of our model including the specificity and accuracy in the results of the logistic regression function. Given that the output of the logistic function was between 0 (indicating no improvement in ambulation) and 1 (indicating an improvement in ambulation), it was considered as the prognostic factor for functional recovery.
Results
Of the 4665 admitted patients, 1446 were included in this study. Eligibility for rtPA was directed by 2009 and 2013 revisions of the AHA guidelines for the early management of patients with acute ischemic stroke [Citation34,Citation35]. Likewise, stroke patients who received rtPA and subsequently underwent endovascular therapy were excluded from the study. Of the 1446 patients qualified to receive rtPA, 595 were given thrombolytic therapy, while the remaining 851 were excluded based on the AHA guideline criteria. All patients eligible for rtPA were admitted to a Prisma Health Upstate (formerly Greenville Health System, SC, USA) facility within 4.5 h of symptom presentation.
Characteristics of patients that received rtPA versus those who did not receive rtPA are presented in . Patients treated with rtPA were significantly younger, on average, than patients in the no rtPA group (65.7 ± 15 years and 70 ± 14.7 years, respectively), were less likely to have a history of atrial fibrillation/flutter, carotid artery stenosis, congestive heart failure or previous stroke, but were more likely to smoke. Moreover, patients treated with rtPA were less likely to ambulate independently upon admission, had higher NIH stroke scale scores and were more likely to present with weakness/paresis or Broca’s aphasia/language disturbance as an initial examination finding. The rtPA group was more likely to be transported to the hospital by the emergency medical services as opposed to private transportation or transfer from another hospital. There was no statistically significant difference in rtPA administration between Caucasian and African–American patients; however, patients of Hispanic ethnicity were more likely to receive rtPA than those of non-Hispanic ethnicity.
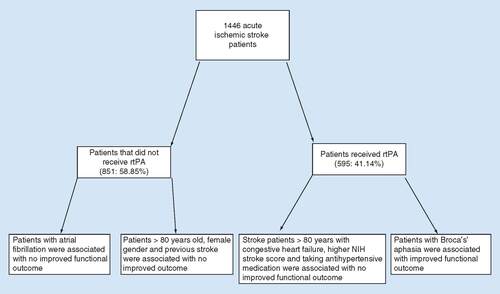
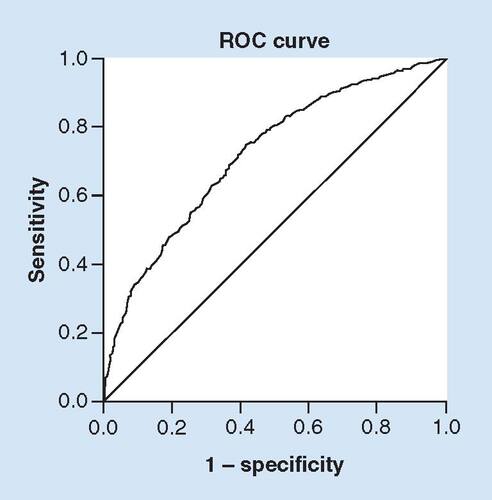
To predict whether patients would have improved outcomes with or without receipt of rtPA, the regression model was developed. The final model considered all significant variables shown in ; the result is expressed in . Patients with no improvement in functional ambulation following rtPA were more likely to be of increased age (>80 years) (OR: 0.967; p < 0.001; 95% CI: 0.97–0.99), present with atrial fibrillation (OR: 0.668; p = 0.024; 95% CI: 0.47–0.94), carotid artery stenosis (OR: 0.511; p = 0.023; 95% CI: 0.29–0.91), a history of previous stroke (OR: 0.479; p < 0.001; 95% CI: 0.36–0.65), with a calculated risk of mortality GWTG (OR: 0.933; p = 0.014; 95% CI: 0.88–0.99) or with an increased time from symptom onset to presentation (OR: 0.993; p < 0.001; 95% CI: 0.99–0.995). Factors more likely to be associated with an improved functional ambulatory outcome following rtPA were more likely to be Hispanic in ethnicity (OR: 2.808; p = 0.034; 95% CI: 1.08–7.30), present with high National Institute of Health Stroke Scale (NIHSS) (OR: 1.112; p ≤ 0.001; 95% CI: 1.06–1.17), present with weakness/paresis (OR: 1.796; p = 0.005; 95% CI: 1.19–2.71), Broca's aphasia (OR: 1.571; p = 0.003; 95% CI: 1.16–2.12) or are on antihypertensive medication (OR: 1.530; p = 0.034; 95% CI: 1.03–2.26). The ROC curve is presented in , indicating that 74.2% of the total population were correctly classified, demonstrating a good internal validity for our model.
