Abstract
Strong evidence exists supporting the effect of lack of physical activity on the risk of developing breast cancer. However, studies examining the effects of physical activity on breast cancer outcomes, including survival and prognosis have been inconclusive. Therefore, the aim of the current study was to provide a systematic review and meta-analysis of studies investigating the association between physical activity and breast cancer recurrence and death.
Methods. PubMed, EMBASE, and CENTRAL databases were searched up to 18 October 2014. Reference lists of retrieved articles and relevant previous reviews were also searched. Observational studies that reported risk estimates for all-cause and/or breast cancer-related death and/or breast cancer recurrences by levels of physical activity, were included in the review. Random effects models were used to calculate pooled hazard ratios (HR) and 95% confidence intervals (CI) and to incorporate variation between studies. The Newcastle-Ottawa scale was used to critically appraise the risk of bias across studies.
Results. Twenty-two prospective cohort studies were eligible in this meta-analysis. During average follow-up periods ranging from 4.3 to 12.7 years there were 123 574 participants, 6898 all-cause deaths and 5462 breast cancer outcomes (i.e. breast cancer-related deaths or recurrences). The average Newcastle-Ottawa score was six stars (range 4–8). Compared to those who reported low/no lifetime recreational pre-diagnosis physical activity, participants who reported high lifetime recreational pre-diagnosis physical activity levels had a significantly lower risk of all-cause (HR = 0.82, 95% CI 0.70–0.96, p < 0.05) and breast cancer-related death (HR = 0.73, 95% CI 0.54–0.98, p < 0.05). Significant risk reductions for all-cause and breast cancer-related death was also demonstrated for more recent pre-diagnosis recreational physical activity (HR = 0.73, 95% CI 0.65–0.82, p < 0.001; and HR = 0.84, 95% CI 0.73–0.97, p < 0.05, respectively), post-diagnosis physical activity (HR = 0.52, 95% CI 0.43–0.64, p < 0.01; and HR = 0.59, 95% CI 0.45–0.78, p < 0.05, respectively) and meeting recommended physical activity guidelines (i.e. ≥ 8 MET-h/wk) post-diagnosis (HR = 0.54, 95% CI 0.38–0.76, p < 0.01; and HR = 0.67, 95% CI 0.50–0.90, p < 0.01, respectively). However, there was evidence of heterogeneity across lifetime recreational pre- and post-diagnosis physical activity analyses. Both pre-diagnosis (lifetime and more recent combined) and post-diagnosis physical activity were also associated with reduced risk of breast cancer events (breast cancer progression, new primaries and recurrence combined) (HR = 0.72 95% CI 0.56–0.91, p < 0.01; and HR = 0.79, 95% CI 0.63–0.98, p < 0.05, respectively).
Conclusion. There is an inverse relationship between physical activity and all-cause, breast cancer-related death and breast cancer events. The current meta-analysis supports the notion that appropriate physical activity may be an important intervention for reducing death and breast cancer events among breast cancer survivors.
Breast cancer is the most commonly diagnosed cancer and a leading cause of death from cancer in women; breast cancer is responsible for 23% of total cancer cases and 14% of cancer deaths worldwide [Citation1]. While incidence rates vary markedly across the world regions, it is the most common cancer in women in both more and less developed regions with higher number of cases in less developed (883 000 cases) than in more developed (794 000) regions [Citation2]. Breast cancer is now also the most common cause of cancer death in women in less developed regions (324 000 deaths) and the second highest in more developed regions (198 000 deaths). Globally, both breast cancer incidence and mortality have increased from 2008 to 2012 (20% and 14% increase, respectively) [Citation2]. In the UK, female breast cancer had the highest incidence rate of all cancers, with an average European age standardised rate of 124 cases per 100 000 population each year between 2007 and 2009 [Citation3]. Recent projections have estimated that new cases of breast cancer in England will rise by 44% from 2001 figures to 2020 [Citation4].
Risk factors associated with an increased risk of developing breast cancer are typically categorised into non-modifiable factors, such as age and genetic predisposition, and modifiable factors, such as alcohol consumption, overweight/obesity and lack of physical activity (defined as any bodily movement produced by the contraction of skeletal muscle that increases energy expenditure above a basal level). Twenty-nine (40%) observational studies have found a statistically significant reduction in breast cancer risk amongst the most physically active when compared to the least active women [Citation5]. A number of mechanisms have been proposed to explain how physical activity can potentially lower the risk of breast cancer, which include reduced exposure to oestrogen and androgens, insulin-related factors, adipokines and inflammation [Citation6–9]. These same mechanisms may also act in breast cancer survivors to reduce overall and disease-specific mortality and recurrence. Therefore, the aim of this systematic review was to evaluate the available literature pertaining to the effects of physical activity on all-cause and breast cancer-related death as well as recurrence in women diagnosed with breast cancer.
