Abstract
Purpose: Sub-lethal temperature elevations in the tumour incurred during laser cancer therapy can induce heat shock protein (HSP) expression leading to enhanced tumour survival and recurrence. Nanoshells utilised in combination with laser therapy can potentially enable selective heat deposition, greater thermal injury, and diminished HSP expression in the tumour. The study objective was to measure the distribution of temperature and HSP expression in prostate tumours in response to laser therapy alone or with nanoshells to determine if these combinatorial therapies can minimise HSP expression.
Methods: PC3 cells were inoculated in the backs of CB17-Prkd c SCID/J mice and treated with external laser irradiation (wavelength of 810 nm, irradiance of 5 W/cm2, spot size of 5 mm, and heating duration of 3 min) alone or in combination with gold nanoshells (diameter of 55 nm and outer gold shell thickness of 10 nm) introduced into the tumour 24 h prior to laser treatment. Magnetic resonance temperature imaging was used to measure the distribution of temperature elevation in the tumours during laser treatment. Tumours were sectioned 16 h following laser treatment, stained for Hsp27 and Hsp70, imaged with a confocal microscope, and HSP expression levels were quantified as a function of depth in the tumours.
Results: Maximum temperature elevations at the tumour surface were 28°C for laser treatment only and 50°C for laser heating in combination with gold nanoshells. Laser therapy alone caused significant induction of HSP expression in the first few millimeters of the tumour depth, whereas decreasing HSP expression occurred with greater tumour depth. Tumours treated with laser and nanoshells experienced substantial temperatures (73–78°C) at the tumour surface and temperatures greater than 53°C in the first few millimeters which eliminated HSP expression.
Conclusion: Inclusion of nanoshells in laser therapy can provide a mechanism for enhancing heat deposition capable of eliminating HSP expression within a larger tumour region compared to laser heating alone.
Introduction
Laser ablative therapies can provide a minimally invasive and potentially more effective treatment than conventional resection procedures for cancer therapy Citation[1–5]. Treatment volumes and durations are dependent upon the temperature distribution generated by the geometry of the fibre tip and the expanding thermal boundary. Therefore, laser therapy effectiveness is limited due to non-specific heating, which can potentially lead to undesirable healthy tissue injury. Treatment of larger tissue volumes with this method requires thermal diffusion from the source, resulting in extended procedural durations and poorly defined lesion boundaries. The efficacy of laser therapies can be further compromised due to induction of molecular chaperones known as heat shock proteins (HSPs) in regions of the tumour where non-lethal temperature elevation occurs resulting from either insufficient or non-selective thermal deposition Citation[6–10]. Temperatures greater than 43°C can induce HSP expression and cause thermal necrosis. Up-regulation of HSP expression by tumour cells can increase tumour recurrence by enhancing cell viability and diminishing effectiveness of chemotherapy and radiation treatments that are employed in conjunction with hyperthermia Citation[11–14]. HSPs have been implicated in multi-drug resistance Citation[15–17], regulation of apoptosis Citation[18–22], and modulation of p53 Citation[18], Citation[23], Citation[24] for a broad range of neoplastic tissues.
Hsp27 and Hsp70 have been demonstrated to be significantly up-regulated in response to thermal stress with the magnitude of expression directly dependent on temperature elevation and heating duration Citation[6–9], Citation[25–32]. Elevated expression of Hsp27 is a poor prognostic marker for invasive prostatic carcinoma Citation[13] and is associated with tumour cell protection by inhibition of apoptosis Citation[19–24]. Increased expression of Hsp70 mitigates the damaging effects of stress Citation[33], Citation[34] with enhanced survival documented for breast and cervical cancers Citation[24], Citation[35]. The cellular protection provided by HSPs may be attributed to their prominent role in regulation of cell proliferation, prognosis, and drug resistance Citation[17], Citation[36], Citation[37]. A study performed by Beckham et al. illustrated that Hsp70 is critical for cell survival by comparing the responses of Hsp70 knockout murine embryonic fibroblasts (MEF) and control MEF to various mild heat treatments Citation[37]. Recent evidence suggests Hsp70 has a role in the control of cell cycling and growth Citation[38–40]. A positive correlation between Hsp70 levels and proliferative activity has been demonstrated in immunohistochemical studies of breast tumours Citation[12]. Hsp27 and Hsp70 regulate reactive oxygen species by a glutathione-dependent pathway Citation[41], Citation[42], providing protection for intracellular proteins and partially explaining their protective effect against chemotherapeutic agents Citation[14], Citation[15], Citation[38], Citation[43], Citation[44].
Photoabsorbing nanoparticles targeted to tumour cells and used in combination with laser therapy may enable selective control of heat deposition, thermal injury, and HSP expression in the tumour and healthy tissue. Ultimately this control can permit reduced injury to normal surrounding tissue and mitigate the protective effects of HSPs, leading to reduced tumour recurrence Citation[1], Citation[7], Citation[8], Citation[45–52]. Nanoparticles such as gold nanoshells Citation[1], Citation[45–50], single and multi-walled carbon nanotubes Citation[7], Citation[8], Citation[51–56], and single walled carbon nanohorns Citation[56–60] have been shown to possess significant optical absorption in the near infrared wavelength range of 800–1200 nm where light penetration in tissue is in the order of 1 centimeter. When these particles are included in vitro and in vivo and coupled with laser irradiation, greater temperature elevation and tumour cell death is achieved with a lower laser dose Citation[1], Citation[7], Citation[8], Citation[45–60].