Table 1. Clinical, hospital-based factors and patient demographics were compared between recombinant tissue plasminogen activator and no recombinant tissue plasminogen activator groups.
Table 2. Factors associated with improvement or no improvement in ambulation in total stroke population (recombinant tissue plasminogen activator or no recombinant tissue plasminogen activator) following an adjustment for the confounding effects of demographics, past medical history and clinical characteristics, as determined by multivariate binary logistic regression and compared.
The discrimination threshold of the model, as shown by the ROC curve, indicates a good fit with area under ROC curve of 74.2% for the total population.
ROC: Receiver operating curve.
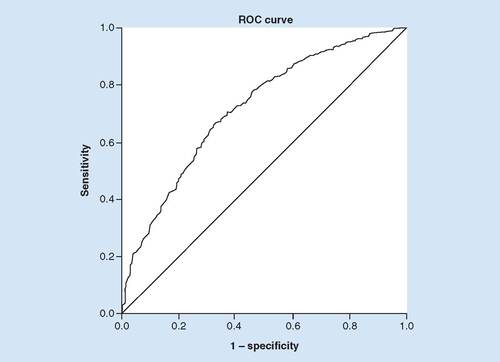
For the subset of patients not receiving rtPA, increasing age (OR: 0.961; p < 0.001; 95% CI: 0.95–0.98), female gender (OR: 0.686; p = 0.028; 95% CI: 0.49–0.96), previous stroke (OR: 0.538; p = 0.002; 95% CI: 0.37–0.79) and high NIHSS (OR: 0.899; p = 0.005; 95% CI: 0.83–0.97) were associated with a nonimproved ambulation outcome. However, atrial fibrillation (OR: 2.133; p = 0.001; 95% CI: 1.34–3.83) was the only factor found to be associated with improved functional outcome at discharge (). The area under ROC indicates 72.2% (95% CI: 0.6715–0.7718) of accurate classification, suggesting a good fit for the multivariate model as shown in the discriminating ability of the model ().
Table 3. Factors associated with functional outcome at discharge for the population of patients with no recombinant tissue plasminogen activator treatment, following an adjustment for the confounding effects of demographics, past medical history and clinical characteristics, as determined by multivariate binary logistic regression and compared.
The discriminating ability of the model as shown by the ROC curve indicates a good fit with area under ROC curve of 72.2% for the no-rtPA group, correctly classified.
ROC: Receiver operating curve; rtPA: Recombinant tissue plasminogen activator.
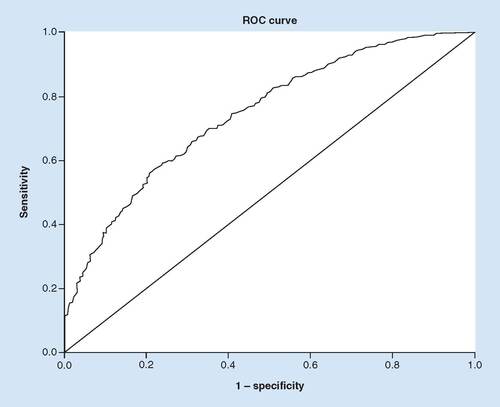
For patients that received rtPA, Broca’s aphasia (OR: 2.270; p = 0.002; 95% CI: 1.34–3.83) was associated with improved functional outcome. Contrarily, stroke patients with increasing age (OR: 0.942; p < 0.001; 95% CI:92–0.96), congestive heart failure (OR: 0.496; p = 0.040; 95% CI: 0.25–0.97), calculated higher NIHSS (0.876; p = 0.001; 95% CI: 0.80–0.95) and patients taking antihypertensive medication (OR: 0.436; p = 0.023; 95% CI: 0.21–0.89) were associated with no improvement in functional outcome (). The discriminating ability of the model was good as shown by the ROC (), with area under the ROC of 71.7% (95% CI: 0.6639–0.7710).
