Abstract
Sentinel lymph node biopsy (SLNB) is a diagnostic staging procedure. The procedure aims to identify the first draining lymph node(s), which are most likely to contain metastases. SLNB is applied in various cancers, but not currently in thyroid carcinoma. However, treatment strategies are changing, making SLNB clinically relevant. SLNB may lead to more accurate staging, prevent unnecessary treatment and help achieve earlier curation. 68Ga-tilmanocept PET/computed tomography (CT) can better localize sentinel lymph nodes (SLNs) near the primary tumor than planar scintigraphy and single-photon emission computed tomography (SPECT)/CT. This paper describes the rationale and design of a study investigating SLNB using 68Ga-tilmanocept PET/CT and indocyanine-green-99mTc-nanocolloid in ten differentiated and medullary thyroid carcinoma patients. Localization and number of SLNs, pathology result, optimal scan protocol, surgical time and surgeon’s experience are examined.
Clinical Trial Registration: 2021-002470-42 (EudraCT).
Plain Language Summary
Sentinel lymph node biopsy (SLNB) can detect or rule out metastases in lymph nodes. SLNB is used in various cancers but not in thyroid cancer. However, due to changing treatment strategies, SLNB might also become valuable in thyroid cancer and lead to more accurate staging, prevent unnecessary treatment and help achieve earlier curation. 68Ga-tilmanocept PET/CT, a new imaging modality, can better localize lymph nodes near the primary tumor than previous imaging modalities, which is essential for SLNB in thyroid carcinoma. This study investigates the feasibility of SLNB in thyroid carcinoma.
Differentiated thyroid carcinoma (DTC) derives from the follicular epithelial cells of the thyroid gland and is the most common (>90%) type of thyroid cancer. DTC has a relatively good prognosis; 10-year survival rates are over 90% [Citation1]. Nevertheless, patients have up to a 40% chance of developing structural recurrent disease. Usually, this involves cervical lymph node metastases which were not detected at the time of initial treatment. Surgical treatment consists of hemi- or total thyroidectomy, depending on multiple factors including tumor size and stage. In case of preoperatively proven metastases, lymph node dissection is also performed [Citation2]. Total thyroidectomy is often followed by adjuvant therapy using radioactive iodine (RAI), which treats possible (micro)metastases [Citation2,Citation3]. However, because of side effects of RAI (including nausea, salivary gland damage and lacrimal gland damage) there is a trend to use less RAI [Citation4]. Studies have shown that in low-risk patients, low-dose RAI is equally effective as high-dose RAI [Citation5–7]. A large randomized trial investigating disease-free survival in low-risk patients that receive RAI versus patients that do not receive RAI is still ongoing [Citation8]. The authors of these aforementioned studies advocate minimalizing the use of RAI for low-risk DTC, by decreasing the dose or not treating with RAI at all [Citation5–8].
Medullary thyroid carcinoma (MTC) arises from the parafollicular C-cells of the thyroid gland, unlike the other forms of thyroid carcinoma. In most patients, MTC occurs sporadically. In the remaining 25–30% of patients MTC occurs as part of the hereditary tumor syndrome multiple endocrine neoplasia type 2 [Citation9]. MTC accounts for 4% of all thyroid carcinomas, while it leads to 13% of thyroid cancer related deaths [Citation10]. Ten-year survival rates are 100%, 93%, 71% and 21%, for stage I, II, III and IV, respectively [Citation11]. Surgical resection is the cornerstone of MTC treatment and adjuvant therapy is of limited value. RAI is not useful in the treatment of MTC and its metastases because MTC tumor cells, contrary to DTC, do not take up iodine [Citation12]. Treatment with curative intent consists of total thyroidectomy combined with central neck dissection. The rationale for this is that the majority of thyroid tumors first metastasize to the central compartment, before spreading any further [Citation11]. However, in 61% of patients, lymph node metastases cannot be demonstrated in the resected tissue after central neck dissection. This suggests that these patients underwent unnecessary lymph node dissection, accompanied by risk of complications [Citation13]. Furthermore, in most patients treated with curative intent, levels of the tumor marker calcitonin remain elevated, indicating that not all active tumor tissue is resected [Citation14,Citation15].
So, for both DTC and MTC patients, care can be improved by creating more personalized treatment plans. To determine whether a specific patients needs a less or more extensive treatment, accurate staging is essential.
