Abstract
Aim: The cost–effectiveness of avelumab first-line maintenance treatment for locally advanced or metastatic urothelial carcinoma in Scotland was assessed. Materials & methods: A partitioned survival model was developed comparing avelumab plus best supportive care (BSC) versus BSC alone, incorporating JAVELIN Bladder 100 trial data, costs from national databases and published literature and clinical expert validation of assumptions. Incremental cost–effectiveness ratio (ICER) was estimated using lifetime costs and quality-adjusted life-years (QALY). Results: Avelumab plus BSC had incremental costs of £9446 and a QALY gain of 0.63, leading to a base-case (deterministic) ICER of £15,046 per QALY gained, supported by robust sensitivity analyses. Conclusion: Avelumab first-line maintenance is likely to be a cost-effective treatment for locally advanced or metastatic urothelial carcinoma in Scotland.
Plain language summary
What is this article about?
This study looked at the costs of avelumab when given as maintenance treatment for people in Scotland with advanced urothelial carcinoma, compared with the longer survival and other benefits that it provides.
How was this done?
Researchers estimated the costs and treatment benefits expected with avelumab using data from a clinical trial called JAVELIN Bladder 100, national databases, data from previously published studies and expert opinions.
What were the results?
Costs associated with using avelumab maintenance treatment for people with advanced urothelial carcinoma in Scotland were considered to be acceptable based on the benefits it provides.
What do the results of the study mean?
These results support the use of avelumab first-line maintenance as a standard treatment for people with advanced urothelial carcinoma in Scotland.
Tweetable abstract
Avelumab first-line maintenance is a cost-effective treatment option for patients with locally advanced or metastatic urothelial carcinoma in Scotland #BladderCancer
Urothelial carcinoma (UC) includes cancers of the bladder, ureter and renal pelvis. It is the most common type of bladder cancer in the UK, accounting for >90% of cases [Citation1]. In 2017, an estimated 830 people in Scotland were diagnosed with bladder cancer, with 52.7% of cases diagnosed in people aged ≥75 years [Citation2]. Prognosis is poor for patients with locally advanced or metastatic UC (LA/mUC), with 1- and 5-year survival estimated to be 35.7 and <5%, respectively. While recent therapeutic advances including immunotherapy and antibody-drug conjugates have improved survival outcomes for some, in the real-world setting, both median and mean life expectancies are generally recognized to be less than 2 years [Citation3–6].
In Scotland, the current standard of care for LA/mUC (occurring anywhere in the urinary tract) is first-line systemic platinum-based chemotherapy (ie, cisplatin or carboplatin combined with gemcitabine) [Citation7]. Those who have ongoing clinical benefit at the end of chemotherapy will then enter a period of surveillance during which treatment is restricted to best supportive care (BSC). This is typically followed by second-line systemic anticancer therapy (typically, immunotherapy such as pembrolizumab, or chemotherapy) once the cancer progresses. While treatment options are limited for LA/mUC, current treatments have three main therapeutic aims: to delay disease progression, to improve and maintain health-related quality of life (HRQoL), and to extend life.
Avelumab is a human immunoglobulin G1 monoclonal antibody that activates immune responses against cancer cells by inhibiting the immune checkpoint protein PD-L1. It is licensed as first-line maintenance treatment for patients with LA/mUC, as well as monotherapy for patients with metastatic Merkel cell carcinoma and in combination with axitinib for patients with advanced renal cell carcinoma [Citation8,Citation9]. The European Society for Medical Oncology Bladder Cancer and European Association of Urology Guidelines have been updated to recommend the use of avelumab as maintenance in LA/mUC [Citation10,Citation11].
The efficacy and safety of avelumab first-line maintenance was explored in the LA/mUC setting in the pivotal phase III JAVELIN Bladder 100 trial (NCT02603432) [Citation12]. JAVELIN Bladder 100 was an international, multicenter, randomized controlled trial in 700 patients with LA/mUC whose disease had not progressed following first-line platinum-based chemotherapy and who were randomized 1:1 to receive either avelumab plus BSC (n = 350) or BSC alone (n = 350). Findings from the 21 October 2019, interim data cut demonstrated that avelumab provided a well-tolerated and efficacious treatment for patients with LA/mUC, with a median overall survival (OS) of 21.4 months with avelumab plus BSC versus 14.3 months with BSC alone; (hazard ratio [HR]: 0.69; 95% CI: 0.56 to 0.86) [Citation13]. Further analysis of patient-reported outcomes found that the extension in OS did not compromise quality of life [Citation14]. Although response rates to chemotherapy are relatively high, these responses are generally not durable, and most patients progress within a few months of completing chemotherapy, at which point they may be considered for second-line immunotherapy with pembrolizumab. However, response rates in the second-line setting are modest, and quality of life generally deteriorates rapidly after disease progression occurs.
