Abstract
Background. Chemotherapy-induced peripheral neuropathy (CIPN) is a dose-limiting side effect of oxaliplatin which can negatively influence quality of life. We aimed to study the influence of cumulative dose, dose schedule and dose reductions of adjuvant oxaliplatin on long-term severity and prevalence of CIPN among colorectal cancer (CRC) survivors.
Material and methods. In total 207 patients, diagnosed with CRC between 2000 and 2009 who underwent adjuvant treatment with oxaliplatin, were included. They completed the EORTC QLQ-CIPN20 2–11 years after diagnosis. Data on oxaliplatin administration and acute neuropathy during treatment were extracted from the medical files. Subscales were analyzed with analysis of covariance and neuropathy symptoms with logistic regression analysis.
Results. Patients who received cumulative oxaliplatin dose of ≥ 842 mg/m2 had a significantly worse EORTC QLQ-CIPN20 sensory score compared to those who received a low cumulative dose of < 421 mg/m2 (mean 19 vs. 8; p = 0.02). They more often reported tingling toes/feet (13% vs. 2%, respectively; p = 0.01). Dose intensity and time delay did not influence the occurrence of CIPN. Patients receiving a dose reduction because of neuropathy (N = 50) reported a significantly worse sensory score at very similar cumulative doses, than those who did not receive a dose reduction because of neuropathy (N = 96) (mean 21 vs. 15; p = 0.01).
Conclusion. Cumulative dose of oxaliplatin is associated with long-term CIPN. The risk of developing long-term CIPN could only be reduced by decreasing the cumulative dose, whereas delay probably is not beneficial. Patients receiving a dose reduction because of acute neuropathy are still at risk of developing long-term CIPN. Future studies should focus on identifying patients who are at risk of developing CIPN.
Treatment of colorectal cancer (CRC) has considerably changed over the past decades. Oxaliplatin first successfully used in the management of advanced CRC, is nowadays the regimen of choice for adjuvant treatment of patients with curatively resected node-positive CRC with improved survival rates as a consequence [Citation1]. Therefore the use of oxaliplatin has been increased and more patients are living with its long-term side effects. Especially chemotherapy- induced peripheral neuropathy (CIPN), as the most common dose limiting side effect of oxaliplatin, is of major concern and can have a negative influence on patients’ quality of life [Citation2,Citation3].
Axonal hyperexcitability and repetitive discharges due to changes in voltage-gated sodium and/or potassium channels and accumulation of oxaliplatin in the dorsal root ganglia with neuronal damage seem to cause the symptoms of acute and chronic oxaliplatin-induced neuropathy, respectively [Citation4–7]. The symptoms of acute neuropathy, which occur in approximately 90% of patients, reverse characteristically within a week [Citation1]. Chronic neuropathy, however, persists in 60% of patients a year or longer after the cessation of chemotherapy [Citation2,Citation8,Citation9].
The severity of CIPN is dependent on cumulative dose, duration of administration and dose intensity [Citation9–11]. Previously, we reported that neuropathy symptoms were still seen 2–11 years after oxaliplatin treatment in CRC patients [Citation2]. However, it is difficult to identify patients at risk for severe CIPN and information about cumulative doses, dose intensity and dose schedule was not available in our previous study. Therefore, we next aimed to study the influence of cumulative dose, dose schedule and dose reductions on long-term severity and prevalence of CIPN among CRC survivors.
Material and methods
Setting and participants
Our prospective yearly survey was set up in December 2010 with the goal to evaluate various patient-reported outcomes, including neuropathy, among CRC survivors. All individuals diagnosed with CRC between 2000 and 2009 as registered in the Eindhoven Cancer Registry (ECR) in the Netherlands received the second yearly questionnaire in December 2011. The data presented in this paper are thus based upon the second data collection wave which included a CIPN questionnaire. A detailed description of the data collection was published earlier [Citation2]. Data collection was performed within PROFILES [Citation12]. Of the respondents, 506 (31%) patients were treated with chemotherapy, including 207 (41%) patients who underwent adjuvant treatment with oxaliplatin. After permission from the attending oncologists, we additionally collected information on the actually delivered oxaliplatin treatment for those adjuvantly treated according to the ECR (N = 207), and these data are presented in this paper. This study was approved by a certified Medical Ethics Committee. All patients gave written informed consent.