Introduction to the trial
This paper describes the rationale and design of a monocenter, proof-of-concept study, investigating sentinel lymph node (SLN) biopsy (SLNB) in DTC and MTC using 68Ga-tilmanocept PET with integrated computed tomography (PET/CT) and indocyanine green (ICG)-99mTc-nanocolloid.
Background & rationale
SLNB is a diagnostic staging procedure which is applied in various cancers, but is not currently used for the staging of thyroid carcinoma. The aim of the SLNB is to identify the first draining lymph node(s) from the primary tumor, known as the SLN(s), which are most likely to contain metastases if present. The histopathological status of the SLN should reflect the histopathological status of the rest of the lymph node level, and proven metastases justify further lymph node dissection or additional treatment. Contrarily, a negative SLN would reflect no metastasized disease and justifies less extensive treatment [Citation16]. There are multiple techniques available to perform SLNB. For preoperative identification, imaging modalities such as lymphoscintigraphy, single-photon emission computed tomography (SPECT) and PET/CT can be used. Preoperative imaging is very useful, but intraoperative identification is essential. SLNs can be intraoperatively identified using a collimated gamma probe in combination with a radiotracer, or visually using dye or fluorescence. Often multiple techniques are combined in one patient.
SLNB, using different techniques, has been investigated in DTC [Citation17–23]. Due to the former standard treatment with RAI, SLNB has not been considered of added value in DTC. However, in the era of de-escalating treatment, RAI is being used less and in a lower dose, potentially leaving undiagnosed (micro)metastases untreated. Considering this, SLNB could be used as a valuable selection tool to decide which patients should receive RAI ablation and in which patients this can be omitted. In addition, since low-risk DTC is nowadays often treated with hemithyroidectomy only, positive SLNB could be used as an indication to continue with a total thyroidectomy followed by RAI.
Only a few papers have been published about SLNB in MTC patients [Citation24–26]. For MTC patients, negative SLNB could give reason to omit the now standard executed central neck dissection, minimalizing unnecessary complications. A positive SLNB in the lateral compartment could select patients for an additional lateral neck lymph node dissection, possibly leading to curation. So, SLNB might not only help to more accurately stage thyroid carcinoma, but also achieve earlier curation and prevent unnecessary complications as well as reoperations.
A short distance between the primary tumor and the draining lymph nodes, which is specifically the case in thyroid carcinoma, might cause difficulties in identifying the SLNs. Activity from the radiotracer at the peritumoral injection site produces a large hotspot on conventional gamma cameras or SPECT imaging, overshadowing smaller hotspots produced by SLNs. This is referred to as the ‘shine-through’ or ‘overshine’ phenomenon. Accordingly, SLNs may be missed and second echelon lymph nodes may erroneously be considered as SLNs. Therefore, technical improvements are needed to bring SLNB to a higher level. More precise localization of the SLN(s) may reduce operating time and risk of vascular or nerve damage, which improves patient safety during surgery. Furthermore, less extensive exploration will lead to less fibrosis, which hampers a subsequent neck dissection and will presumably result in a reduction of complications and unintended sacrificed structures in the neck following complementary neck dissection [Citation27]. Moreover, if the shine-through phenomenon can be limited, the false-negative rate concerning SLNB will be reduced, which will eventually result in a better oncological outcome in these patients [Citation28].
A potential solution to this issue regards the use of PET/CT. PET/CT provides 3D information at a higher spatial resolution, compared with the resolution provided by gamma cameras or SPECT imaging. Consequently, the use of PET/CT limits the shine-through effect and improves anatomic localization, which is imperative in the complex anatomy of the neck and its abundant lymph nodes. Moreover, improved visualization of lymphatic vessels may improve differentiation between first- and second-echelon lymph nodes.
