To the Editor,
Breast conserving treatment (BCT) with radiotherapy has replaced mastectomy as the standard care for early stage breast cancer in the last few decades. A rare and severe treatment complication is the occurrence of secondary angiosarcoma which has been associated to radiotherapy and lymphedema after a median latency period of 4–8 years [Citation1–4]. Even though the absolute risk of secondary angiosarcoma after radiotherapy for breast cancer is estimated to be < 0.5%, improved breast cancer survival rates imply that an increasing number of individuals are at risk of this side effect.
Angiosarcomas are highly invasive tumors and grow in a multifocal pattern; the risk of local recurrence and metastasis after surgery is high [Citation1,Citation5,Citation6]. The effect of adjuvant systemic treatment is unclear although recent studies of targeted therapies have shown promising results [Citation7]. Earlier case series have reported better tumor control with aggressive surgical approaches [Citation5,Citation8], but the extent of the resection needed is neither determined nor described in detail. Here, we present long-term survival in six patients with secondary angiosarcoma after breast cancer treated with resection of all irradiated skin and associated extra thoracic soft tissue.
Six women with localized secondary angiosarcoma of the thoracic wall after breast cancer treatment were included in this series, all treated at our Sarcoma Center in Lund, Sweden. The patients were diagnosed with breast cancer between ages 42 and 67, treated with BCT and adjuvant radiotherapy with 60 Gy over 25 fractions. Two patients also received post-operative chemotherapy (six cycles of CMF or FEC, respectively). The latency time to secondary angiosarcoma was 5–9 years (Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.983657).
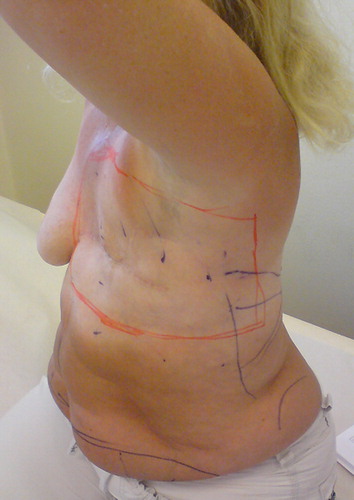
The surgical treatment of the secondary angiosarcoma was planned for inclusion of all irradiated skin using radiotherapy coordination tattoos and the patient's radiation dose planning charts (). Pre-operative magnetic resonance imaging (MRI) was used to determine the resection depth needed to achieve wide surgical margins in relation to the thoracic wall. The latter varied from including the deep fascia and superficial muscle tissue to include all extra thoracic muscles and sometimes periosteal stripping of the ribs. Reconstructions were performed using pedicled m. latissimus dorsi or m. rectus abdominis flaps, and split thickness skin grafts (). The resected area was 15–20 cm by 25–30 cm. All wounds healed with the primary reconstructions. In one patient complementary cosmetic reconstructive surgery was performed.
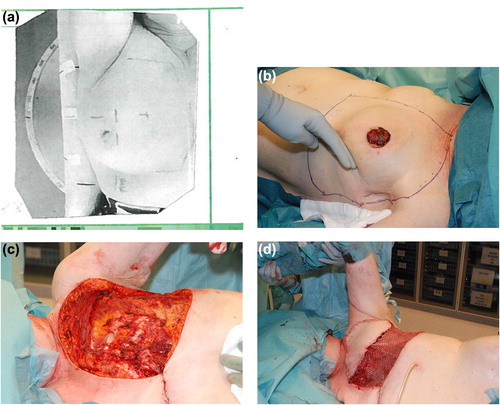
Four patients were treated with resection of all irradiated tissue for the first presentation of secondary angiosarcoma and three of them had no local or distant tumor recurrence and were alive without evidence of disease at latest follow-up (FU) at 1.8, 6 and 6.5 years, respectively (). The fourth patient was diagnosed with pulmonary metastases one month after surgery and died six months later.
For two patients, the initial treatment with local resection was performed in other hospitals and they were treated with resection of all irradiated tissue due to local recurrence. Both had further recurrences after the resection of all irradiated tissue. In the first case, the patient developed a metastasis in the contralateral axillary lymph nodes nine months after surgery. The lymph node metastasis was resected and the area treated with radiotherapy (50 Gy over 25 fractions). At latest FU the patient was disease-free at 5.5 years. The second patient developed a local recurrence after 2.5 years. The tumor was excised with wide margins and the patient treated with adjuvant radiotherapy (50 Gy over 25 fractions). At latest FU, she was disease-free at seven years.