Methods
A review of literature on the association between physical activity and all-cause and breast cancer-related death as well as recurrence in women diagnosed with breast cancer was conducted using PubMed articles from 1966 up to October 2014, EMBASE (1980 to October 2014) articles, Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 10) articles, and a search of the reference lists of previous studies and reviews for potentially relevant additional articles. The database search strategy is available in Supplementary 1, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.998275. The reporting of this systematic review is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [Citation10].
Eligibility criteria for considering studies for the review
Based on the current literature search there are at present no randomised controlled trials addressing the effects of physical activity on all-cause or breast cancer-related death or recurrence in breast cancer survivors. Therefore, a priori was given to observational studies such as cohort and case-control studies for inclusion in the review. Studies which measured and investigated the effects of either pre- and/or post-diagnosis physical activity on all-cause death, breast cancer-related death and/or breast cancer recurrence were included. Studies which included cancer patients but did not provide a sub-analysis of breast cancer patients were excluded. Studies which controlled for at least one confounding variable known to influence the risk of death and/or recurrence were eligible. The search strategy was limited to English language articles that described studies in humans.
Search methods for identification of studies
Two authors (IML and GSM) independently screened and assessed the records for eligibility. All the titles and abstracts of the studies resulting from the searches of PubMed, EMBASE and CENTRAL databases and reference lists were reviewed and articles that were clearly irrelevant were excluded. Disagreements on study eligibility were resolved through consensus or, when necessary, there was a meeting with a third review author not involved in the particular assessment (AMN). Potentially eligible studies from each database were merged and duplicates were removed. Full-text copies of all trials were retrieved if a trial possibly or definitely met the inclusion criteria. The retrieved full-text articles were reviewed using the defined eligibility criteria, and were included if the eligibility criteria were met. If there was a need for clarification of any detail of a trial, the trial authors were contacted to obtain such clarification for a complete assessment of the trial's relevance for the current systematic review. Missing data were retrieved by asking study authors for the necessary data via electronic mail.
Study collection and data extraction
For each eligible trial, information on the characteristics of studies, study population, physical activity assessment and breast cancer outcomes were extracted, in addition to details of the comparisons made within each study. Characteristics of studies extracted included study design, country of origin, years patients were recruited/diagnosed with breast cancer and follow-up time. Characteristics of study population included participants’ age, menopausal status, body mass index (BMI), race, percentage with family history of breast cancer, percentage of participants who had received chemotherapy and hormonal therapy, breast cancer staging information and hormone-receptor status. Characteristics of physical activity assessment extracted included the time period between diagnosis and assessment, the period(s) of time that physical activity was assessed, the mode of physical activity assessment and a description of the assessment tool used. In addition, information on physical activity variables and categories, comparisons and their statistical results [hazard ration (HR) and 95% confidence interval (CI)] were extracted for the effects of physical activity on all-cause and breast cancer-related death as well as recurrence. Details of any physical activity sub-analyses were also extracted from studies. IML and GSM independently extracted the data and any conflicts not due to extractor error were arbitrated by AMN.
Measurement of outcome
Our primary outcomes for all studies were all-cause death, breast cancer-related death and breast cancer recurrence. Each of these outcomes is described as time-to-event data and as such the result was expressed as a HR with 95% CI in all studies. The HR is a measure of relative risk over time, and in this case it is the risk of suffering death or breast cancer recurrence over a particular time period [Citation11]. In the current review, the HR provides a measure of how high the risks of these events are in one group (group 1; reference group of those who perform no/low levels of physical activity) compared to another group (group 2; those who are sufficiently physically active, i.e. performing at least 150 min/wk of moderate or 75 min/wk of vigorous physical activity). We reported the ratios of treatment effects for response so that HRs less than 1.0 favour higher physical activity and HRs greater than 1.0 favour no/low physical activity.