There are multiple modalities available for pre- and intraoperative detection of SLNs. Additionally, multiple modalities can be combined to verify the identification of an SLN. ICG is US FDA-approved and can be made visible using a near-IR fluorescence camera [Citation29]. For SLNB in breast cancer, the use of ICG is preferred over blue dye, due to the better identification rate [Citation30]. On its own, ICG passes easily through lymph nodes due to its small molecular structure. When linked to 99mTc-nanocolloid, which is also valuable for SLN detection, ICG is retained in lymph nodes, enabling the use of ICG for SLNB [Citation16]. 99mTc-nanocolloid is the most used radiotracer for intraoperative SLN detection in Europe [Citation31]. To combine preoperative PET/CT imaging with intraoperative detection using a 99mTc-tracer and a gamma probe, a PET-tracer with a short half-life is needed. 68-Gallium (68Ga) is a good candidate considering the short half-life of 68 min and that it can be produced without the use of a cyclotron [Citation32,Citation33]. Whereas labelling of nanocolloids with 68Ga has experienced some difficulties, 68Ga has been successfully labelled to tilmanocept in our laboratory. Since tilmanocept has been deliberately designed for SLN mapping, the characteristics of tilmanocept are specifically suited to the needs of the procedure. Tilmanocept is cleared rapidly from the injection site, which limits the shine-through effect. Furthermore, it is retained specifically in SLNs without transport to second-echelon nodes [Citation34,Citation35]. SLN identification using 68Ga-labelled tilmanocept imaging has been successfully performed in animal models and 68Ga-tilmanocept PET/CT has been used in patient studies before in our institute [Citation34,Citation36–38].
In this proof-of-concept study, the feasibility of SLNB in thyroid carcinoma will be studied using preoperative 68Ga-tilmanocept PET/CT imaging and intraoperative identification using ICG-99mTc-nanocolloid.
Design
Study design
The trial protocol described here is a monocenter, proof-of-concept study.
Eligibility criteria
A subject must meet all of the following criteria to be eligible for participation in this study:
The patient has provided written informed consent authorization before participating in the study.
The patient has a cytologic diagnosis of DTC (Bethesda 6) and will undergo a hemi- or total thyroidectomy, or a cytologic diagnosis of MTC (Bethesda 6) and will undergo total thyroidectomy.
The patient is ≥18 years of age at the time of consent.
The patient has an Eastern Cooperative Oncology Group performance status of grade 0–2
A potential subject will be excluded in case of any of the following criteria:
The patient is incapacitated.
The patient is pregnant or lactating.
The patient has a history of neck dissection, gross injury or radiotherapy to the neck that would preclude reasonable surgical dissection for this trial.
The patient will undergo minimally invasive thyroid surgery (via the axilla or trans-oral approach).
The patient is actively receiving systemic cytotoxic chemotherapy.
The patient is on immunosuppressive, antimonocyte or immunomodulatory therapy.
The patient has a preoperatively histologically proven multifocal tumor.
Planned sample size
Since this is a proof-of-concept study, ten patients will be included.
Planned study period
Ten adult patients highly suspected of DTC or MTC (Bethesda 6) will be included. Considering the number of patients undergoing surgery for thyroid carcinoma in the University Medical Center Utrecht, the study is estimated to last 2 years.
Study procedures
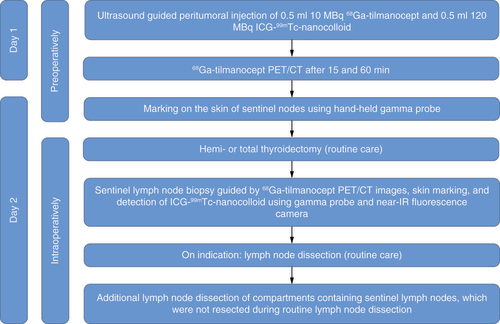
The procedure is identical for DTC and MTC patients. Included patients will pay an extra visit to the hospital the day prior to the planned surgery for thyroid carcinoma. The patients will undergo multiple (two to eight) consecutive, ultrasound guided, intra- or peritumoral injections of 0.5 ml (10 MBq) 68Ga-tilmanocept and 0.5 ml (120 MBq) ICG-99mTc-nanocolloid. The ICG-99mTc-nanocolloid complex is prepared by adding 0.050 ml of a solution of 5 mg/ml ICG in water for injection (0.250 mg ICG) to 1 ml 99mTc-nanocolloid. Approximately 40–60% of the ICG binds noncovalent to nanocolloid, forming a stable complex. The uptake of 68Ga-tilmanocept and ICG-99mTc-nanocolloid is not influenced by each another. After injection patients will undergo 68Ga-tilmanocept PET/CT 15 and 60 min after injection. The execution of each 68Ga-tilmanocept PET/CT will take 5 min. The next day, the approximate location of the SLNs will be preoperatively assessed using a hand-held gamma probe and marked on the skin. During regular surgery (hemi- or total thyroidectomy, with or without neck dissection), the SLNs will be surgically removed with the help of the 68Ga-tilmanocept PET/CT images, skin markings, hand-held gamma probe and fluorescence camera. Hereafter, the corresponding lymph node level will be removed, in order to investigate whether there are no positive lymph nodes in a level corresponding to a negative sentinel node. Since corresponding lymph node levels will be removed, independently of pathology results, frozen sections will not be performed. A detailed flowchart of the performed study procedures is shown in .