Metal nanoshells that consist of a spherical dielectric core (e.g. silica) surrounded by a metal shell (e.g. gold) have been demonstrated as promising photoabsorbers for laser based cancer treatment Citation[1], Citation[45–50]. Nanoshells possess a highly tunable plasmon resonance that determines the particle's scattering and absorbing properties. The plasmon resonance, and in turn the nanoshell's optical properties, can be tuned across a broad range of the optical spectrum from the ultraviolet to the infrared by controlling the relative core and shell thickness Citation[1], Citation[45–50], Citation[61–63]. Previous studies by O’Neal and Hirsch et al. have demonstrated significantly increased temperature elevation measured with magnetic resonance temperature imaging (MRTI) (ΔT = 37°C for nanoshells + laser irradiation and ΔT = 10°C for laser irradiation only) and tumour regression following treatment with a continuous wave diode laser (wavelengths of 808 or 820 nm at an irradiance of 4 W/cm2 for durations of 3–6 min) in combination with gold nanoshells compared to laser heating alone Citation[47], Citation[48]. Other groups have compared the heating kinetics, spatial temperature distribution, and morphological alterations in tissue following hypodermic and intramuscular injections of gold nanoshells in combination with irradiation with a continuous wave (wavelength of 810 nm, power of 2 W, duration of 10–360 s) or pulsed diode laser (wavelength of 810 nm, peak power of 8 W, on–off time ratio of 0.25, and pulse duration of 1 ms) Citation[49], Citation[64]. These studies demonstrated the capability of nanoshells in combination with irradiation by a continuous wave or pulsed laser to achieve a temperature rise of 46–50°C within a duration of 40 s and cause targeted tumour destruction at a depth of 4 mm while preserving overlying skin. Gold nanoshells combined with silica rattles and drugs can permit multifunctional photothermal treatment and chemotherapy delivery Citation[65]. Computational models have been created to predict temperature elevation and thermal injury associated with nanoshell inclusion in therapies using continuous wave or pulsed lasers, and confirmed their capability for enhanced heat generation and tumour destruction Citation[64], Citation[66], Citation[67].
Although previous studies have provided knowledge of the relationship of nanoshell properties and laser parameters with optical absorption, temperature elevation, and thermal injury Citation[1], Citation[45–50], the spatial distribution of HSP expression in tumours following treatment with laser irradiation and gold nanoshells has not been measured. This information is critical for accurately predicting the likelihood of tumour survival and recurrence following hyperthermia therapies. We have characterised the thermally induced kinetics of Hsp27 and Hsp70 levels following hyperthermia applied with water bath heating of normal (RWPE-1 cells) and cancerous prostate cells (PC3 cells) for elevated temperatures of 44–60°C and periods of 1–30 min Citation[26]. We have also determined the HSP expression and cell viability of kidney and prostate cancer cells in response to laser heating and multi-walled carbon nanotubes Citation[7]. In order to accurately predict the response of in vivo kidney tumours to laser therapy and multi-walled carbon nanotubes, we have measured the spatial distribution of Hsp27, Hsp70, and Hsp90 and correlated this with MRTI measured temperature and tumour regression Citation[8]. Other studies have utilised a bioluminescent reporter gene to measure Hsp70 expression, determine the zone of thermal damage Citation[68], and provide more comprehensive characterisation of cell death kinetics after heat shock Citation[8], Citation[9]. This technique can be adapted to in vivo models, which is imperative in determining effective thermal therapy protocols Citation[10].
This is the first study measuring and quantifying HSP expression associated with laser irradiation of prostate tumours in combination with nanoshells and correlating this information with temperature distribution measured with MRTI. This paper will explore whether inclusion of nanoshells in laser therapy can effectively minimise HSP expression in the tumour compared to laser heating alone.
Methods
Tumour preparation
Human prostate cancer cells (PC-3) (CRL-1435, American Type Culture Collection (ATCC), Manassas, VA) were cultured with Ham's F12 medium (30-2004, ATCC) supplemented with 10% FBS (30-2020, ATCC) and 5% penicillin-streptomycin (15140-122, Gibco, Carlsbad, CA). On the day of tumour inoculation, PC-3 cells were harvested from culture and counted in a haemocytometer with trypan blue to determine cell number and viability. Cells were centrifuged at 1500 rpm for 7 min. Supernatant was removed and 3 × 107 PC3 cells were resuspended in 1400 µl ice cold BD Matrigel™ Matrix to promote tumour cell growth. Mice were anaesthetised with isoflurane. PC3 cells were inoculated into the backs of 4–6 week old female CB17-Prkd c SCID/J mice with an injection volume of 0.2 mL containing 5 × 106 PC3 cells in Matrigel for each mouse. Prostate tumours were grown to a tumour burden of 1.0 cm in diameter (∼3 weeks). Twenty mice were employed: five controls with no treatment, five with introduction of nanoshells, five with laser heating only, and five with nanoshells and laser therapy.
Laser heating protocols
External laser irradiation was employed rather than intratumoural heating to prevent perturbation of the tissue associated with probe insertion, which could elicit HSP expression due to tissue trauma and inflammation as described previously Citation[48]. The laser parameters employed were laser wavelength of 810 nm, laser irradiance of 5 W/cm2, laser spot size of 5 mm, and heating duration of 3 min. The chosen laser parameters enabled induction of HSP expression gradients and varying levels of necrosis in tumours exposed to laser alone or in combination with nanoshells. For treatments utilising nanoshells and laser heating, nanoshells were injected into the mouse tail vein 24 h prior to laser irradiation to enable adequate nanoshell accumulation in the tumour volume as confirmed by prior studies with laser treatment with nanoshells and multi-walled carbon nanotubes Citation[8], Citation[47], Citation[48]. Nanoshells furnished by Nanospectra Biosciences (Houston, TX) and composed of a silica core with a diameter of 55 nm and an outer gold shell thickness of 10 nm were utilised due to their capability for absorption at 808 nm and significant temperature elevation and tumour regression when laser irradiated Citation[48].