The main treatment of radiation-associated secondary angiosarcoma after breast cancer treatment is surgery. The effect of adjuvant systemic treatment is unclear [Citation7]. Despite free surgical margins, the prognosis is dismal with five years survival rates of 30–50% [Citation5,Citation9–11]. Using more aggressive surgical approaches, earlier center-based case series have shown that long-term survival is possible to achieve [Citation4,Citation5,Citation8,Citation11–14]. However, few studies have defined the extent of surgical margins needed and only rarely is the median size of the excision reported [Citation5,Citation14]. Most studies describe that the operations were performed as mastectomies or as wide excisions (when the patient had undergone mastectomy as part of the previous breast cancer treatment).
Here we present a small case series with long-term survival in five of six patients treated with an extensive surgical approach for secondary angiosarcoma. All irradiated skin and subcutaneous tissue, determined according to radiotherapy coordination tattoos and dose planning charts, was excised. Deep surgical margins were planned in relation the extension of tumor as evaluated by MRI, and had to include all extra thoracic muscle tissue in some cases. Despite recurrences in two patients, long-term survival appears to have been achieved. Although our series is small it presents a treatment strategy for secondary angiosarcomas after breast cancer treatment. The outcome suggests that this aggressive surgical approach is motivated and that aggressive treatment of solitary tumor recurrences also should be considered.
We recommend that patients with suspected secondary angiosarcoma after breast cancer treatment are referred to a multidisciplinary sarcoma center for evaluation, diagnosis and treatment.
Supplementary material available online
Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.983657.
ionc_a_983657_sm8208.xlsx
Download MS Excel (10.7 KB)Acknowledgments
Financial support was grated from the Swedish Research Council, the Swedish Cancer Fund, the Faculty of Medicine, Lund University, Region Skåne, the John och Augusta Perssons stiftelse and the Maggie Stephens stiftelse.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Styring E, Fernebro J, Jonsson PE, Ehinger A, Engellau J, Rissler P, et al. Changing clinical presentation of angiosarcomas after breast cancer: From late tumors in edematous arms to earlier tumors on the thoracic wall. Breast Cancer Res Treat 2010;122:883–7.
- Body G, Sauvanet E, Calais G, Fignon A, Fetissof F, Lansac J. [Cutaneous angiosarcoma of the breast following surgery and irradiation of breast adenocarcinoma]. J Gynecol Obstet Biol Reprod 1987;16:479–83.
- Stewart FW, Treves N. Lymphangiosarcoma in postmastectomy lymphedema; a report of six cases in elephantiasis chirurgica. Cancer 1948;1:64–81.
- Billings SD, McKenney JK, Folpe AL, Hardacre MC, Weiss SW. Cutaneous angiosarcoma following breast- conserving surgery and radiation: An analysis of 27 cases. Am J Surg Pathol 2004;28:781–8.
- Seinen JM, Styring E, Verstappen V, Vult von Steyern F, Rydholm A, Suurmeijer AJ, et al. Radiation-associated angiosarcoma after breast cancer: High recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol 2012;19:2700–6.
- Neuhaus SJ, Pinnock N, Giblin V, Fisher C, Thway K, Thomas JM, et al. Treatment and outcome of radiation- induced soft-tissue sarcomas at a specialist institution. Eur J Surg Oncol 2009;35:654–9.
- Maki RG, D’Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol 2009; 27:3133–40.
- Yap J, Chuba PJ, Thomas R, Aref A, Lucas D, Severson RK, et al. Sarcoma as a second malignancy after treatment for breast cancer. Int J Radiat Oncol Biol Phys 2002; 52:1231–7.
- Vorburger SA, Xing Y, Hunt KK, Lakin GE, Benjamin RS, Feig BW, et al. Angiosarcoma of the breast. Cancer 2005;104:2682–8.
- Cha C, Antonescu CR, Quan ML, Maru S, Brennan MF. Long-term results with resection of radiation-induced soft tissue sarcomas. Ann Surg 2004;239:903–9; discussion 9–10.
- Torres KE, Ravi V, Kin K, Yi M, Guadagnolo BA, May CD, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol 2013;20:1267–74.
- Lahat G, Dhuka AR, Hallevi H, Xiao L, Zou C, Smith KD, et al. Angiosarcoma: Clinical and molecular insights. Ann Surg 2010;251:1098–106.
- Sheth GR, Cranmer LD, Smith BD, Grasso-Lebeau L, Lang JE. Radiation-induced sarcoma of the breast: A systematic review. Oncologist 2012;17:405–18.
- Jallali N, James S, Searle A, Ghattaura A, Hayes A, Harris P. Surgical management of radiation-induced angiosarcoma after breast conservation therapy. Am J Surg 2012;203:156–61.