Table 4. Clinical factors that were associated with functional outcome for acute ischemic that received recombinant tissue plasminogen activator.
The discrimination threshold of the model, as shown by the ROC curve, indicates a good fit with area under ROC curve of 71.7% for the rtPA group, correctly classified.
ROC: Receiver Operating Characteristic; rtPA: Recombinant tissue plasminogen activator.
Discussion
The current study focused on the identification of independent predictors of outcome measured by ambulatory change, which is known to be a sensitive and reliable tool for functional outcomes [Citation36]. The goal was to identify clinical variables that contributed to functional ambulatory outcome following thrombolytic therapy. It is important to be able to identify clinical risk factors for which thrombolytic intervention will produce the greatest benefit in the context of ambulatory recovery to ultimately improve the effectiveness of thrombolytic therapy. Our model was able to successfully identify clinical variables that were associated with improvement or nonimprovement in ambulatory status following thrombolytic therapy within the whole ischemic stroke population along with the rtPA- and no rtPA-treated subpopulations. It is important to emphasize that in the whole stroke population (i.e., rtPA group and no rtPA groups), our model predicted increasing age, atrial fibrillation, carotid artery stenosis, a history of the previous stroke, calculated risk of mortality GWTG and an increased time from symptom onset to presentation to be associated with no improvement in functional ambulatory status.
Patients with Hispanic ethnicity, increased stroke severity (based on calculated NIHSS), presentation of weakness/paresis, Broca’s aphasia and a history of receiving antihypertensive medication were more likely to be associated with an improvement in functional ambulatory outcome after rtPA therapy. Furthermore, our model predicted the independent prognostic role of increasing age on rtPA and functional outcome. Increasing age was associated with nonimprovement in functional ambulation in the whole stroke population. The adjusted analysis did not eliminate the effect of age as the sustained effect of increasing age manifested in the rtPA group. Our model predicted lower chances of recovery from a stroke within octogenarian and nonagenarian patients, due to their higher risk of subsequent complications [Citation37,Citation38]. This finding is consistent with observational studies in which octogenarians and nonagenarians have lower chances of achieving favorable outcomes than patients <80 years old [Citation16,Citation39–41]. The nonimproved or poor ambulatory outcomes of elderly patients may be in part due to their numerous comorbidities [Citation42–44], as well as their decreased likelihood of regaining motoric functions following thrombolytic therapy [Citation45–47]. Additionally, our finding of poor or no improvement in functional ambulatory outcomes among patients who received rtPA and presented with congestive heart failure, higher NIH scores and were taking antihypertensive medication agrees with several studies [Citation24,Citation48–50]; advanced age subgroups within these studies had significantly higher rates of comorbidities, including hypertension, congestive heart failure and ischemic heart disease.
Baseline NIHSS score is a strong predictor of functional outcome and represents a major evaluation tool to assess the efficacy of rtPA treatment [Citation46,Citation47,Citation51]. In this study, stroke severity was between 0 and 10 for most of the patients excluded for rtPA. This suggests that the severity of stroke by itself alone does not provide an explanation for rtPA exclusion. Other variables [Citation52–55], including demographic factors, maybe also be associated with functional outcome in ischemic stroke patients from thrombolytic therapy.