Temperature measurement
Magnetic resonance imaging (MRI) was used for planning, localisation, and monitoring of the temperature distribution during laser treatment. The distribution of temperature increase was measured by magnetic resonance temperature imaging (MRTI) via the proton-resonance frequency-shift method Citation[48], Citation[69–72] to facilitate correlation of thermal dose with HSP expression distribution. Imaging was performed in a 1.5 Tesla MR scanner (Signa Echospeed, General Electric Medical Systems, Milwaukee, WI) using a 3-inch (7.62 cm) receive-only surface coil specially designed for small animal imaging (courtesy of R. Giaquinto, General Electric Corporate Research and Development, Schenectady, NY). T1- and T2-weighted images were used to plan and localise the treatment by verifying the position of the laser fibre (via a fiducial marker placed on the fibre) relative to the imaged region prior to irradiation. MRTI was performed by using a complex phase-difference technique applied to a fast, 2-D radiofrequency-spoiled gradient-recalled echo sequence (TR/TE = 74 ms/10 ms, flip angle = 30°, bandwidth = 9.62 kHz). To achieve a 5-s per image scan time, a rectilinear field of view (4 × 2 cm2) was used with a prescribed 256 × 128 matrix and acquired voxel size was 0.16 × 0.31 × 3 mm3. Change in temperature from baseline after N images (ΔTN) was extrapolated from the complex-valued MRTI data (S) by using the temperature dependence of the proton resonance frequency shift Citation[72] and a temperature sensitivity coefficient (α) of −0.01 ppm/°C Citation[72] according to equation 1:where δTi is the temperature difference measured between the i and (i − 1) images, γB0 is the resonance frequency (63.87 MHz), and TE is the sequence echo time Citation[72]. The images were smoothed with a 3 × 3 Wiener filter. The temperature resolution for MRTI measured temperature difference is less than 1°C.
HSP expression measurement
Mice were sacrificed 16 hours following laser treatment based on our earlier work, which demonstrated that maximum HSP expression was induced following thermal stress at this time Citation[7], Citation[8], Citation[26]. This time-frame also enabled the maximum HSP expression signal to be measured which is critical to allow sufficient comparison between tumours treated under varying conditions and between different locations within a single tumour. Subsequently, tumours were flash-frozen and embedded into OCT cryo-matrix. At every 1 mm increment within the tumour, five sections with a thickness of 5 microns were obtained. Tumour sections were adhered to poly-lysine coated, wide format glass slides and fixed in cold acetone for 10 min. The location of the laser fibre was denoted on each section as a reference for analysis to enable correlation of HSP expression with probe position and corresponding temperature distribution. Tissue sections were enumerated in a grid-format (e.g. 1.1, 1.2, 1.3, etc.) to ensure that the spatial location of each tissue fragment was known in relation to the distribution of the immunofluorescence staining in each corresponding slice.
Immunofluorescence staining of frozen tissue samples was employed to localise and quantify HSP expression following heating. Tissue sections were incubated with Triton X-100 solution for 15 min to permeabilise cell membranes to HSP antibodies and fluorochromes. All incubations were conducted in an incubator (T = 37°C and 5% CO2). Primary monoclonal isotype-specific antibodies were employed to increase specificity of epitope binding and decrease background fluorescence. Next, tissue sections underwent blocking in 1.5% goat anti-mouse sera (SC-2043, Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h to decrease non-specific binding. Tissues were then incubated with Hsp70 primary mouse monoclonal IgG2A antibody (SC-24, Santa Cruz Biotechnology) for 1 h followed by incubation with its secondary antibody Rhodamine Red-X (IgG2A) (115-295-206, Jackson ImmunoResearch, West Grove, PA) for 1 h. Next, tissue sections were incubated with biotinylated Hsp27 mouse monoclonal IgG1 primary antibody (ADI-SPA-800B, Assay Designs, Ann Arbor, MI) for 1 h followed by incubation with its secondary antibody, Cy-2 conjugated streptavidin antibody (016-220-084, Jackson ImmunoResearch) for 1 h. Mounting medium was added, tissues were enclosed in cover glass, and samples were stored at 8°C.
Observation of HSP expression levels was accomplished with a 3D laser scanning confocal microscope (Leica SP2 AOBS). An argon laser (λ = 488 nm) was employed for excitation of the Cy2-streptavidin conjugate to enable visualisation and quantification of Hsp27 expression. A helium-neon laser (λ = 543 nm) was utilised for excitation of the Rhodamine Red-X dye permitting visualisation and quantification of Hsp70 expression. HSP expression was quantified using the Leica Lite image processing software. Histograms of fluorescence in the field of view from five different regions in five separate samples were generated and the mean fluorescence of each image was averaged for all five images. Normalised HSP expression concentration, HSPNorm, was calculated with the following equation:where HSPFinal is the HSP concentration of the tumour following laser heating with or without nanoshells, HSPControl is the HSP concentration for the unheated tumour, FIFm is the mean fluorescence intensity following laser heating with or without nanoshells, and FICm is the mean fluorescence intensity for the untreated tumour. FIFm and FICm were measured using confocal microscopy and Leica Lite software.