In 2021, the Scottish Medicines Consortium (SMC) initiated its assessment of the clinical effectiveness and cost–effectiveness of avelumab within its marketing authorization for the treatment of LA/mUC in the maintenance setting (SMC2359) [Citation7]. The SMC published its outcome in August 2021, accepting avelumab as a treatment for routine use within NHS Scotland, and the appraisal considered modifiers for medicines that improve survival otherwise limited to less than 3 years [Citation7,Citation15]. As part of this assessment, a cost–effectiveness analysis (CEA) was conducted to inform the SMC’s decision-making.
In this study, we provide a detailed description of the economic model and associated inputs used to inform the SMC assessment beyond the information available in the public submission documentation. This is the first CEA of avelumab maintenance from a Scottish perspective that utilizes patient-level data from the pivotal JAVELIN Bladder 100 study.
Materials & methods
Model overview
A 3-state partitioned survival analysis model was constructed to assess the cost–effectiveness of avelumab plus BSC versus BSC alone. This model structure is commonly used to assess late-stage cancer interventions, including other checkpoint inhibitors in the same disease area [Citation16–18]. The model health states defined patients as either pre-progression, post-progression, or dead. Transitions between pre- and post-progression health states were inferred by extrapolated OS and progression-free survival (PFS) curves, and each health state was associated with different costs and HRQoL parameters. In addition to OS and PFS data, data concerning the time on treatment (ToT) with avelumab were also included in the model to capture the costs of treatment and its administration more accurately. A model schematic is presented in Supplementary Figure 1.
The outcomes of interest from the model were the differences in costs, life-years (LY) and quality-adjusted life-years (QALY), as well as the incremental cost–effectiveness ratio (ICER, or cost per QALY gained). The model was constructed from a Scottish NHS perspective and, aligned with Scottish guidance, both costs and QALYs were discounted at an annual rate of 3.5% [Citation19]. A time horizon of up to 25 years was adopted, with a cycle length of 1 week. The time horizon was considered sufficiently long to ensure that nearly 100% of patients had died by the end of the model (and thus considered a lifetime horizon), and a cycle length of 7 days was considered short enough to avoid the need for a half-cycle correction and to accurately reflect cost and health outcomes, and also correspond with the avelumab dosing regimen (800 mg intravenous [iv.] infusion once every 2 weeks).
Clinical model parameters
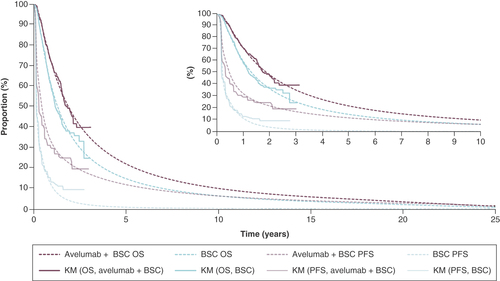
Data from the 21 October 2019, interim data cut of the pivotal phase III JAVELIN Bladder 100 trial were analyzed to populate the inputs of the three-state partitioned survival analysis model. Although this was an interim data cut, the pivotal phase III study data demonstrated that avelumab maintenance treatment extended the benefit of first-line platinum-based chemotherapy, with a statistically significant and clinically meaningful 7.1-month improvement in median OS (HR: 0.69; 95% CI: 0.56–0.86), as well as a significant improvement in PFS (HR: 0.62; 95% CI: 0.52–0.75). This data cut constitutes an interim analysis where the efficacy boundaries were crossed and thus represents the final analysis. Standard parametric survival models (PSM) were fitted to OS and ToT, while spline-based PSMs were implemented for PFS to better capture the shape of the Kaplan–Meier estimates (which falls at week 8 due to the protocol-driven first radiographical tumor assessment). Further details related to the PSMs (including log-cumulative hazard plots [Supplementary Figures 2–10]) and statistical goodness-of-fit scores [Supplementary Tables 1 & 2]) can be found in the electronic supplementary material.
In all instances, independent PSMs were considered due to the lack of evidence in support of a time-invariant treatment expressed either as a hazard or time ratio.
The fitted models were used to extrapolate these time-to-event outcomes over the 25-year time horizon. Where appropriate, OS extrapolations were adjusted to consider background mortality estimates (to ensure that the risk of death in the age- and sex-matched general population was never higher than that of the patients in the JAVELIN Bladder 100 cohort) [Citation20].
In line with published guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit, the selection of the best-fitting PSM for each outcome was based on statistical goodness of fit, the visual fit to the data and the long-term plausibility of the extrapolation [Citation21]. Please refer to the Supplementary Appendix for presentation of the PSM extrapolations (Supplementary Figures 2–10). Clinical input was obtained to ascertain when patients would be expected to discontinue treatment in the longer term, beyond the maximum duration of follow-up in JAVELIN Bladder 100. Eight practicing UK oncologists with a specialist interest in the management of urothelial cancer indicated that no more than 5% of patients would be expected to remain on treatment after 2 years and that by 5 years, all patients would have discontinued treatment. Therefore, adjustments were made to the ToT extrapolations to reflect this clinical opinion, and scenario analysis was conducted to explore uncertainty associated with earlier discontinuation of treatment and any possible implications in terms of survival projections.