Data collection
Socio-demographic and clinical characteristics
Survivors’ socio-demographic and clinical information were available from the ECR which routinely collects data like date of diagnosis, tumor stage and treatment. Comorbidity at time of the study was assessed with the adapted Self-administered Comorbidity Questionnaire.
Treatment with oxaliplatin
Complementary information about the oxaliplatin administration, such as combination regimen, number of received chemotherapy cycles, dose of oxaliplatin per cycle, and timing and reasons for dose reduction were collected from patients’ medical files.
Chemotherapy-induced neuropathy
Long-term CIPN was assessed with the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-CIPN20 [Citation13] which contains three subscales assessing sensory, motor, and autonomic symptoms. Each item is measured on a Likert scale ranging from ‘not at all’ (1) to ‘very much’ (4). Scores were transformed to a 0–100 scale with higher scores representing more complaints.
In addition, data about acute neuropathy complaints shortly after treatment with oxaliplatin were collected from the patients’ medical files. It was defined as having any kind of sensory, motor or autonomic neuropathy as documented by the oncologist during outpatient clinical contact and/or reported according to the National Cancer Institute (NCI)-Common Toxicity Criteria (CTC) after treatment with oxaliplatin.
Statistical analyses
Patients were divided into categories of received cumulative dose; low < 421 mg/m2; moderate 421–842 mg/m2; high ≥ 842 mg/m2 [Citation8,Citation14–16], which correspond approximately to 1–3, 3–6 and 7 or more cycles of oxaliplatin in combination with capecitabine (CAPOX) and 1–5, 5–10 or ≥ 10 cycles of oxaliplatin in combination with 5-fluorouracil/leucovorin (5-FU/LV) (FOLFOX). Clinical characteristics were compared between categories of cumulative dose using ANOVA for continuous data and χ2 analyses for categorical variables. Neuropathy symptoms that bothered patients the most were reported according the EORTC QLQ-CIPN20 answer categories ‘quite a bit’ or ‘very much’ combined. Logistic regression analyses were performed to detect differences regarding experienced neuropathy problems in the past week between the different categories of cumulative doses, treatment regimens and between patients with and without documented acute neuropathy and applied dose reduction. Mean total scores on the EORTC QLQ-CIPN20 were compared between categories of cumulative dose with analysis of covariance (ANCOVA). In addition, subgroup analysis of the EORTC QLQ-CIPN20 subscales and separate questions in patients treated with CAPOX or FOLFOX were done. Confounding background variables that might interfere with general nervous system or physical functioning were determined a priori and chosen to be diabetes mellitus (DM), osteoarthritis and rheumatoid arthritis. Furthermore, analyses were adjusted for time since last course with chemotherapy treatment as this influences the evolution of CIPN. Logistic regression analyses between regimens of chemotherapy and those for applied dose reduction were also adjusted for received cumulative dose, but not for rheumatoid arthritis. All test were two-sided, considered significant if p < 0.05, and were performed using SPSS (version 19.0).
Results
Socio-demographic and clinical characteristics
In total, 1643 CRC patients were included in the initial study with a response rate of 83%. There were no differences in sociodemographic and clinical characteristics between respondents, non-respondents and those with unverified addresses [Citation2]. Patients who were treated with chemotherapy (N = 506) were significantly younger, diagnosed more recently, and more often with colon cancer compared with those treated without chemotherapy [Citation2]. Patients, who underwent treatment with oxaliplatin (N = 207), were in 83% diagnosed with colon cancer, with a mean 4.0 years (SD 1.2) follow-up since diagnosis and 84% had stage III disease. Patients who received a higher cumulative dose of oxaliplatin were more often male (66% vs. 36%; p = 0.01) and were significantly younger than patients who received a lower cumulative dose (63 vs. 69 years; p = 0.01) ().
Table I. Sociodemographic and clinical characteristics of colorectal cancer survivorsa stratified by categories of cumulative dose of oxaliplatinb.