Results
Laser heating only case
Temperature distribution
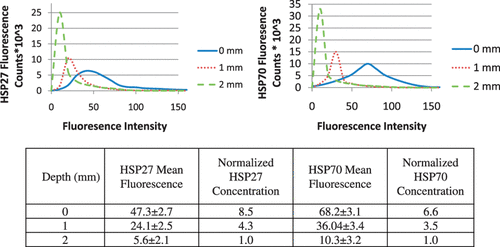
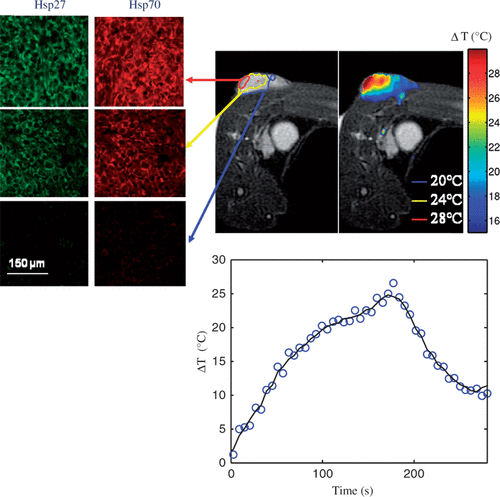
shows the distribution of maximum temperature elevation measured with MRTI (top right) and maximum temperature increase as a function of time at the tumour surface (bottom centre) during laser treatment (wavelength of 810 nm, irradiance of 5 W/cm2, and pulse duration of 3 min) and HSP expression measured 16 h post-treatment (top left) for varying tumour depths. All temperature measurements represent the mean value for five mice (top right). The initial tumour temperature was measured with a thermocouple to be in the range of 23–28°C and the maximum temperature rise was determined by Equation 1. Significant levels of temperature elevation were induced in the tumour surface (ΔT = 25–28°C) with maximum measured temperatures in the range of 48–51°C for all mice. With increasing distance from the tumour surface and incident laser probe, the temperature elevation decreased with temperatures at the base of the tumour of 36–39°C. The average change in temperature versus time at the surface of the tumour (bottom centre) demonstrated sub-lethal temperature elevations.
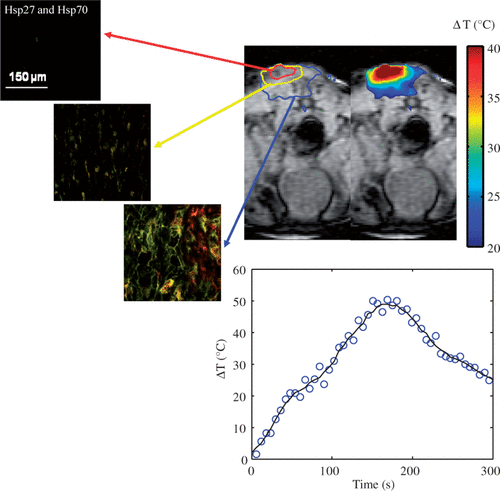
Figure 1. Hsp27 and Hsp70 expression measured 16 h following laser irradiation for three tumour depths with distinct temperature elevation zones (top left) and corresponding mean MRTI measured maximum temperature elevation during laser irradiation of five mice (top right). Mean measured temperature elevation as a function of time at the tumour surface (bottom centre).
HSP quantification
Three distinct temperature elevation zones were identified as indicated by the red, yellow, and blue circles representing 1 mm incremental distances in tumour depth (). Hsp27 and Hsp70 expression was measured and quantified in these regions 16 h following laser treatment using immunofluorescence. Green and red fluorescence denote Hsp27 and Hsp70 respectively. Significant levels of Hsp27 and Hsp70 expression were induced in the tumour surface (denoted by the red circle) where maximum temperatures of 48–51°C (temperature elevations of 25–27°C) occurred for sufficient time. With increasing depth, the level of HSP expression diminished due to decreasing temperature elevation which was less efficient in inducing expression. The 1 mm depth (yellow region) experienced a maximum temperature of 47°C which is capable of inducing HSP expression. The 2 mm region (denoted by blue circle) only reached a maximum temperature of 39°C which is below the threshold of 43°C necessary to elicit HSP expression and therefore this level exhibits only basal HSP expression.
shows histograms of fluorescence counts (i.e. number of cells with a given fluorescence intensity) as a function of fluorescence intensity for varying depth from the tumour surface. For both HSPs, the tumour surface (0 mm) possesses the broadest fluorescence distribution which encompasses the greatest number of fluorescent counts with high fluorescence magnitudes. With increasing depth, the distribution narrows causing the fluorescence counts to be associated with lower fluorescence intensity. The decreasing fluorescence intensity with depth is expected since the temperature elevation diminishes with distance from the tumour surface which is less conducive to inducing HSP expression. The mean fluorescence (FIFm) at each tissue depth for all of the five mice was determined using the histograms and analysis methods in Leica Lite image processing software. The normalised HSP concentration was then determined for each mouse using Equation 2. The measured fluorescence at 2 mm was nearly identical to the basal level of fluorescence so it was employed for the basal value. The FIFm values at each tissue depth for both Hsp27 and Hsp70 were averaged for all five mice. The average FIFm, the associated standard deviation, and calculated normalised Hsp27 and Hsp70 concentrations are shown in . The mean fluorescence level for Hsp27 and Hsp70 at the surface (0 mm) is more than twice the level of fluorescence measured at 1 mm. The mean fluorescence intensity decreased approximately 77% and 72% for Hsp27 and Hsp70 respectively as the measurement region moves from 1 mm to 2 mm.