Scenarios exploring a possible treatment-waning effect were implemented by assuming that from a given (arbitrary) time point, the effective HR was 1 (i.e., the hazard of an event with avelumab plus BSC is set to be the same as the modeled hazard of an event with BSC alone).
A summary of the efficacy applied in the base-case model is provided in .
BSC: Best supportive care; KM: Kaplan–Meier; OS: Overall survival; PFS: Progression-free survival.
To help ensure that the impact of adverse events (AE) was captured, the model also included cost and HRQoL impacts associated with the occurrence of AEs. The rates of AEs for both arms of the JAVELIN Bladder 100 trial were considered relevant for inclusion in the model if they occurred at grade ≥3 and in ≥2% of patients in either arm. Overall, the incidence of AEs of grade ≥3 in the JAVELIN Bladder 100 study was 47.4 and 25.2% for the avelumab plus BSC and BSC arms, respectively. However, on an individual AE basis, the occurrence of AEs was similar across both arms, indicating that the addition of avelumab was generally well tolerated. This is in line with other evidence available for avelumab in alternative treatment settings, which also indicates that avelumab is a well-tolerated treatment [Citation22,Citation23].
Health state utility values were applied within the model with a value of 0.772 for patients in PFS and 0.698 for progressed disease. The same utilities were applied irrespective of treatment arm in the base-case settings (with treatment-specific values considered in scenario analysis). HRQoL values were assessed for patients in JAVELIN Bladder 100 via the EQ-5D-5L questionnaire. In line with SMC guidance, EQ-5D-5L responses were converted into EQ-5D-5L utility scores using the ‘crosswalk’ algorithm by van Hout et al. [Citation24]. Mixed-effects linear regression with a random intercept for each patient (to account for the clustering of multiple observations for each patient) was used to estimate the utilities for both of the following health states: progression-free and progressed disease. Age-adjusted utilities were incorporated using an algorithm developed by Ara and Brazier to account for the natural decline in the population’s HRQoL over time [Citation25].
Disutilities due to the occurrence of AEs were incorporated within the model and applied as a one-off decrement in the first model cycle. To do this, treatment-emergent AEs of Grade ≥3 occurring in >1% of patients on either treatment arm of JAVELIN Bladder 100 were included, and a loss of QALYs was calculated based on the duration of days in which each AE event was expected to be experienced for, and a published disutility value. Disutility values and durations were based on published literature and/or assumption (please see Supplementary Table 3 for AE-specific input parameters) [Citation26–31]. The total decrement applied was based on the sum of each individual AE observed in JAVELIN Bladder 100.
Cost model parameters
Direct medical costs were included within the economic model, including costs associated with the acquisition of treatments, medical visits, laboratory tests and medical management associated with the occurrence of grade ≥3 AEs occurring in ≥2% of patients in either arm of the JAVELIN Bladder 100 trial.
Avelumab is available in a 200 mg/10 ml vial priced at £768.00 at the time of analysis, although in Scotland it is also available with a confidential patient access scheme discount, which was included in this analysis [Citation7,Citation32]. Per the summary of product characteristics, avelumab is administered as an iv. infusion once every 2 weeks at a dose of 800 mg [Citation8]. Because the comparator for avelumab plus BSC is BSC alone, no active drug costs were considered in the comparator arm.
Medical resource use (MRU) and monitoring assumptions were informed by a combination of prior UK-based Health Technology Assessment submissions and UK clinical experts, including Scotland-based clinicians, to ensure suitability of assumptions for Scottish practice [Citation33–35]. The average of the clinician estimates was taken and used to inform MRU estimates in the base case, with scenario analysis informed by a prior UK submission [Citation33]. MRU estimates differed by progression status, with patients in a pre-progression state incurring slightly greater MRU. No differences were assumed by treatment arm, as MRU was assumed to be linked to disease state only. Unit costs for MRU items were taken from the national reference costs database and the Personal Social Services Research Unit using the most recent versions available at the time of construction (cost year 2018–2019) [Citation36,Citation37]. An outpatient iv. administration cost was applied for iv. treatments, in accordance with the national reference costs database [Citation37]. Unit costs for the resolution of grade ≥3 AEs were also identified from the national reference costs database. Costs for AEs were applied in the same way as per the application of AE-related disutilities (i.e., as a one-off cost in the first model cycle based on the proportion of patients experiencing a given event on each arm in JAVELIN Bladder 100). AE-specific costs are provided in Supplementary Table 3.