In patients that did not receive rtPA, our model predicted poor or no improvement in ambulatory outcomes for patients with increasing age, patients who presented with atrial fibrillation, carotid artery stenosis, history of previous stroke and calculated risk of mortality GWTG. Precisely, female stroke patients with increasing age, previous stroke and atrial fibrillation were associated with a nonimprovement in their ambulatory status in the adjusted analysis. These factors are known to influence functional outcome in longitudinal studies and are more frequent in female stroke patients [Citation26,Citation56–58].
In the total stroke population, patients with Broca’s aphasia that received rtPA were more likely to be associated with an improvement in functional ambulatory outcome, and this result was retained in the adjusted analysis for the subset group of patients treated with thrombolytic therapy. At face value, this finding would suggest a positive association between speech deficits, thrombolytic therapy and motor recovery after ischemic stroke. It is important to point out that while Broca’s aphasia is caused by a lesion to the inferior frontal lobe of the dominant hemisphere and not within the primary motor cortex, Broca’s area overlaps, at least in part, with the ventral premotor cortex and is typically associated with a smaller lesion than Wernicke’s or global aphasia [Citation59], which may explain the outcome association. Although this may explain the outcome association, this stroke-related deficit would not independently be a reliable indicator of ambulatory functional recovery after stroke. Nevertheless, the link between these variables is not completely understood nor was the focus of this study. Other types of aphasia – conductive, receptive (Wernicke’s) and mixed aphasia – were not included in this study because they were not enumerated within the dataset used in the study. Further analysis of the different types of aphasia and its impact on ambulatory functional status would benefit from further investigation.
In general, outcomes of the logistic function were used to indicate the probability of correctly assigning patients from the whole ischemic stroke population to either improved or nonimproved functional ambulatory outcome. The accuracy of our model was generally strong and, therefore, indicates a good discriminatory performance in terms of both sensitivity and specificity. Thus, the internal validation of our results and the computed logistic function appears to indicate a good estimate of probability of ambulatory outcome at discharge. A major finding in this study is that patients with Hispanic ethnicity that received rtPA were predicted by our model to more likely be associated with an ambulatory improvement following thrombolytic therapy.
As a limitation, our model only accounts for patients that were treated within the 4.5 h of thrombolytic therapy. In addition, the time of recovery was limited to 14 days. Therefore, there is a need for the development of more models that take into consideration patients treated in 4.5–6 h of stroke onset and 3 or 6 months’ postrecovery. Moreover, a lack of data on mortality may provide an inaccurate estimate for the model performance, as the internal validation used in this study tends to over evaluate the model fitness. Nevertheless, our model has predicted an important association of thrombolytic therapy and improved functional motor outcome among Hispanic patients.
Future perspective
The accurate prediction of motor recovery for stroke patients who are most likely to benefit from thrombolytic therapy will help clinicians to plan rehabilitation programs and provide realistic goals for the patient. Moreover, since the heterogeneity of stroke manifestations provide a source of variability that could be amenable to clinical and motor parsing, the accurate prediction of motor recovery following an ischemic stroke represents a future and strong tool to evaluate functional and treatment outcome following thrombolytic therapy.
We developed a logistic regression model that predicts functional outcomes following thrombolytic therapy within an ischemic stroke population.
We identified demographic and baseline clinical factors that predicted ambulatory improvement or nonimprovement within this stroke population treated with recombinant tissue plasminogen activator.
In thrombolytic treated patients, Broca’s aphasia was associated with improved functional outcome.
Author contributions
Statistical analysis was performed by J Gainey. Critical interpretation of the results was performed by all authors. The manuscript was drafted by TIN and revised by M Scalise, L Brechtel, B Bailes and Z Conn.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
Informed consent was obtained from all participants in the stroke registry.
References
- HunterSM, Johansen-BergH, WardNet al.Functional strength training and movement performance therapy for upper limb recovery early poststroke-efficacy, neural correlates, predictive markers, and cost-effectiveness: FAST-INdiCATE trial. Front. Neurol.8, 733 (2018).
- PowersWJ, RabinsteinAA, AckersonTet al.2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke49(3), e46–e110 (2018).