Influence of nanoshell inclusion on HSP expression
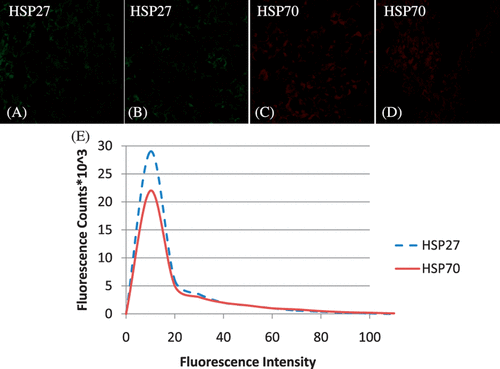
Hsp27 and Hsp70 expression in tumours was measured before ( and ) and following nanoshell introduction ( and ) to determine whether nanoshell inclusion caused sufficient perturbation to induce HSP expression. Tumours with and without nanoshell inclusion showed identical and faint expression of both Hsp27 and Hsp70 expression, confirming nanoshell inclusion did not cause a difference in HSP expression. The sections shown were acquired from the 1 mm tumour depth and are representative of the entire tumour. A histogram showing fluorescence counts for Hsp27 and Hsp70 as a function of fluorescence intensity for the tumour with nanoshell inclusion depicts a very narrow fluorescence distribution which is nearly identical to the basal level of fluorescence shown in the laser heated tissue for the 2 mm level in .
Nanoshell + laser heating case
Temperature distribution
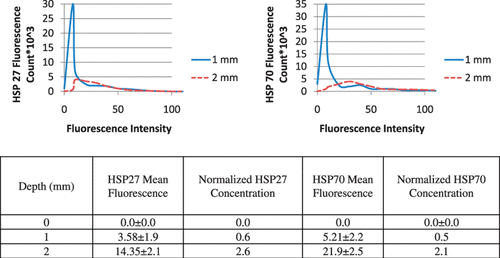
shows the distribution of maximum temperature elevation measured with MRTI (top right) and maximum change in temperature as a function of time at the tumour surface (bottom centre) during laser treatment (wavelength of 810 nm, irradiance of 5 W/cm2, and pulse duration of 3 min) in combination with nanoshells and corresponding HSP expression measured 16 h post-treatment (top left). All temperature measurements represent the mean value for five mice (top right). The initial tumour temperature was measured to be 23–28°C and the maximum temperature increase was determined by Equation 1. Inclusion of nanoshells in the laser therapy caused more significant temperature elevations over a larger tumour region compared to tumours treated with laser only. Temperature elevations at the tumour surface (denoted by red circle) were in the range of 40–50°C with corresponding maximum temperatures of 73–78°C for sufficient time to cause tissue necrosis. The minimum measured temperature at the tumour base was approximately 46°C.
Figure 4. Hsp27 and Hsp70 expression dual stained image for three distinct tumour depths (top left) measured 16 h following laser irradiation in combination with nanoshells and corresponding MRTI measured mean maximum temperature increase for five mice (top right). Average change in temperature versus time at the tumour surface (bottom centre).
HSP quantification
Hsp27 and Hsp70 expression was quantified 16 h following laser treatment for the three regions denoted in the MRT image in where green and red fluorescence denote Hsp27 and Hsp70 respectively. The tumour surface denoted by the red circle experienced severe temperatures of 73–78°C for sufficient time to cause complete cell death, protein denaturation, and no visible HSP expression. The middle 1 mm region, denoted by the yellow circle experienced significant temperature elevation ranging from 53–56°C. This region experienced significant thermal denaturation of proteins and cell damage yielding very little HSP expression. The bottom tumour level experienced temperatures of 46–50°C which is conducive for HSP expression induction over the period of exposure.
Histograms for quantification of average fluorescence of five mice for the three tumour depths were generated and representative histograms are shown in . Due to the significant temperature elevation at the tumour surface of approximately 73°C, the tumour surface (0 mm) exhibited no fluorescence, which is expected due to significant protein denaturation which eliminated HSP expression. As a result, the histogram for the tumour surface is not shown. At the tissue depth of 1 mm, the histogram has a narrow distribution with the majority of fluorescence counts attributed to low fluorescence intensity due to thermal denaturation of proteins characteristic of temperatures of 56°C. At the 2 mm depth, the histogram distribution widens such that a greater number of fluorescence counts are associated with higher fluorescence intensity. This is expected since temperatures of 46°C are effective at inducing HSP expression. Hsp27 and Hsp70 mean fluorescence, standard deviation, and concentration were determined for each tumour level for all five mice using the Leica Lite software and Equation 2, and are shown in .
Discussion
This is the first study measuring and quantifying Hsp27 and Hsp70 expression in prostate tumours following laser therapy alone or in combination with gold nanoshells. HSP expression for varying tumour depths was correlated with corresponding temperature distributions measured with MRTI in response to therapy. Knowledge of the relationship between temperature distribution and spatial HSP expression in vivo is critical for accurately predicting regions of treatment success or failure, since HSP expression is an indirect measure of thermal dose and an important indicator of the likelihood of tumour survival and recurrence Citation[6–10]. Ultimately, determination of the thermal response and HSP expression profile in tumours for a given set of treatment parameters can permit selection of laser regimens (e.g. irradiance, duration, and wavelength) and nanoparticle properties (e.g. concentration, type, and delivery method) that create thermal dose distributions that maximise tumour necrosis and minimise HSP expression within the targeted tumour volume.