Subsequent active treatments were modeled separately for avelumab plus BSC and BSC alone based on data from the JAVELIN Bladder 100 trial, with adjustments made based on expectations in Scottish clinical practice (i.e., any discordance between observed subsequent therapy use in the trial vs the Scottish LA/mUC treatment pathway based on the availability of treatments accepted by the SMC). Although patients in the trial received subsequent PD-1 and PD-L1 inhibitors, some of these treatments were not considered standard of care in routine practice in Scotland. Therefore, a simplifying assumption was made on the basis of clinical advice from eight practicing UK oncologists, costing these patients as receiving pembrolizumab (a PD-1 inhibitor), which is available in Scottish clinical practice [Citation17]. The only immunotherapy available in Scotland in the next line of therapy is pembrolizumab. Six different chemotherapy regimens were included: cisplatin, carboplatin, gemcitabine, docetaxel, paclitaxel and pemetrexed. The dosing regimens for each of the subsequent therapies were aligned with each product’s marketing authorization. In total, 68.52% of patients on the avelumab plus BSC arm and 86.06% of patients on the BSC arm in JAVELIN Bladder 100 went on to receive a subsequent therapy. For these patients, in accordance with the dosing regimens, market share estimates, and estimated durations, the cost of subsequent therapies was modeled as a weighted distribution of treatments relevant to Scottish practice.
The duration of subsequent therapy was estimated using data from JAVELIN Bladder 100 separately based on whether patients previously received avelumab plus BSC or BSC alone and based on subsequent use of immunotherapy (i.e., pembrolizumab) or chemotherapy. However, avelumab plus BSC patients were assumed to not receive subsequent pembrolizumab. For subsequent pembrolizumab, the average duration of treatment was 25.12 weeks (BSC alone). For chemotherapy, the average durations were 15.99 weeks (avelumab plus BSC) and 16.97 weeks (BSC alone). The cost of any oral medication administration was assumed to be zero, and the cost for administration of any iv. treatment was assumed to be the same as per avelumab.
A one-off terminal care cost taken from Round et al. and uplifted to 2019 values (using the Personal Social Services Research Unit inflation indices) was applied within the CEA, which was assumed to cover costs of supporting patients at a palliative stage before death [Citation38]. The same cost was applied to both treatment arms based on the proportion of patients who died in each model cycle.
A full list of model inputs (and corresponding uncertainty information) is provided in Supplementary Table 3 [Citation26–28,Citation30,Citation31,Citation36–39].
Model outputs
As previously described, the main outputs from the model were the total costs, QALYs and LYs associated with each treatment arm, which could then be used to calculate the ICER. Owing to the confidential nature of the patient access scheme discount for avelumab, the full breakdown of costs is not provided as part of the results of this study. The base-case results of the model were then investigated further through the exploration of sensitivity analyses, including a probabilistic sensitivity analysis (PSA), wherein each model parameter was varied within its plausible bounds, and deterministic sensitivity analyses, which explore the impact of individual settings and assumptions on model results. PSA was conducted by running 2000 probabilistic iterations.
Model validation
Model predictions of long-term outcomes were presented to eight UK-based practicing oncologists to verify that projections were reflective of clinical expectation and likely outcomes for patients with LA/mUC across the UK more widely. In addition, further opinion on the use of subsequent therapy was sought from two additional Scotland-based clinical experts.
Results
Base-case results
The base-case results are provided in . Avelumab plus BSC was estimated to provide an additional 12.3 months in a pre-progression health state, with a mean OS benefit of 12.0 months. These benefits translated to an additional 0.63 QALYs (equivalent to an additional 7.5 months in a state of full health) compared with BSC alone. The base-case ICER was £15,046, and consequently, the SMC considered avelumab to be a cost-effective treatment option for patients with LA/mUC in Scotland (though no explicit willingness-to-pay threshold was stated within the guidance produced by the SMC).
Table 1. Base-case results.
Probabilistic sensitivity analysis
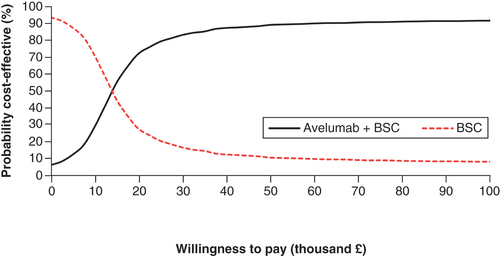
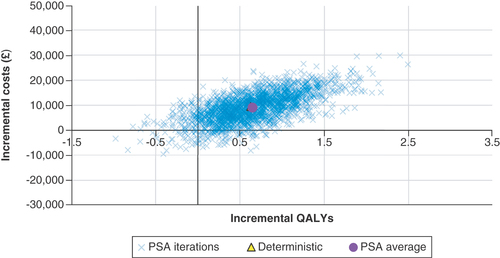
The results of the PSA are presented in , with a cost–effectiveness plot and corresponding cost–effectiveness acceptability curve provided in & .
Of the 2000 iterations, 88.90% had a resulting ICER that was below £30,000 per QALY gained, indicating that avelumab plus BSC was cost-effective. In total, 4.80% of the iterations indicated that BSC would dominate avelumab and 4.40% indicated that avelumab plus BSC would dominate BSC.
BSC: Best supportive care; ICER: Incremental cost–effectiveness ratio; PSA: Probabilistic sensitivity analysis; QALY: Quality-adjusted life-year.