A meta-analysis was conducted to determine the direction and size of the possible effect that physical activity has on all-cause mortality and breast cancer-related mortality in breast cancer patients. There are conflicting results from the available data, and therefore, by combining these data the power (i.e. ability to detect a real effect as statistically significant if it exists) and precision (i.e. improve the accuracy of the effect estimate) can be improved. Comparisons were made between: 1) lifetime pre-diagnosis physical activity; 2) more recent (≤ 12 years) pre-diagnosis total physical activity; 3) post-diagnosis recreational physical activity; and 4) meeting recommended physical activity guidelines post-diagnosis (defined as ≥ 8 MET-h/wk), and all-cause and breast cancer-related death and recurrence.
Assessment of study quality and publication bias
IML and GSM independently and in duplicate assessed the quality of the studies using a modified Newcastle-Ottawa scale for cohort studies [Citation12]. Conflicts were resolved via consensus or, when necessary, arbitrated by AMN. Key quality areas that were assessed included: 1) selection of exposed and non-exposed cohorts, which included four items related to the representativeness of the exposed cohort, selection of the non-exposed cohort, assessment of the exposure (i.e. physical activity) and demonstration of the presence of the outcome (breast cancer in this instance); 2) comparability of the groups, which requires the selection of confounders considered to be the most important (age, cancer stage/nodal status and treatment were chosen in the current review due to their prognostic importance); 3) assessment of the outcome, which included three items related to the assessment of the outcomes of interest (i.e. all-cause and breast cancer-related death and breast cancer recurrence), whether the duration of the follow-up period was long enough for outcome to occur (≥ 5 years follow-up was considered to be adequate) [Citation13] and the adequacy of the follow-up process (i.e. how complete was the follow-up process; lost to follow-up < 20% and/or the completeness of vital statistics data < 10%) [Citation14]. A star was awarded for high quality in each area. Therefore, the maximum possible study quality score was nine.
To investigate publication bias, we prepared funnel plots using RevMan (version 5.2). Tests for funnel-plot asymmetry, such as the Peters test or the Egger regression test [Citation15,Citation16], were not used because only one of the comparisons performed in the meta-analysis had above 10 studies. A small number of studies lowers test power to a point where it is too low to distinguish chance from real asymmetry, which leads to an increase in the likelihood of a “statistically significant” result when in reality there is no association between study size and intervention effects [Citation17]. Therefore, publication bias was interpreted in the context of visual inspection of funnel plots. Effect estimates from smaller studies should scatter along the bottom of the funnel plot because these studies are usually less precise [i.e. have larger standard errors (SE)] and are highly subject to random variation, while the spread among larger more precise (i.e. smaller SEs) studies should narrow [Citation17]. Thus, publication bias was deemed to be absent if the funnel plot resembled a symmetrical inverted funnel shape. Publication bias was suspected if there were studies with markedly different intervention effect estimates, if smaller studies tended to lead to more or less beneficial effect estimates, and if the funnel plot was skewed and asymmetrical (i.e. gaps in bottom right and left corners indicating “missing studies”).
Statistical analysis
To perform a meta-analysis of time-to-event outcomes, log HR (comparison group relative to reference group) and its SE must be obtained. HRs and their 95% CI were extracted from the relevant trials, and were then transformed by taking their natural logarithms (log base e, written “ln”). SE were calculated from the corresponding 95% CI as follows: (ln[upper limit of CI] - ln[lower limit of CI])/3.92 [Citation18]. This transformation was conducted due to the asymmetry of risk ratios. The lowest value that a ratio can be is zero while the highest value is infinity. By ln transforming the HR, the “no effect” value becomes 0. The HR and 95% CI extracted from the studies included and used in the analysis, was the estimate that adjusted for the maximum number of covariates or the estimate that was identified as the primary adjusted model by the authors (see Supplementary 2, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.998275, for adjustments for potential confounding variables across the studies). To estimate a pooled effect and corresponding 95% CI, the HRs were weighted by the inverse of their variance. For ease of interpretation the ln transformations of summary estimates and their 95% CI were converted back to their ratio (i.e. eln(HR)) and presented in the results section.