MRTI enabled spatial measurement of temperature within the tumour volume and confirmed that the inclusion of gold nanoshells significantly increased temperature elevation compared to laser heating alone. Previous studies have utilised MRTI to measure temperature distribution in prostate and kidney tumours treated with laser irradiation in combination with gold nanoshells Citation[48] and multi-walled carbon nanotubes Citation[8]. In this study a laser wavelength of 810 nm, irradiance of 5 W/cm2, spot size of 5 mm, and heating duration of 3 min were employed to achieve maximum temperature elevations of 25–28°C for laser only and 40–50°C for laser heating in combination with gold nanoshells. In an earlier study by Hirsch et al. tumours were treated with a wavelength of 810 nm, irradiance of 4 W/cm2, beam diameter of 5 mm for 3 min, alone or in combination with gold nanoshells (identical geometry and concentration as the current study). Temperature elevations of 35°C for nanoshells + laser and 10°C for laser alone were measured with MRTI. The larger temperature elevations achieved in our study are attributed to the higher laser irradiance employed. Temperature elevations attained within the tumours treated with nanoparticles and laser therapy have been shown to cause tumour death for both in vitro Citation[7], Citation[8], Citation[48] and in vivo studies Citation[47], Citation[48] resulting in effective prostate Citation[47], Citation[48] and kidney tumour regression Citation[8]. Temperature elevations measured with MRTI for laser and laser + nanoshell treatments also correspond closely with predictions made by published computational models Citation[64], Citation[66], Citation[67].
HSP expression can enable determination of tumour regions with a high likelihood of treatment failure due to their documented role in enhancing cell viability and promoting tumour recurrence Citation[6–16]. In tumours treated with laser alone, maximal Hsp27 and Hsp70 expression was observed at the surface of the tumour proximal to the incident laser which corresponded with peak temperatures in the range of 48–51°C. With decreasing distance from the tumour surface, HSP expression levels diminished corresponding with lower temperature increases which were less conducive to HSP expression induction. In contrast, tumours treated with laser and nanoshells experienced substantial temperatures of 73–78°C at the tumour surface and 53–56°C in the first few millimeters for sufficient time to cause coagulative necrosis and denaturation of HSPs. Induction of HSPs were observed deeper in the tumour at the interface between the tumour and normal tissue. In a prior study, similar HSP expression trends and expression levels were observed for kidney tumours treated alone or in combination with multi-walled carbon nanotubes and 1064 nm continuous wave laser irradiation Citation[8]. Therefore, it is evident that inclusion of nanoshells in laser therapy can provide a mechanism for enhancing heat generation necessary to eliminate HSP expression within a large tumour region. More precise control of HSP expression within the tissue could be achieved through targeting of nanoshells to the tumour cells.
Acknowledgements
This research was funded by the following sources: National Science Foundation Grants CBET 0955072 and 0731108 and National Institute of Health Grant 1 R21 CA135230-01.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Stern JM, Stanfield J, Kabbani W, Hsieh JT, Cadeddu JA. Selective prostate cancer thermal ablation with laser activated gold nanoshells. J Urol 2008; 179: 748–753
- Mertyna P, Goldberg W, Yang W, Goldberg SN. Thermal ablation: A comparison of thermal dose required for radiofrequency, microwave, and laser-induced coagulation in an ex vivo bovine liver model. Acad Radiol 2009; 16: 1539–1548
- Chen W, Liu H, Richey J. Effect of different components of laser immunotherapy in treatments of metastatic tumors in rats. Cancer Res 2002; 62: 4295–4299
- Vlastos G, Verkooijecn A. Minimally invasive approaches for diagnosis and treatment of early-stage breast cancer. Oncologist 2007; 12: 1–10
- Harries SA, Amin Z, Smith ME, Lees WR, Cooke J, Cooke MG, Scurr JH, Kissin MW, Bown SG. Interstitial laser photocoagulation as a treatment for breast cancer. Br J Surg 1994; 81: 1617–1619
- Madersabacher S, Grobl M, Kramer G, Dirnhoger S, Steiner G, Marberger M. Regulation of heat shock protein 27 expression of prostastic cells in response to heat treatment. Prostate 1998; 37: 174–181
- Fisher J, Buchanan C, Szot C, Sarkar S, Rylander C, Rylander MN. Photothermal response of human and murine cancer cells to multiwalled carbon nanotubes and laser irradiation. Cancer Res 2010; 70: 1–10
- Burke A, Ding X, Singh R, Kraft RA, Rylander MN, Szot C, Buchanan C, Whitney J, Fisher J, Levi-Polyachenko N, et al. Rapid thermal treatment of kidney tumors with multi-walled carbon nanotubes results in long term survival. Proc Nat Acad Sci 2009; 4: 12897–12902
- Beckham JT, Mackanos MA, Crooke C, Takahashi T, O’Connel-Rodwell C, Contag CH, Jansen ED. Assessment of cellular response to thermal laser injury through bioluminescence imaging of heat shock protein 70. Photochem Photobiol 2004; 79: 76–85
- O’Connell-Rodwell CE, Mackanos MA, Simanovskii DM, Cao YA, Bachmann MH, Schwettman HA, Contag CH. In vivo analysis of heat-shock-protein-70 induction following pulsed laser irradiation in transgenic reporter mouse. J Biomed Opt 2008; 13: 030501