Treatment with oxaliplatin
Oxaliplatin was mainly given in combination with CAPOX (N = 173, 84%) in a mean cumulative dose of 695 mg/m2 (SD 273) (). Any dose reduction had taken place in 144 patients (70%) and was applied because of neurotoxicity in 38% of patients.
Table II. Clinical characteristics of oxaliplatin administration, N = 207.
Chemotherapy-induced neuropathy
The neuropathy subscale-related symptoms that bothered patients the most during the past week were mainly sensory and consisted of tingling toes or feet (30%), numbness in toes or feet (19%), tingling hands or fingers (15%), burning or shooting pain in the toes or feet (13%), trouble hearing (13%) and the autonomic symptom trouble getting or maintaining an erection (36% of male patients).
Analysis among categories of cumulative doses of oxaliplatin showed that patients who received a cumulative oxaliplatin dose of ≥ 842 mg/m2 had significantly worse EORTC QLQ-CIPN20 sensory scores than those who received a low cumulative dose of < 421 mg/m2 (mean 19 vs. 8; p = 0.02) (). No differences in the other EORTC QLQ-CIPN20 subscales were observed.
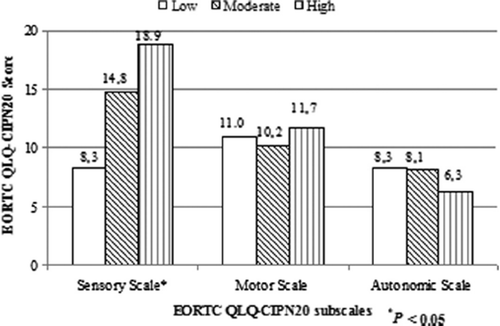
Logistic regression analysis of the individual EORTC QLQ-CIPN20 questions showed that tingling toes or feet were more often reported by patients who received a higher cumulative dose in comparison with low cumulative dose respectively (13 vs. 2%; p = 0.01) (Supplementary Table I, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.980912). In addition, trouble with standing or walking was significantly more often observed in patients treated with a high vs. moderate cumulative dose (6 vs. 3%; p = 0.03).
The EORTC QLQ-CIPN20 subscales and questions did not differ between number of received cycles after adjustment for time between first and last cycle of oxaliplatin and cumulative dose. Moreover, dose intensity did not contribute to the EORTC QLQ-CIPN20 subscales and questions (data not shown). In addition, there was no difference in mean sensory scale score between patients who did (N = 144) or did not (N = 56) receive a dose reduction (15 vs. 17; p = 0.61). However, patients who received a dose reduction after the sixth cycle of oxaliplatin (N = 35) reported more severe neuropathy symptoms than patients who received a dose reduction earlier (N = 90), resulting in a worse mean sensory scale (mean 25 vs. 11; p = 0.001).
There was a trend of worse mean sensory scores in patients with documented acute neuropathy (N = 155, 75%) when comparing patients who received a higher cumulative dose (N = 55) to lower cumulative dose (N = 11) (mean 20 vs. 7; p = 0.09). Moreover, patients with documented acute neuropathy during treatment who received a dose reduction because of this neuropathy (N = 50) still reported significantly worse scores on the sensory scale at very similar cumulative doses, than those who did not receive a dose reduction because of neuropathy (N = 96) (mean 21 vs. 15; p = 0.01).
There were no significant differences between those treated with CAPOX (n = 173) or FOLFOX (n = 27). Patients who received a higher cumulative dose of oxaliplatin in combination with capecitabine had worse EORTC QLQ-CIPN20 sensory scores than those who received a low cumulative dose (mean 18 vs. 9; p = 0.06). Patients treated with FOLFOX reported similar results (mean 20 vs. 7; p = 0.31). No differences in the other EORTC QLQ-CIPN20 subscales were observed in the subgroup analysis. Furthermore, patients treated with CAPOX who received a higher cumulative dose reported significantly more tingling toes or feet in comparison with low cumulative dose (14% vs. 1%; p = 0.01). FOLFOX-treated patients reported similar results in tingling toes and feet (12% vs. 4%).