- IchijoM, IwasawaE, NumasawaYet al.Significance of development and reversion of collaterals on MRI in early neurologic improvement and long-term functional outcome after intravenous thrombolysis for ischemic stroke. AJNR Am. J. Neuroradiol.36(10), 1839–1845 (2015).
- AlawiehA, VargasJ, FargenKMet al.Impact of procedure time on outcomes of thrombectomy for stroke. J. Am. Coll. Cardiol.73(8), 879–890 (2019).
- FuentesCalderón MA, MirallesAN, PimientaMJ, EstellaJMG, LedesmaMJS. Analysis of the factors related to the effectiveness of transcranial current stimulation in upper limb motor function recovery after stroke: a systematic review. J. Med. Syst.43(3), 69 (2019).
- FirthN, BarkerRN, HaywardKS, BernhardtJ, BellinganM, GunnarssonR. Safety and efficacy of recovery-promoting drugs for motor function after stroke: a systematic review of randomized controlled trials. J. Rehabil. Med.51(5), 319–330 (2019).
- LopezND, PereiraEM, CentenoEJ, PageJCM. Motor imagery as a complementary technique for functional recovery after stroke: a systematic review. Top. Stroke Rehabil.26(8), 576–587 (2019).
- DrozdowskaBA, SinghS, QuinnTJ. Thinking about the future: a review of prognostic scales used in acute stroke. Front. Neurol.10, 274 (2019).
- Magdon-IsmailZ, LednevaT, SunMZet al.Factors associated with 1-year mortality after discharge for acute stroke: what matters?Top. Stroke Rehabil.25(8), 576–583 (2018).
- MeretojaA, StrbianD, PutaalaJ, KasteM, TatlisumakT, HelsinkiStroke T. Hemiplegia and thrombolysis. Eur. J. Neurol.19(9), 1235–1238 (2012).
- BembenekJP, KurczychK, KarlinskiM, CzlonkowskaA. The prognostic value of motor-evoked potentials in motor recovery and functional outcome after stroke – a systematic review of the literature. Funct. Neurol.27(2), 79–84 (2012).
- Colla-MachadoPE, PigrettiSG, LuzziAA, BalianNR, CristianoE, Zurru-GanenMC. Is endovenous thrombolysis safety and efficacy in ischemic stroke comparable between patients aged over and above 80 years? Experience from an Argentinean cohort. Rev. Neurol.64(8), 347–352 (2017).
- KimSY, FinkMA, PeriniMet al.Age 80 years and over is not associated with increased morbidity and mortality following pancreaticoduodenectomy. ANZ J. Surg.88(5), E445–E450 (2018).
- PegoPM, NunesAP, FerreiraP, SousaC, Amaral-SilvaA. Thrombolysis in patients aged over 80 years is equally effective and safe. J. Stroke Cerebrovasc. Dis.25(6), 1532–1538 (2016).
- DoshiR, PatelV, ShahP. Comparison of in-hospital outcomes between octogenarians and nonagenarians undergoing transcatheter aortic valve replacement: a propensity matched analysis. J. Geriatr. Cardiol.15(2), 123–130 (2018).
- BehrouzR, Masjuan-VallejoJ, VeraRet al.Outcomes of nonagenarians with acute ischemic stroke treated with intravenous thrombolytics. J. Stroke Cerebrovasc. Dis.27(1), 246–256 (2018).
- StinearCM, ByblowWD, AckerleySJ, SmithMC, BorgesVM, BarberPA. Proportional motor recovery after stroke: implications for trial design. Stroke48(3), 795–798 (2017).
- ColemanER, MoudgalR, LangKet al.Early rehabilitation after stroke: a narrative review. Curr. Atheroscler. Rep.19(12), 59 (2017).
- AliaC, SpallettiC, LaiSet al.Neuroplastic changes following brain ischemia and their contribution to stroke recovery: novel approaches in neurorehabilitation. Front. Cell. Neurosci.11, 76 (2017).
- KuemmererD, StockertA, WredeKet al.Dynamics of language reorganization after stroke in patients with left frontal lesions. Procedia Soc. Behav. Sci.94, 220–221 (2013).