- Vertrees RA, Jordan JM, Zwischenberger JB. Hyperthermia and chemotherapy: The Science. Current Clinical Oncology: Intraperitoneal Cancer Therapy, CW Helm, RP Edwards. Humana Press, Totowa 2007; 71–100
- Vargus-Roig LM, Fanelli MA, Lopez LA, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock proteins and cell proliferation in human breast cancer biopsy samples. Cancer Detect Prev 1997; 21: 441–451
- Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y, Foster CS. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res 2000; 60: 7099–7105
- Richards EH, Hickey E, Weber L, Masters JR. Effects of over-expression of small heat shock protein Hsp27 on the heat and drug sensitivities of human testis tumor cells. Cancer Res 1996; 56: 2446–2451
- Ciocca DR, Clark GM, Tandon AK, Fuqua SAN, Welch WJ, McGuire WL. Heat shock protein Hsp70 in patients with axillary lymph node-negative breast cancer: Prognostic implications. J Natl Cancer Inst 1993; 85: 570–574
- Fuqua SA, Oesterreich S, Hilsenbeck SG, Von Hoff DD, Eckardt J, Osborne CK. Heat shock proteins and drug resistance. Breast Cancer Res Treat 1994; 32: 67–71
- Landriscina M, Amoroso MR, Piscazzi A, Esposito F, Heat shock proteins, cell survival and drug resistance: The mitochondrial chaperone TRAP1, a potential novel target for ovarian cancer therapy. Gynecol Oncol 2010;117:177–182
- Tomei LD, Cope FO. Apoptosis: The Molecular Basis of Cell Death. Cold Spring Harbor Laboratory Press, New York 1991
- Gibbons NB, Watson RWG, Coffey RNT, Brady HP, Fitzpatrick JM. Heat-shock proteins inhibit induction of prostate cancer cell apoptosis. Prostate 2000; 45: 58–65
- Creagh EM, Sheehan D, Cotter TG. Heat shock proteins – Modulators of apoptosis in tumour cells. Leukemia 2000; 14: 1161–1173
- Garrido C, Solary E. A role of HSPs in apoptosis through ‘protein triage’?. Cell Death Differ 2003; 10: 619–620
- Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene 2003; 22: 9041–9047
- Levine AJ, Momand J, Finlay CA. The p53 tumor supressor gene. Nature 1991; 351: 453–456
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005; 10: 86–103
- Rylander MN, Diller KR, Wang S, Aggarwal S. Correlation of Hsp70 expression and cell viability following thermal stimulation of bovine aortic endothelial cells. J Biomech Eng 2005; 127: 751–757
- Rylander MN, Feng Y, Zimmermann K, Diller KR. Measurement and mathematical modeling of thermally induced injury and heat shock protein expression kinetics in normal and cancerous prostate cells. Int J Hyperthermia 2010; 26: 748–764
- Wang S, Xie W, Rylander MN, Tucker PW, Aggarwal S, Diller KR. Hsp70 kinetics study by continuous observation of HSP-GFP fusion protein expression on a perfusion heating stage. Biotechnol Bioeng 2007; 99: 146–154
- Wang S, Aggarwal S, Diller KR. Heat shock protein 70 expression kinetics. J Biomech Eng 2003; 125: 794–797
- Rylander MN, Feng Y, Bass J, Diller KR. Coordinated modeling of thermal stress induced cell injury and heat shock protein expression. Ann N Y Acad Sci 2005; 1066: 222–242
- Rylander MN, Feng Y, Zhang Y, Bass J, Stafford RJ, Volgin A, Hazle JD, Diller KR. Optimizing heat shock protein expression induced by prostate cancer laser therapy through predictive computational models. J Biomed Opt 2006; 11: 041113
- Rylander MN, Feng Y, Bass J, Diller KR. Heat shock protein expression and injury optimization for laser therapy design. Lasers Surg Med 2007; 39: 731–746
- Rieger TR, Morimoto RI, Hatzimanikatis V. Mathematical modeling of the eukaryotic heat-shock response: Dynamics of the hsp70 promoter. Biophys J 2005; 88: 1646–1658
- Barnes JA, Dix DJ, Collins BW, Luft C, Allen JW. Expression of inducible Hsp70 enhances the proliferation of MCF-7 breast cancer cells and protects against the cytotoxic effects of hyperthermia. Cell Stress Chaperones 2001; 6: 316–325
- Beckham JT, Wilmink GJ, Mackanos MA, Takahashi K, Contag CH, Takahashi T, Jansen ED. Role of Hsp70 in cellular thermotolerance. Lasers Surg Med 2008; 40: 704–715
- Georgopoulous C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol 1993; 9: 601–634
- Craig EA, Weissman JS, Horwich AL. Heat shock proteins and molecular chaperones: Mediators of protein conformation and turnover in the cell. Cell 1994; 78: 365–372
- Kurahashi T, Miyake H, Hara I, Fujisawa M. Expression of major heat shock proteins in prostate cancer: Correlation with clinicopathological outcomes in patients undergoing radical prostatectomy. J Urol 2007; 177: 757–761
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trends Biochem Sci 2006; 31: 164–172
- Tiara T, Narita T, Iguchi-Ariga H. A novel G1-specific enhancer identified in the human heat shock protein 70 gene. Nucleic Acids Res 1997; 25: 1975–1983
- Daugaard M, Jaattela M, Rohde M. Hsp70-2 is required for tumor cell growth and survival. Cell Cycle 2005; 4: 877–880
- Mehlen P, Kretz-Remy C, Preville X, Arigo AP. Human Hsp27, Drosophillia Hsp27, and human ab-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNF-a-induced cell death. EMBO J 1996; 15: 2695–2706
- Singh J, Kaur G. Hsp70 induction and oxidative stress protection mediated by a subtoxic dose of NMDA in the retinoic acid-differentiated C6 glioma cell line. Brain Res Bull 2006; 69: 37–47