Outcome measures/end points
To determine the feasibility of SLNB in thyroid carcinoma, the number of SLNs determined on 68Ga-tilmanocept PET/CT and the number of resected SLNs are considered the primary end points. Secondary end points consist of the localization of the SLNs, the pathology result of SLNs compared with the pathology result of the rest of corresponding lymph node levels and the optimal scan protocol. The optimal scan protocol is determined by comparing the two 68Ga-tilmanocept PET/CTs to assess which scan is most valuable: the 68Ga-tilmanocept PET/CT performed after 15 min, after 60 min or whether both scans are of added value in identifying the SLNs. Other secondary end points are the (additional) surgical time, and a questionnaire directed to the surgeons about complexity, feasibility and additional value of various identification methods used during the SLNB.
Statistics
Data will be expressed as median with ranges, for continuous variables. Number of cases and percentages will be represented for categorical variables. Missing data will not be imputed.
Conclusion
Introduction of SLNB in thyroid carcinoma may lead to more accurate staging, prevent unnecessary treatment and accompanying complications and help achieve (earlier) curation. The new promising imaging modality 68Ga-tilmanocept PET/CT limits the shine-through effect and can better localize SLNs located near the primary tumor than previous imaging modalities, which is essential in thyroid carcinoma.
Background & rationale
Sentinel lymph node (SLN) biopsy (SLNB) is a diagnostic staging procedure, which is applied in various cancers, but is not currently used in thyroid carcinoma. SLNB aims to identify the first draining lymph nodes (SLNs), which are most likely to contain metastases, if present. The histopathological status of the SLN should reflect the histopathological status of the rest of the lymph node level, and presence of metastases justifies more extensive treatment.
Detecting SLNs can be challenging; the peritumorally injected radiotracer produces a large hotspot on imaging, potentially hiding nearby SLNs (shine-through effect). 68Ga-tilmanocept PET/computed tomography (PET/CT), a new promising imaging modality with high resolution, limits this effect and can better localize SLNs located near the injection site than previous imaging modalities, which is essential in thyroid carcinoma.
After surgery, differentiated thyroid carcinoma (DTC) patients often undergo adjuvant therapy using radioactive iodine (RAI), which treats possible (micro)metastases. Therefore, SLNB has not been of added value in these patients. However, recent studies emphasize the disadvantages and side effects of RAI, and advocate minimizing the use of RAI for low-risk DTC by decreasing the dose or not treating with RAI at all. Considering this, SLNB could be used to select patients for RAI and total thyroidectomy, or hemithyroidectomy alone.
In case of curative intent, total thyroidectomy with central neck dissection is the treatment for patients with medullary thyroid carcinoma (MTC). In 61% of patients, lymph node metastases cannot be demonstrated after central neck dissection, suggesting unnecessary lymph node dissection was performed [Citation13]. A negative SLNB could give reason to omit the central neck dissection, minimalizing unnecessary complications. A positive SLNB in the lateral compartment could select patients for an additional lateral neck lymph node dissection, possibly leading to curation.
Study design
A monocenter, proof-of-concept study investigating SLNB in ten patients with Bethesda 6 DTC or MTC is described. During regular surgery (thyroidectomy with/without lymph node dissection), SLNB will be performed using the 68Ga-tilmanocept PET/CT images, skin marking, fluorescence camera and gamma probe.
Outcomes
Location and number of detected and resected SLNs, pathology results, optimal scan protocol, surgical time and surgeon’s experience are examined.
Conclusion
Introduction of SLNB in thyroid carcinoma may lead to more accurate staging, prevent unnecessary treatment and help achieve earlier cure.