Moreover, overall there were no differences between categories of age at time of diagnosis (data not shown). In addition, recurrence of disease between patients who received a low or a high cumulative dose did not differ (data not shown).
Discussion
Median four years after diagnosis, neuropathy symptoms are still frequently reported by CRC patients who underwent adjuvant treatment with oxaliplatin. Symptoms were mainly sensory and consisted of tingling toes or feet (30%), numbness in toes or feet (19%), tingling hands or fingers (15%) and burning or shooting pain in the toes or feet (13%). The actually delivered cumulative dose of oxaliplatin was associated with CIPN, and especially sensory complaints in toes and feet were significantly more often reported in patients who received a higher cumulative dose. Dose intensity did not contribute to severity of long-term CIPN. Therefore, decreasing the total cumulative dose is supposed to reduce long-term neuropathy, whereas delaying chemotherapy cycles probably is not beneficial. Patients who received a dose reduction because of neuropathy during oxaliplatin treatment, at very similar cumulative doses, still reported more long-term neuropathy symptoms. Furthermore, dose reduction after the sixth cycle of oxaliplatin was associated with more severe neuropathy symptoms. Therefore, it is important to decrease cumulative dose in patients with presenting neuropathy as soon as possible.
Since the introduction of CRC screening, more patients are faced with early stage CRC. Adjuvant treatment with oxaliplatin in combination with 5-FU/LV or capecitabine, showed an absolute risk reduction of approximately 5% on disease-free and overall survival [Citation1,Citation17], and became standard care for stage II and III CRC [Citation14]. Accordingly, more CRC survivors are confronted with the long-term side effects of oxaliplatin, in particular CIPN, which can have a substantial impact on patient's quality of life [Citation2]. The MOSAIC and NSABP C-07 trials reported the development of CIPN after four years and 27 months, respectively, in 15% and 10% of patients after a mean cumulative dose of 810 mg/m2 and 667 mg/m2 [Citation14,Citation15]. However, CIPN was not a primary endpoint and no association with different cumulative doses was reported. A recent review demonstrated that only few studies directly investigated the influence of cumulative dose on persistent CIPN more than a year after treatment [Citation11]. This review reports that two retrospective studies in CRC patients found that CIPN was associated with a median cumulative dose of ≥ 834 and 850 mg/m2, respectively, and four other studies found similar results. Our results are similar, as patients who received more than 842 mg/m2 had more severe CIPN than patients who received less oxaliplatin. Therefore the results from the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) and the TOSCA non-inferiority studies, which compare disease-free survival between standard six versus alternative three months (planned cumulative dose of 1030 mg/m2 vs. 520 mg/m2 oxaliplatin, respectively) of adjuvant treatment for CRC with oxaliplatin, are important [Citation18,Citation19]. These studies will prospectively demonstrate the optimal number of adjuvant courses with oxaliplatin in relation to both effectiveness and toxicity and might justify treatment with only six cycles of FOLFOX or four cycles of CAPOX with lower associated cumulative doses of oxaliplatin, which is supposed to result in a reduction of persistent CIPN.
Unlike other studies, the majority of patients included in this study were treated with CAPOX instead of FOLFOX [Citation11]. Studies investigating CIPN in patients treated with CAPOX are scarce. However, type of combination therapy may influence the degree of neuropathy. One study reported that FOLFOX was associated with increased incidence of CIPN compared with CAPOX at a similar cumulative dose [Citation20]. Another study reported more grade ≥ 3 neuropathy (26% vs. 11%; p < 0.001) in patients treated with FOLFOX versus CAPOX [Citation21]. In our study, EORTC QLQ-CIPN20 subscales were similar between regimens. However, patients were mainly treated with CAPOX and a minority with FOLFOX, which makes drawing conclusions on CIPN due to FOLOX difficult.