One-way sensitivity analysis
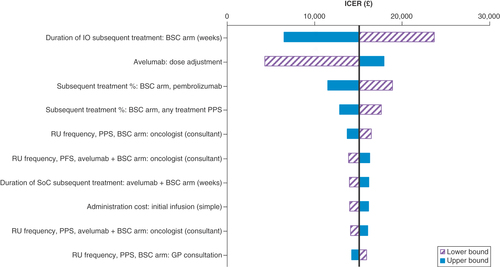
The results of the one-way sensitivity analysis are presented in , which shows the parameters that had the largest impact on the ICER when varied in isolation. The parameter with the greatest influence on the cost–effectiveness outcome was the duration of subsequent therapy on the BSC arm. Other relevant parameters of interest were assumptions related to resource use and subsequent treatment.
Note: Drug costs are considered fixed in this one-way sensitivity analysis, and so the acquisition cost is not reflected within the tornado diagram.
BSC: Best supportive care; admin: Administration; GP: General practitioner; ICER: Incremental cost–effectiveness ratio; IO: Immunotherapy; PFS: Progression-free survival; PPS: Post-progression survival; prog: Progression; RU: Resource use; SoC: Standard of care; subs: Subsequent; tx: Treatment.
Deterministic sensitivity analyses
Extensive deterministic sensitivity analyses were performed to understand key areas of uncertainty within the CEA. This included various alternative assumptions regarding the PFS and OS of patients receiving avelumab, the approach to HRQoL data and the application of a treatment-waning effect. The results of the scenarios are presented in .
Table 2. Scenario analysis results.
The scenarios found to have the largest impact on results were related to OS distribution assumed. Changes to the choice of survival models for the time-to-event outcomes also had the potential to increase the ICER, although alternative choices for extrapolations of OS were contradictory to clinical opinion received. The approach to informing the distributions of subsequent therapies after progression also had the potential to increase the ICER. Assuming a stopping rule with and without the application of a treatment-waning effect did not lead to a large difference in the ICER, suggesting that the unadjusted projections of both arms yielded similar long-term hazards. Treatment-specific utility values were also explored, which resulted in a small variation in the ICER.
Discussion
In this study, we present the details and subsequent findings of a CEA of avelumab plus BSC versus BSC alone for the treatment of LA/mUC in Scotland. This analysis resulted in the SMC considering avelumab a clinically effective and cost-effective treatment option for patients with LA/mUC in Scotland. The analysis adds to the growing body of evidence supporting its availability in a range of countries where it is the newly established standard of care for patients treated with first-line platinum-based chemotherapy whose disease has not progressed (including recommendation from NICE, with similar requirements to the SMC) [Citation40]. The survival benefits attributable to avelumab in this setting are unprecedented within the context of this treatment setting, where current BSC consists of no active treatment and a surveillance strategy, considering further treatment only at the point of disease progression.
The CEA was informed primarily by patient-level data collected as part of the pivotal JAVELIN Bladder 100 trial. Findings were consistent with those of previously published CEAs in other jurisdictions, with consistent LY and QALY gains associated with treating patients with avelumab [Citation41–43]. However, several simplifications were factored into the model due to limitations of the evidence available to inform model parameters, both for our analysis and previous analyses. For example, BSC costs were omitted since these vary across individual patients and over time, though ultimately were not expected to influence cost–effectiveness results greatly.
The JAVELIN Bladder 100 trial provides data regarding the safety and efficacy of avelumab plus BSC versus an appropriate comparator relevant for decision-making in Scotland. Although the CEA is based on an interim analysis with immature OS data, which is a limitation of the analysis, the CEA made full use of the extensive data reported within the trial, including efficacy and safety data, subsequent treatment duration and HRQoL information. Where appropriate, adjustments were made at the validation stage based on feedback from UK clinicians. While data from JAVELIN Bladder 100 were considered broadly generalizable to Scottish practice, the use of subsequent immunotherapies after avelumab was considered a limitation, as consecutive immunotherapy treatments are not considered as part of clinical practice in Scotland. However, consecutive use of immunotherapy only occurred for 6.3% of the intention-to-treat population. Second-line immunotherapies are available in England, Wales and Scotland; therefore, the decision problem is the same across the UK, and the cost–effectiveness outcomes result should be considered as generalizable to England and Wales, as further demonstrated by the recent NICE recommendation for avelumab in mUC [Citation40]. Although costs were adapted within the model, it was challenging to ascertain how the use of subsequent therapy may have influenced efficacy estimates. Scenario analysis indicated that while subsequent therapies may be a limitation of the Scottish CEA, adapting inputs to reflect local practice was not a key driver in cost–effectiveness outcomes, and the overarching results still indicated avelumab was a cost-effective treatment.