Author contributions
All authors contributed to the article and approved the submitted version.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
Acknowledgments
The authors would like to thank Professor R de Bree for sharing his knowledge and advice on sentinel lymph node biopsy.
References
- Durante C , HaddyN, BaudinEet al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab.91(8), 2892–2899 (2006).
- Haugen BR , AlexanderEK, BibleKCet al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid26(1), 1–133 (2016).
- Shah MH , GoldnerWS, BensonABet al. Neuroendocrine and adrenal tumors, version 2.2021. J. Natl Compr. Canc. Netw.19(7), 839–868 (2021).
- Van Nostrand D . The benefits and risks of I-131 therapy in patients with well-differentiated thyroid cancer. Thyroid19(12), 1381–1391 (2009).
- Mallick U , HarmerC, HackshawA. The HiLo trial: a multicentre randomised trial of high- versus low-dose radioiodine, with or without recombinant human thyroid stimulating hormone, for remnant ablation after surgery for differentiated thyroid cancer. Clin. Oncol.20(5), 325–326 (2008).
- Schlumberger M , BogdanC, BorgetIet al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N. Engl. J. Med.366(18), 1663–1673 (2012).
- Schlumberger M , LeboulleuxS, CatargiBet al. Outcome after ablation in patients with low-risk thyroid cancer (ESTIMABL1): 5-year follow-up results of a randomised, phase 3, equivalence trial. Lancet Diabetes Endocrinol.6(8), 618–626 (2018).
- Mallick U , HarmerC, HackshawA, MossL. Iodine or not (IoN) for low-risk differentiated thyroid cancer: the next UK National Cancer Research Network randomised trial following HiLo. Clin. Oncol.24(3), 159–161 (2012).
- Cakir M , GrossmanAB. At the cutting edge. Medullary thyroid cancer: molecular biology and novel molecular therapies. Neuroendocrinology90(4), 323–348 (2009).
- Kebebew E , ItuartePHG, SipersteinAE, DuhQ-Y, ClarkOH. Medullary thyroid carcinoma. Clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer88(5), 1139–1148 (2000).
- Wells SA , AsaSL, DralleHet al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid25(6), 567–610 (2015).
- Meijer JAA , BakkerLEH, ValkGDet al. Radioactive iodine in the treatment of medullary thyroid carcinoma: a controlled multicenter study. Eur. J. Endocrinol.168(5), 779–786 (2013).
- van Beek D-J , AlmquistM, BergenfelzAOet al. Complications after medullary thyroid carcinoma surgery: multicentre study of the SQRTPA and EUROCRINE® databases. Br. J. Surg.108(6), 691–701 (2020).
- Scollo C , BaudinE, TravagliJ-PTet al. Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J. Clin. Endocrinol. Metab.88(5), 2070–2075 (2003).
- Kuo EJ , ShoS, LiN, ZanoccoKA, YehMW, LivhitsMJ. Risk factors associated with reoperation and disease-specific mortality in patients with medullary thyroid carcinoma. JAMA Surg.153(1), 52–59 (2018).
- Schilling C , StoeckliSJ, VigiliMGet al. Surgical consensus guidelines on sentinel node biopsy (SNB) in patients with oral cancer. Head Neck41(8), 2655–2664 (2019).
- Albers MB , NordenströmE, WohlfahrtJ, BergenfelzA, AlmquistM. Sentinel lymph node biopsy in thyroid cancer. World J. Surg.44(1), 142–147 (2020).
- Puccini M , MancaG, NeriCMet al. Effect of sentinel node biopsy in clinically N0, BRAF V600E-mutated, small papillary thyroid carcinoma: a pilot study. Clin. Nucl. Med.44(5), 359–364 (2019).
- Garau LM , RubelloD, MorgantiRet al. Sentinel lymph node biopsy in small papillary thyroid cancer: a meta-analysis. Clin. Nucl. Med.44(2), 108–118 (2019).
- González Ó , ZafonC, CaubetEet al. Selective sentinel lymph node biopsy in papillary thyroid carcinoma in patients with no preoperative evidence of lymph node metastasis. Endocrinol. Diabetes Nutr.64(8), 451–455 (2017).