Acute neuropathy is unlikely to be caused by structural damage of axons and is more likely to be caused by changes in voltage-dependent sodium and/or potassium-channels in comparison with persistent CIPN [Citation4,Citation7]. However, patients with acute neuropathy and those who received a dose reduction because of acute neuropathy also had complaints of long-term CIPN. This suggests that acute neurotoxicity is associated with the development of persistent CIPN. A possible explanation might be that the changes in the ion-channels lead to axonal damage. This is in concordance with the hypothesis that following to changes in Na+-channels, calcium enters and accumulates in the axons leading to destruction, irreversible damage and therefore persistent CIPN [Citation22]. Furthermore, recently single nucleotide polymorphisms of voltage-gated Na+-channels genes or oxaliplatin metabolism were associated with an increased incidence of oxaliplatin-induced neurotoxicity [Citation5,Citation6,Citation23]. In addition, knockout of SCN9a, which encodes for the Nav1.7-channel, demonstrated to cause anosmia in mice [Citation24] and loss of smelling during oxaliplatin treatment might thus predispose development of CIPN. Nonetheless, loss of smelling is not an item in the most commonly used EORTC QLQ-CIPN20 questionnaire. Discovering specific neuropathy symptoms that predispose the development of CIPN would be of great interest.
We were able to collect data regarding cumulative dose, oxaliplatin administration and acute neuropathy during treatment for most of the patients. However, the retrospective collection of this data is a limitation of the study. The NCI-CTC was lacking in more than 50% of patients and acute neuropathy was defined as having any documented neuropathy symptoms during treatment. Moreover, we had no information about the presence of neuropathy symptoms before treatment with oxaliplatin. Furthermore, data on possible recurrence of disease with complementary chemotherapy within the years after diagnosis was lacking for patients that did not initially receive oxaliplatin. In addition, we have adjusted the analysis for the presence of DM and other co-morbidities. However, selection bias could have occurred because patients with DM are less often treated with chemotherapy compared to patients without DM [Citation25].
We believe that our study contributes to the knowledge of persistent CIPN after adjuvant treatment with oxaliplatin, with the majority of patients treated with CAPOX, especially since there is no proven therapy to treat CIPN. Our study demonstrates that a higher cumulative dose is associated with the development of long-term CIPN. Monitoring symptoms of neuropathy during treatment is important as the risk of developing persistent CIPN may only be reduced by decreasing the cumulative dose of oxaliplatin on time, whereas delaying chemotherapy cycles probably is not beneficial. In addition, clinicians should be aware that acute neuropathy seems to be associated with long-term CIPN and that patients, in spite of receiving a dose reduction because of acute neuropathy, are still at risk of developing long-term CIPN. Patients should be informed on this matter. Future studies should focus on identifying patients with an increased risk of developing CIPN. In that way, cancer treatment might become more tailored as physicians might decide to restrain oxaliplatin treatment for certain patients.
Supplementary material available online
Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.980912
ionc_a_980912_sm2268.pdf
Download PDF (32.7 KB)Acknowledgements
We would like to thank all patients and their doctors for their participation in the study. We want to thank the following hospitals for their cooperation: Amphia Hospital, Breda; Bernhoven Hospital, Veghel and Oss; Catharina Hospital, Eindhoven; Elkerliek Hospital, Helmond; Jeroen Bosch Hospital, ‘s Hertogenbosch; Máxima Medical Centre, Eindhoven and Veldhoven; Sint Anna Hospital, Geldrop; St. Elisabeth Hospital, Tilburg; Twee Steden Hospital, Tilburg and Waalwijk; VieCuri Hospital, Venlo and Venray. This work was supported by the Netherlands Organization for Scientific Research [VENI grant number 451-10-041 to F.M.] The Hague, The Netherlands.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51.
- Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV. Chemotherapy- induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: Results from the population-based PROFILES registry. J Clin Oncol 2013;31:2699–707.
- Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: A systematic review. Support Care Cancer 2014;22:2261–9.
- Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC. Dose effects of oxaliplatin on persistent and transient Na+ conductances and the development of neurotoxicity. PLoS One 2011;6:e18469.
- Faber CG, Hoeijmakers JG, Ahn HS, Cheng X, Han C, Choi JS, et al. Gain of function Nanu1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 2012;71: 26–39.
- Faber CG, Lauria G, Merkies IS, Cheng X, Han C, Ahn HS, et al. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc Natl Acad Sci U S A 2012;109:19444–9.
- Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C. The chemotherapeutic oxaliplatin alters voltage-gated Na(+) channel kinetics on rat sensory neurons. Eur J Pharmacol 2000;406:25–32.
- Matsumoto S, Nishimura T, Kanai M, Mori Y, Nagayama S, Kawamura J, et al. Safety and efficacy of modified FOLFOX6 for treatment of metastatic or locally advanced colorectal cancer. A single-institution outcome study. Chemotherapy 2008;54:395–403.
- Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC. Long-term neuropathy after oxaliplatin treatment: Challenging the dictum of reversibility. Oncologist 2011;16:708–16.
- Cavaletti G, Tredici G, Petruccioli MG, Donde E, Tredici P, Marmiroli P, et al. Effects of different schedules of oxaliplatin treatment on the peripheral nervous system of the rat. Eur J Cancer 2001;37:2457–63.
- Beijers AJ, Mols F, Vreugdenhil G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer 2014;22:1999–2007.
- van de Poll-Franse LV, Horevoorts N, van Eenbergen M, Denollet J, Roukema JA, Aaronson NK, et al. The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: Scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer 2011;47:2188–94.
- Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur J Cancer 2005;41:1135–9.
- Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16.
- Land SR, Kopec JA, Cecchini RS, Ganz PA, Wieand HS, Colangelo LH, et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol 2007;25:2205–11.
- Storey DJ, Sakala M, McLean CM, Phillips HA, Dawson LK, Wall LR, et al. Capecitabine combined with oxaliplatin (CapOx) in clinical practice: How significant is peripheral neuropathy? Ann Oncol 2010;21: 1657–61.
- Schmoll HJ, Cartwright T, Tabernero J, Nowacki MP, Figer A, Maroun J, et al. Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: A planned safety analysis in 1,864 patients. J Clin Oncol 2007;25:102–9.
- Andre T, Iveson T, Labianca R, Meyerhardt JA, Souglakos I, Yoshino T, et al. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) collaboration: Prospective combined analysis of phase III trials investigating duration of adjuvant therapy with the FOLFOX (FOLFOX4 or modified FOLFOX6) or XELOX (3 versus 6 months) regimen for patients with stage III colon cancer: Trial design and current status. Curr Colorectal Cancer Rep 2013;9:261–9.
- Labianca R. FOLFOX-4 3 months versus 6 months and bevacizumab as adjuvant therapy for patients with stage II/III colon cancer (TOSCA). In: Gruppo Italiano per lo studio dei Carcinomi dell’Apparato Digerente 2014. [updated 2013 Sep 3; cited 2014 Jul 22]. Available from: http://www.clinicaltrials.gov/show/NCT00646607
- Argyriou AA, Velasco R, Briani C, Cavaletti G, Bruna J, Alberti P, et al. Peripheral neurotoxicity of oxaliplatin in combination with 5-fluorouracil (FOLFOX) or capecitabine (XELOX): A prospective evaluation of 150 colorectal cancer patients. Ann Oncol 2012;23:3116–22.
- Ducreux M, Bennouna J, Hebbar M, Ychou M, Lledo G, Conroy T, et al. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer. Int J Cancer 2011;128:682–90.
- LoPachin RM, Lehning EJ. Mechanism of calcium entry during axon injury and degeneration. Toxicol Appl Pharmacol 1997;143:233–44.
- Argyriou AA, Cavaletti G, Antonacopoulou A, Genazzani AA, Briani C, Bruna J, et al. Voltage-gated sodium channel polymorphisms play a pivotal role in the development of oxaliplatin-induced peripheral neurotoxicity: Results from a prospective multicenter study. Cancer 2013;119:3570–7.
- Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The Na(V)1.7 sodium channel: From molecule to man. Nat Rev Neurosci 2013;14:49–62.
- Zanders MM, van Steenbergen LN, Haak HR, Rutten HJ, Pruijt JF, Poortmans PM, et al. Diminishing differences in treatment between patients with colorectal cancer with and without diabetes: A population-based study. Diabet Med 2013;30:1181–8.