- SaurD, BaumgaertnerA, LangeR, SchraknepperV, RijntjesM, WeillerC. Dynamics of reorganisation in the language system after stroke: an MRI-follow-up study from the acute to the chronic phase. Brain Lang.95(1), 8–9 (2005).
- SaurD, LangeR, BaumgaertnerAet al.Dynamics of language reorganization after stroke. Brain129(6), 1371–1384 (2006).
- von ElmE, AltmanDG, EggerMet al.The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int. J. Surg.12(12), 1495–1499 (2014).
- NathanielTI, CochranT, ChavesJet al.Co-morbid conditions in use of recombinant tissue plasminogen activator (rtPA) for the treatment of acute ischaemic stroke. Brain Inj.30(10), 1261–1265 (2016).
- ColelloMJ, IveyLE, GaineyJet al.Pharmacological thrombolysis for acute ischemic stroke treatment: gender differences in clinical risk factors. Adv. Med. Sci.63(1), 100–106 (2018).
- GaineyJ, BrechtelL, KonklinSet al.In a stroke cohort with incident hypertension; are more women than men likely to be excluded from recombinant tissue-type Plasminogen Activator (rtPA)?J. Neurol. Sci.387, 139–146 (2018).
- SchwammLH, FonarowGC, ReevesMJet al.Get with the guidelines-stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation119(1), 107–115 (2009).
- WhisnantJP, BasfordJR, BernsteinEF. Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases III. Stroke21(4), 637–676 (1990).
- PocoviN. Appraisal of clinical practice guideline: 2018 guidelines for the early management of patients with acute ischemic stroke. J. Physiother.64(3), 199 (2018).
- PowersWJ, RabinsteinAA. Response by Powers and Rabinstein to letter regarding article, “2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association”. Stroke50(9), e277–e278 (2019).
- LawsonT, BrownI, WesterkamDet al.A new diagnostic tool for measuring the effectiveness of intravenous tissue plasminogen activator (r-pa) in the treatment of acute ischemic stroke. International Stroke ConferenceQuebec, Canada, 4(6), P346 (2018).
- SullivanLM, MassaroJM, D’agostmoSr RB. Presentation of multivariate data for clinical use: the Framingham study risk score functions. Stat. Med.23(10), 1631–1660 (2004).
- HanleyJA, McNeilBJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology143(1), 29–36 (1982).
- FurieKL, JayaramanMV. 2018 Guidelines for the early management of patients with acute ischemic stroke. Stroke49(3), 509–510 (2018).
- PowersWJ, RabinsteinAA, AckersonTet al.2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke49(3), e46–e110 (2018).
- KollenB, KwakkelG, LindemanE. Longitudinal robustness of variables predicting independent gait following severe middle cerebral artery stroke: a prospective cohort study. Clin. Rehabil.20(3), 262–268 (2006).
- BlumB, BrechtelL, NathanielT. Thrombolysis therapy in specialized and non-specialized stroke units. Arch. Med. Res.49(8), 588–597 (2018).
- BlumB, PenwellA, WormackL, WalkerB, LariS, NathanielTI. Gender and thrombolysis therapy in acute ischemic stroke patients with incidence of obesity. Neurol. Sci.40(9), 1829–1839 (2019).
- CochoD, YarlequeS, BoltesAet al.Clinical outcome of ischemic stroke in old patients versus oldest-old. J. Stroke Cerebrovasc. Dis.27(12), 3657–3661 (2018).
- HeitschLE, PanagosPD. Treating the elderly stroke patient complications, controversies, and best care metrics. Clin. Geriatr. Med.29(1), 231–255 (2013).
- PouporeN, StratD, MackeyT, NathanielIT. The association between an antecedent of transient ischemic attack prior to onset of stroke and functional ambulatory outcome. Clin. Appl. Thromb. Hemost.26, 1076029620906867 (2020).