The cost–effectiveness model adopted a simple yet intuitive structure, allowing for transparent interpretation of results from the JAVELIN Bladder 100 trial. A comprehensive set of sensitivity analyses (beyond those presented within the SMC guidance) further demonstrated the robustness of the evidence and modeling assumptions. The results were most sensitive to alternative survival extrapolations, which is typical of an oncology product’s appraisal with maturing evidence. Relatedly, the key limitations of this study were related to the certainty of OS and ToT estimates associated with avelumab as a maintenance therapy. With the availability of longer-term data from the JAVELIN Bladder 100 trial, the uncertainty regarding long-term survival outcomes for patients treated with avelumab will diminish [Citation44]. Despite uncertainty linked to OS and ToT, extensive scenario analyses were conducted, and avelumab was shown to consistently offer an incremental LY and QALY gain compared with BSC, and model results showed avelumab to be a cost-effective treatment strategy.
Conclusion
Avelumab first-line maintenance immunotherapy with BSC was considered by the SMC to be a cost-effective treatment strategy for Scottish patients with LA/mUC that has not progressed on or after first-line platinum-based chemotherapy. The use of avelumab as a maintenance therapy represents a step-change in the management of LA/mUC, offering a clinically and cost-effective therapy for patients in NHS Scotland practice who are otherwise faced with no active treatment alternatives until disease progression.
In international treatment guidelines, platinum-based chemotherapy, followed by avelumab maintenance in patients without disease progression, is the standard-of-care first-line treatment for locally advanced or metastatic urothelial cancer (LA/mUC).
We describe the economic model and associated inputs that informed the Scottish Medicines Consortium’s assessment of the cost–effectiveness of avelumab first-line maintenance treatment for patients with LA/mUC.
A partitioned survival analysis model was constructed to assess the cost–effectiveness of avelumab plus best supportive care (BSC) compared with BSC alone.
The model incorporated data from the pivotal JAVELIN Bladder 100 phase III trial, with parametric survival models fitted for key efficacy endpoints.
Direct medical costs incorporated in the model included treatment acquisition costs, medical visits, laboratory tests, costs associated with managing grade ≥3 adverse events and costs of subsequent therapies.
Avelumab plus BSC was estimated to provide an additional 12.3 months in a pre-progression health state and a mean OS benefit of 12.0 months, translating into an additional 0.63 quality-adjusted life-years (additional 7.5 months in a state of full health) versus BSC alone.
The base-case incremental cost–effectiveness ratio was £15,046.
Comprehensive sensitivity analyses further demonstrated the robustness of the evidence and modeling assumptions.
Based on these analyses, the Scottish Medicines Consortium considered avelumab to be a cost-effective treatment option for patients with LA/mUC in Scotland.
Author contributions
Concept and design: S Critchlow, A Bullement, Y Xiao, V Kapetanakis, Á Benedict, J Chang, and M Kearney. Analysis and interpretation of data: S Critchlow, A Bullement, Y Xiao, V Kapetanakis, Á Benedict, J Chang, and M Kearney. Drafting of the manuscript: S Critchlow, A Bullement, J Chang, and M Kearney. Statistical analysis: S Critchlow, A Bullement, S Crabb, R Jones, K Christoforou, A Amin, Y Xiao, V Kapetanakis, Á Benedict, and A Eccleston.
Acknowledgments
The authors thank the patients, families, investigators, co-investigators and study team members of the JAVELIN Bladder 100 trial (NCT02603432), without whom this study would not have been possible.
Financial disclosure
This study was sponsored by Merck (CrossRef Funder ID: 10.13039/100009945) and was previously conducted under an alliance between Merck and Pfizer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
Employees of the sponsor were involved in the model design and validation and interpretation of the results. Employees of the sponsor also participated in the preparation, review, and approval of the manuscript. S Critchlow and A Bullement are employees of Delta Hat Limited, Nottingham, UK, which received funding Merck and Pfizer for conducting this work. S Crabb is an employee of the University Hospital Southampton NHS Foundation Trust, Southampton, UK. He reports consulting or advisory roles for Roche, Janssen Cilag, MSD, Astellas, Bayer, and AstraZeneca, and research funding provision from Astex Pharmaceuticals, AstraZeneca, and Clovis Oncology. R Jones is an employee of the University of Glasgow, UK. He reports consulting fees from Janssen, Astellas, Bayer, Novartis, Pfizer, Merck, MSD, Roche, Ipsen, and BMS; research grants from Exelixis, Astellas, Clovis, and Bayer; speaker honoraria from Janssen, Astellas, Bayer, Pfizer, Merck, MSD, Roche, Ipsen, and BMS; support for travel and attending meetings from Bayer; and participation on a data safety monitoring board or advisory board for Roche. K Christoforou was an employee of Merck Serono Ltd, Feltham, UK, an affiliate of Merck KGaA, at the time of study. A Amin is an employee of Merck Serono Ltd, Feltham, UK, an affiliate of Merck KGaA. Y Xiao and V Kapetanakis are employees of Evidence Synthesis, Modeling & Communication, Evidera, London, UK. Á Benedict is an employee of Evidence Synthesis, Modeling & Communication, Evidera, Budapest, Hungary. Evidera received funding from Merck and Pfizer. J Chang is an employee of Pfizer and owns stock in Pfizer, BMS, and Bayer. M Kearney is an employee of Merck. A Eccleston is an employee of Pfizer. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
Editorial support was provided by Clinical Thinking and funded by Merck and Pfizer.