- Oesterreich S, Weng CN, Qiu M, Hilsenbeck SG, Fuqua SAW. The small heat shock protein Hsp27 is correlated with growth and drug resistance in human breast cancer cell lines. Cancer Res 1993; 53: 4442–4448
- Haraldsdóttir KH, Ivarsson K, Jansner K, Stenram U, Tranberg KG. Changes in immunocompetent cells after interstitial laser thermotherapy of breast cancer. Cancer Immunol Immunother 2011; 60: 847–856
- Hirsch L, Gobin A, Lowery A, Tam F, Drezek R, Halas N, West J. Metal nanoshells. Ann Biomed Eng 2006; 34: 15–22
- Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett 2005; 5: 709–711
- O’Neal D, Hirsch L, Halas N, Payne J, West J. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett 2004; 209: 171–176
- Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Nat Acad Sci 2003; 100: 13549–13554
- Terentyuk G, Maslyakova G, Suleymanova L, Khlebstov BN, Akchurin G, Maksimova I, Tuchin V. Laser-induced tissue hyperthermia mediated by gold nanoparticles: Toward cancer phototherapy. J Biomed Opt 2009; 14: 021016
- Gobin A, Lee M, Halas N, James W, Drezek R, West J. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. 2007; 7: 1929–1934
- Shao N, Lu S, Wickerstrom E, Panchapakesan B. Integrated molecular targeting of IGF1R and HER2 surface receptors and destruction of breast cancer cells using single wall carbon nanotubes. Nanotechnology 2007; 18: 9
- Wong Shi Kam N, O’Connel M, Wisdom J, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA 2005; 102: 11600–11605
- Zhou F, Xing D, Ou Z, Wu B, Resasco D, Chen W. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. J Biomedical Optics 2009; 14: 021009
- Sarkar S, Fisher J, Rylander CG, Rylander MN. Photothermal response of tissue phantoms containing multi-walled carbon nanotubes. J Biomech Eng 2010; 132: 044505
- Torti S, Byrne F, Whelan O, Levi N, Ucer B, Schmid M, Torti F, Akman S, Liu J, Ajayan P, et al. Thermal ablation therapeutics based on CNx multi-walled nanotubes. Int J Nanomed 2007; 2: 707–771
- Sarkar S, Gurjarpadhye A, Rylander C, Rylander MN, Optical properties of breast tumor phantoms containing carbon nanotubes and nanohorns. J Biomed Optics 2011; 16:05i304-1-051304-11
- Miyako E, Nagata H, Hirano K, Makita Y, Nakayama K, Hirotsu T. Near infrared laser triggered carbon nanohorns for selective elimination of microbes. Nanotechnology 2007; 18: 475103–475110
- Whitney J, Sarkar S, Zhang J, Dorn H, Geohegan D, Rylander C, Campbell T, Rylander MN. Carbon nanohorns as photothermal agents for cancer therapy. Lasers Surg Med 2011; 43: 43–51
- Zhang M, Murakami T, Ajima K, Tsuchida K, Sandanayaka A, Ito O, Iijima S, Yudasaka M. Fabrication of ZnPc/protein nanohorns for double photodynamic and hyperthermic cancer phototherapy. Proc Nat Acad Sci 2008; 105: 14773–14778
- Miyako E, Nagata H, Hirano K, Sakamoto K, Makita Y, Nakayama K, Hirotsu T. Photoinduced antiviral carbon nanohorns. Nanotechnology 2008; 19: 075106–075112
- Prevo B, Esakoff S, Mikhailovsky A, Zasadzinski J. Scalable routes to gold nanoshells with tunable sizes and response to near-infrared pulsed-laser irradiation. Small 2008; 4: 1183–1195
- Oldenburg R, Averitt D, Westcott S, Halas N. Nanoengineering of optical resonances. Chem Phys Lett 1998; 288: 243–247
- Lal S, Clare S, Halas N. Nanoscience-enabled cancer therapy: Impending clinical impact. Acc Chem Res 2008; 41: 1842–1851
- Maksimova I, Akchurin G, Khlebtsov B, Terentyuk G, Akchurin G, Ermolaev I, Skaptsov A, Soboleva E, Khlebtsov N, Tuchin V. Near-infrared laser photothermal therapy of cancer by using gold nanoparticles: Computer simulations and experiment. Med Laser Appl 2007; 22: 199–206
- Liu H, Chen D, Li L, Liu T, Tan L, Wu X, Tang F. Multifunctional gold nanoshells on silica nanorattles: A platform for the combination of photothermal therapy and cheomotherapy with low systemic toxicity. Nanomedicine 2011; 50: 891–895
- Sarkar S, Zimmermann K, Leng W, Vikesland P, Zhang J, Dorn H, Diller T, Rylander C, Rylander MN. Measurement of the thermal conductivity of carbon nanotube-tissue phantom composites with the hot wire probe method. Ann Biomed Eng 2011;39:1745–1758.
- Feng Y, Fuentes Y, Hawkins A, Bass J, Rylander MN, Elliott A, Shetty A, Stafford RJ, Oden JT. Nanoshell-mediated laser surgery simulation for prostate cancer treatment. J Eng Comp 2009; 25: 3–13
- O’Connel-Rodwell CE, Shriver D, Simanovskii DM, Mcclure C, Cao YA, Zhang W, Bachmann MH, Beckham JT, Jansen ED, Palanker D, et al. A genetic reporter of thermal stress defines physiologic zones over a defined temperature range. FASEB J. 2004; 18: 264–271
- De Poorter J, De Wagter C, De Deene Y, Thomsen C, Ståhlberg F, Achten E. The proton resonance frequency shift method compared with molecular diffusion for quantitative measurement of two dimensional time dependent temperature distribution in phantom. J Magn Reson 1994; 103: 234–241
- Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Yo, Kuroda K, Suzuki Yu. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med 1995; 34: 814–823
- Olsrud J, Wirestam R, Brockstedt S, Nilsson AMK, Tranberg K-G, Ståhlberg F, Persson BRR. MRI thermometry in phantoms by use of the proton resonance frequency shift method: Application to interstitial laser thermotherapy. Phys Med Biol 1998; 43: 2597–2613
- Hindman JC. Proton resonance shift of water in the gas and liquid states. J Chem Phys 1966; 44: 4582–4592