- Pelizzo MR , ToniatoA, SorgatoNet al. 99Tc nanocolloid sentinel node procedure in papillary thyroid carcinoma: our mono-institutional experience on a large series of patients. Acta Otorhinolaryngol. Ital.29(6), 321–325 (2009).
- Lee SK , ChoiJH, LimHIet al. Sentinel lymph node biopsy in papillary thyroid cancer: comparison study of blue dye method and combined radioisotope and blue dye method in papillary thyroid cancer. Eur. J. Surg. Oncol.35(9), 974–979 (2009).
- Jozaghi Y , RichardsonK, AnandSet al. Frozen section analysis and sentinel lymph node biopsy in well differentiated thyroid cancer. J. Otolaryngol. Head Neck Surg.42(Sep), (2013).
- Puccini M , MancaG, UgoliniCet al. Interest of sentinel node biopsy in apparently intrathyroidal medullary thyroid cancer: a pilot study. J. Endocrinol. Invest.37(9), 829–834 (2014).
- Boni G , MazzarriS, GrossoMet al. Sentinel node radioguided biopsy in surgical management of the medullary thyroid carcinoma a case report. Ann. Ital. Chir.85(ePub), 1–4 (2014).
- Kim MJ , BackK, ChoeJ-H, KimJ-H, KimJS. Feasibility of lateral sentinel lymph node biopsy in medullary thyroid cancer: surrogate tool for determining prophylactic lateral neck dissection – a pilot study. Head Neck43(11), 3276–3286 (2021).
- Bluemel C , RubelloD, CollettiPM, de BreeR, HerrmannK. Sentinel lymph node biopsy in oral and oropharyngeal squamous cell carcinoma: current status and unresolved challenges. Eur. J. Nucl. Med. Mol. Imaging42(9), 1469–1480 (2015).
- de Bree R , TakesRP, ShahJPet al. Elective neck dissection in oral squamous cell carcinoma: past, present and future. Oral Oncol.90, 87–93 (2019).
- Zhang X , ShenY-P, LiJ-G, ChenG. Clinical feasibility of imaging with indocyanine green combined with carbon nanoparticles for sentinel lymph node identification in papillary thyroid microcarcinoma. Medicine (Baltimore)98(36), 1–6 (2019).
- Ahmed M , PurushothamAD, DouekM. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol.15(8), e351–e362 (2014).
- den Toom IJ , MahieuR, van RooijRet al. Sentinel lymph node detection in oral cancer: a within-patient comparison between [99mTc]Tc-tilmanocept and [99mTc]Tc-nanocolloid. Eur. J. Nucl. Med. Mol. Imaging48(3), 851–858 (2021).
- Kasbollah A , EuP, CowellS, DebP. Review on production of 89Zr in a medical cyclotron for PET radiopharmaceuticals. J. Nucl. Med. Technol.41(1), 35–41 (2013).
- Martiniova L , DePalatis L, EtchebehereE, RavizziniG. Gallium-68 in medical imaging. Curr. Radiopharm.9(3), 187–207 (2016).
- Anderson KM , BarbackCV, QinZet al. Molecular imaging of endometrial sentinel lymph nodes utilizing fluorescent-labeled tilmanocept during robotic-assisted surgery in a porcine model. PLOS ONE13(7), 1–13 (2018).
- Qin Z , HohCK, HallDJ, VeraDR. A tri-modal molecular imaging agent for sentinel lymph node mapping. Nucl. Med. Biol.42(12), 917–922 (2015).
- Stroup SP , KaneCJ, Farchshchi-HeydariSet al. Preoperative sentinel lymph node mapping of the prostate using PET/CT fusion imaging and Ga-68-labeled tilmanocept in an animal model. Clin. Exp. Metastasis29(7), 673–680 (2012).
- Mahieu R , KrijgerGC, VerversFFTTet al. [68Ga]Ga-tilmanocept PET/CT lymphoscintigraphy: a novel technique for sentinel lymph node imaging. Eur. J. Nucl. Med. Mol. Imaging48(4), 963–965 (2021).
- Mahieu R , KrijgerGC, VerversFFTTet al. [68Ga]Ga-tilmanocept PET/CT lymphoscintigraphy for sentinel lymph node detection in early-stage oral cavity carcinoma. Eur. J. Nucl. Med. Mol. Imaging48, 3–4 (2020).