Supplementary figure
Download PNG Image (426.9 KB)Supplemental figure
Download PNG Image (330.4 KB)Supplemental figure
Download PNG Image (410.8 KB)Supplemental figure
Download PNG Image (415.9 KB)Supplemental figure
Download PNG Image (172.4 KB)Supplemental figure
Download PNG Image (404.2 KB)Supplemental figure
Download PNG Image (342.1 KB)Supplemental figure
Download PNG Image (464.7 KB)Supplemental figure
Download PNG Image (388.1 KB)Supplemental figure
Download PNG Image (186.2 KB)Supplementary Material
Download MS Word (53.4 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/fon-2023-0372
References
- Cancer.Net . Bladder cancer: introduction. www.cancer.net/cancer-types/bladder-cancer/introduction (Accessed: 21 April 2023 ).
- Public Health Scotland . Cancer statistics: bladder cancer. www.isdscotland.org/Health-Topics/Cancer/Cancer-Statistics/Bladder/ (Accessed: 21 April 2023 ).
- Davies F, Knott C, Kerr C, Lockhat M, Adedokun L. Utilising routine health data to establish a standing cohort of patients with metastatic bladder cancer: initial results and algorithm defining disease progression. europe2020-ispor.ipostersessions.com/Default.aspx?s=95-56-36-01-D0-00-15-AA-7F-9F-D4-84-35-78-E1-C7 (Accessed: 21 April 2023 ).
- Kearney M, Knott C, Lamy FX, Harnett J, Amin A, Verpillat P. Treatment patterns and clinical outcomes in patients with locally advanced or metastatic urothelial cancer in England: results of a longitudinal observational cohort study. Value Health 23(Suppl. 2), S483 (abstract PCN341) (2020).
- Cheeseman S, Thompson M, Sopwith Wet al. Current treatment and outcomes benchmark for locally advanced or metastatic urothelial cancer from a large UK-based single centre. Front. Oncol. 10, 167 (2020).
- Powles T, Huddart RA, Elliott Tet al. Phase III, double-blind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2-positive metastatic bladder cancer. J. Clin. Oncol. 35(1), 48–55 (2017).
- Scottish Medicines Consortium . SMC2359: avelumab 20 mg/ml concentrate for solution for infusion (Bavencio®). www.scottishmedicines.org.uk/media/6187/avelumab-bavencio-final-july-2021-amended-050821-for-website.pdf (Accessed: 21 April 2023 ).
- European Medicines Agency . Bavencio (avelumab). www.ema.europa.eu/en/documents/product-information/bavencio-epar-product-information_en.pdf (Accessed 21: April 2023).
- Electronic Medicines Compendium . Bavencio 20 mg/ml concentrate for solution for infusion. www.medicines.org.uk/emc/product/8453/smpc#grefJ (Accessed: 21 April 2023 ).
- Powles T, Bellmunt J, Comperat Eet al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 33(3), 244–258 (2022).
- Cathomas R, Lorch A, Bruins HMet al. The 2021 updated European Association of Urology guidelines on metastatic urothelial carcinoma. Eur. Urol. 81(1), 95–103 (2022).
- ClinicalTrials.gov . A study of avelumab in patients with locally advanced or metastatic urothelial cancer (JAVELIN Bladder 100). clinicaltrials.gov/ct2/show/NCT02603432 (Accessed: 21 April 2023 ).
- Powles T, Park SH, Voog Eet al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 383(13), 1218–1230 (2020).
- Grivas P, Kopyltsov E, Su PJet al. Patient-reported outcomes from JAVELIN Bladder 100: avelumab first-line maintenance plus best supportive care versus best supportive care alone for advanced urothelial carcinoma. Eur. Urol. 83(4), 320–328 (2023).
- Scottish Medicines Consortium . Avelumab (Bavencio). www.scottishmedicines.org.uk/medicines-advice/avelumab-bavencio-full-smc2359/ (Accessed: 21 April 2023 ).
- Scottish Medicines Consortium . Atezolizumab 1,200mg concentrate for solution for infusion (Tecentriq®). www.scottishmedicines.org.uk/media/3134/atezolizumab-tecentriq-for-uc-final-feb-2018-for-website.pdf (Accessed: 21 April 2023 ).
- Scottish Medicines Consortium . Pembrolizumab 25mg/ml concentrate for solution for infusion and 50mg powder for concentrate for solution for infusion (Keytruda®). www.scottishmedicines.org.uk/media/3121/pembrolizumab_keytruda_final_jan_2018_for_website.pdf (Accessed: 21 April 2023 ).
- Scottish Medicines Consortium . Nivolumab 10mg/ml concentrate for solution for infusion (Opdivo®). www.scottishmedicines.org.uk/media/3114/nivolumab_opvido_final_dec_2017_for_website.pdf (Accessed: 21 April 2023 ).