- SchneiderJR, JacksonCR, HelenowskiIBet al.A comparison of results of carotid endarterectomy in octogenarians and nonagenarians to younger patients from the Mid-America Vascular Study Group and the Society for Vascular Surgery Vascular Quality Initiative. J. Vasc. Surg.65(6), 1643–1652 (2017).
- FazzoneB, MorrisG, BlackLAet al.Exclusion and inclusion criteria for thrombolytic therapy in an ischemic stroke population. J. Neurol. Disord. Stroke4(2), 1112 (2016).
- NathanielIT, WormackL, GaineyJ. Development of a new predictive model for thrombolysis in the telestroke for hypertensive acute ischemic stroke patients. Presented at: 11th World Stroke congress. Montreal, QC, Canada, (2018).
- HospJA, LuftAR. Cortical plasticity during motor learning and recovery after ischemic stroke. Neural Plast.2011, 871296 (2011).
- NathanielTI, UbahC, WormackL, GaineyJ. The telestroke and thrombolysis therapy in diabetic stroke patients. Diabetol. Metab. Syndr.11, 36 (2019).
- NathanielTI, WilliamsJA, FazzoneBet al.Contraindications and exclusion criteria in guidelines for rtPA in acute ischemic stroke: can the new AHA/ASA guideline expand the use of rtPA?Hypertension68 (2016).
- KwakkelG, KollenB, LindemanE. Understanding the pattern of functional recovery after stroke: facts and theories. Restor. Neurol. Neurosci.22(3–5), 281–299 (2004).
- SmithEE, FonarowGC, ReevesMJ. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: findings from get with the guidelines-stroke. Stroke42(11), 3110–3115 (2011).
- YangJ, YuF, LiuH, AnHD, XiongR, HuangDY. A retrospective study of thrombolysis with 0.6 mg/kg recombinant tissue plasminogen activator (rtPA) in mild stroke. Sci. Rep.6, 31344 (2016).
- AokiJ, KimuraK, KogaMet al.NIHSS-time score easily predicts outcomes in rtPA patients: The SAMURAI rtPA registry. J. Neurol. Sci.327(1–2), 6–11 (2013).
- O’BrienEC, KimS, ThomasLet al.Clinical characteristics, oral anticoagulation patterns, and outcomes of medicaid patients with atrial fibrillation: insights from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF I) registry. J. Am. Heart Assoc.5(5), e002721 (2016).
- MadsenTE, ChooEK, SeigelTA, PalmsD, SilverB. Lack of gender disparities in emergency department triage of acute stroke patients. West. J. Emerg. Med.16(1), 203–209 (2015).
- HsiehCY, WuDP, SungSF. Trends in vascular risk factors, stroke performance measures, and outcomes in patients with first-ever ischemic stroke in Taiwan between 2000 and 2012. J. Neurol. Sci.378, 80–84 (2017).
- ZhangS, LiuZ, LiuYL, WangYL, LiuT, CuiXB. Prevalence of stroke and associated risk factors among middle-aged and older farmers in western China. Environ. Health Prev. Med.22, 6 (2017).
- ChenWQ, PanYS, ZhaoXQet al.Intravenous thrombolysis in chinese patients with different subtype of mild stroke: thrombolysis in patients with mild stroke. Sci. Rep.7, 2299 (2017).
- TanYF, ZhanLX, ChenXH, GuoJJ, QinC, XuE. Risk factors, clinical features and prognosis for subtypes of ischemic stroke in a chinese population. Curr. Med. Sci.38(2), 296–303 (2018).
- WapshottT, BlumB, WilliamsK, NathanielTI. Investigation of gender differences and exclusive criteria in a diabetic acute ischemic stroke population treated with recombinant tissue-type plasminogen activator (rtPA). J. Vasc. Interv. Neurol.9(6), 26–32 (2017).
- FridrikssonJ, FillmoreP, GuoDZ, RordenC. Chronic Broca’s aphasia is caused by damage to Broca’s and Wernicke’s areas. Cereb. Cortex25(12), 4689–4696 (2015).