- Scottish Medicines Consortium . Working with SMC: a guide for manufacturers. www.scottishmedicines.org.uk/media/7486/working-with-smc.pdf (Accessed: 21 April 2023 ).
- Office for National Statistics . National life tables: Scotland. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesscotlandreferencetables (Accessed: 21 April 2023 ).
- Latimer N. NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data. www.sheffield.ac.uk/media/34225/download?attachment (Accessed: 21 April 2023 ).
- Kelly K, Infante JR, Taylor MHet al. Safety profile of avelumab in patients with advanced solid tumors: a pooled analysis of data from the phase 1 JAVELIN solid tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer 124(9), 2010–2017 (2018).
- Ascierto PA, Orlova K, Grignani Get al. Avelumab expanded access program in metastatic Merkel cell carcinoma: efficacy and safety findings from patients in Europe and the Middle East. Int. J. Cancer 149(11), 1926–1934 (2021).
- van Hout B, Janssen MF, Feng YSet al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 15(5), 708–715 (2012).
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 13(5), 509–518 (2010).
- Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual. Life Outcomes 6, 84 (2008).
- Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med. Decis. Making. 26(4), 410–420 (2006).
- Beusterien KM, Davies J, Leach Met al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual. Life Outcomes 8, 50 (2010).
- National Institute for Health and Care Excellence . Nivolumab with ipilimumab for untreated advanced renal cell carcinoma. http://www.nice.org.uk/guidance/ta581/history (Accessed: 21 April 2023 ).
- National Institute for Health and Care Excellence . Ramucirumab for treating advanced gastric cancer or gastro-oesophageal junction adenocarcinoma previously treated with chemotherapy. www.nice.org.uk/guidance/ta378/history (Accessed: 21 April 2023 ).
- Gauci ML, Baroudjian B, Zeboulon Cet al. Immune-related hepatitis with immunotherapy: are corticosteroids always needed? J. Hepatol. 69(2), 548–550 (2018).
- National Institute for Health and Care Excellence . BNF: British National Formulary – avelumab. bnf.nice.org.uk/medicinal-forms/avelumab.html (Accessed: 21 April 2023 ).
- National Institute for Health and Care Excellence . Vinflunine for the treatment of advanced or metastatic transitional cell carcinoma of the urothelial tract. www.nice.org.uk/guidance/ta272/history (Accessed: 21 April 2023 ).
- National Institute for Health and Care Excellence . Atezolizumab for untreated PD-L1-positive locally advanced or metastatic urothelial cancer when cisplatin is unsuitable. www.nice.org.uk/guidance/ta492/history (Accessed: 21 April 2023 ).
- National Institute for Health and Care Excellence . Pembrolizumab for untreated PD-L1-positive locally advanced or metastatic urothelial cancer when cisplatin is unsuitable. www.nice.org.uk/guidance/ta522/history (Accessed: 21 April 2023 ).
- Curtis L, Burns A. Unit costs of health and social care 2019. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2019/ (Accessed: 21 April 2023 ).
- NHS England . 2018/19 National Cost Collection data publication. www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication/ (Accessed: 21 April 2023 ).
- Round J, Jones L, Morris S. Estimating the cost of caring for people with cancer at the end of life: a modelling study. Palliat. Med. 29(10), 899–907 (2015).
- National Institute for Health and Care Excellence . BNF: British National Formulary. bnf.nice.org.uk/ (Accessed: 21 April 2023 ).
- National Institute for Health and Care Excellence . Avelumab for maintenance treatment of locally advanced or metastatic urothelial cancer after platinum-based chemotherapy. www.nice.org.uk/guidance/ta788/history (Accessed: 21 April 2023 ).
- Peng Y, She Z, Peng Let al. Cost–effectiveness of avelumab maintenance therapy for advanced or metastatic urothelial carcinoma in the United States. Adv. Ther. 38(12), 5710–5720 (2021).
- Karttunen E, Jaaskelainen S, Hervonen P, Chang J, Kearney M. Cost–effectiveness of avelumab as first-line maintenance treatment for locally advanced or metastatic urothelial carcinoma in Finland. Poster presented at: ISPOR Europe 2021. Virtual, 30 November–3 December 2021. www.ispor.org/heor-resources/presentations-database/presentation/euro2021-3409/112147 (Accessed: 21 April 2023 ).
- Chang WC, Xiao Y, Lin AYet al. Cost–effectiveness analysis of avelumab plus best supportive care (BSC) vs BSC alone as a first-line (1L) maintenance treatment for patients with locally advanced or metastatic urothelial carcinoma in Taiwan. Poster presented at: ISPOR Europe 2021. Virtual, 30 November–3 December 2021. www.ispor.org/heor-resources/presentations-database/presentation/euro2021-3407/112259 (Accessed: 21 April 2023 ).
- Powles T, Park SH, Caserta Cet al. Avelumab first-line maintenance for advanced urothelial carcinoma: results from the JAVELIN Bladder 100 trial after ≥2 years of follow-up. J. Clin. Oncol. 41(19), 3486–3492 (2023).