Based on the ln of the HRs and their corresponding SE, the Cochran's Q test and I2 statistics were used to assess heterogeneity among studies [Citation19]. The significance of the Q statistic was interpreted using the χ2 distribution table. For the Q statistic, a p-value of less than 0.10 was used as an indicator of the presence of heterogeneity (i.e. variation in intervention effects beyond chance), while a p-value of greater 0.10 suggests there is no significant heterogeneity (i.e. effect estimates of studies are similar). The I2 statistic expresses the percentage of variability in the effect estimate due to heterogeneity rather than by chance. We interpreted I2 values of 0–40% as “might not be important”, 30–60% as “may represent moderate heterogeneity”, 50–90% as “may represent substantial heterogeneity” and 75–100% as “considerable heterogeneity” [Citation19].
Study results were combined using the generic inverse-variance method. A random-effects model [Citation20] was used to combine data where heterogeneity was suspected, while the fixed effects model was used when heterogeneity was not suspected. The summary statistic based on either a fixed- or random effects model will be expressed as a HR and 95% CI, and not their ln for ease of comparison with other studies and consistency. If substantial heterogeneity was found we conducted sensitivity analysis by including only studies with a quality score of six and above. Due to the variation in assessment periods, we also conducted a sensitivity analysis of the studies investigating more recent recreational physical activity, which involved analysing only those studies which assessed physical activity in less than five years preceding diagnosis. Subanalyses were conducted when there were sufficient numbers of studies involved (i.e. at least two studies). These subanalyses stratified breast cancer patients by postmenopausal status/age, BMI, breast cancer stage and oestrogen receptor (ER) status. The meta-analyses and accompanying forest plots were carried out using RevMan (version 5.2).
Results
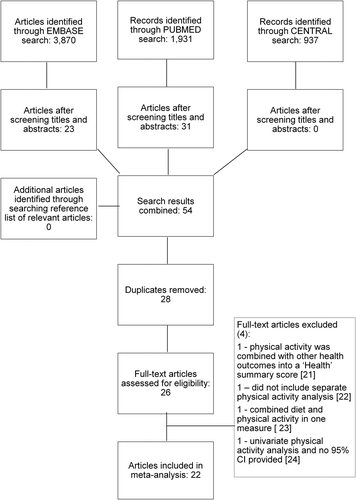
Our primary search identified 6738 potentially relevant references (). Based on the title and abstract, and after duplicates were removed, 26 references were retrieved for full-text screening. Four references were excluded as they did not meet the inclusion criteria and 22 studies were identified as appropriate for inclusion in the current review. lists reasons for excluding these four studies [Citation21–24].
Results of the search
The final selection based on consensus resulted in 22 studies being included in this review [Citation25–46]. We corresponded with and requested additional data from one trial author, and this trial author was able to provide additional data.
Characteristics of included studies
Trial characteristics and physical activity assessment details of eligible trials are summarised in . All studies were published between 1995 and 2014, and study locations included USA, Canada, Europe, Australia and China. Of the 22 included studies, all were prospective cohort studies. Eight studies were prospective cohort follow-ups to case-control studies [Citation25,Citation26,Citation32,Citation35,Citation37,Citation41,Citation42,Citation44] and two were prospective cohort follow-ups of prospective cohort studies [Citation25,Citation29]. The total number of participants across all 22 studies was 123 574, and the mean number of participants in each study was 5617 (s = 16 480; range = 412–79 124). There were a total of 6898 all-cause deaths and 5462 breast cancer events (i.e. breast cancer related-deaths or recurrences). The years in which participants were diagnosed with breast cancer ranged from 1974 to 2006. The mean average (mean/median) follow-up period was 7.7 (s = 2.4) years. Ten of the studies reported that these physical activity questionnaires were interview-administered, and the other 12 used self-administered physical activity questionnaires. Eleven of the 22 studies reported an actual time between diagnosis of breast cancer and physical activity assessment, and there was considerable variation in this duration. The mean average time from diagnosis to assessment was 36 (s = 45, range = 2–138) months. There were insufficient studies investigating non-recreational physical activity, therefore, only recreational physical activity was included in this current review. Seven of the 22 studies investigated the effects of lifetime recreational pre-diagnosis physical activity [Citation26,Citation31,Citation32,Citation34,Citation35,Citation38,Citation41], 13 of the studies assessed more recent pre-diagnosis recreational physical activity [Citation25,Citation26,Citation28–31,Citation34,Citation36,Citation38,Citation40–42,Citation45] and nine studies evaluated post-diagnosis physical activity [Citation23,Citation27,Citation33,Citation36,Citation37,Citation39,Citation43,Citation44,Citation46].
Table I. Characteristics of included studies.
Risk of bias in included studies
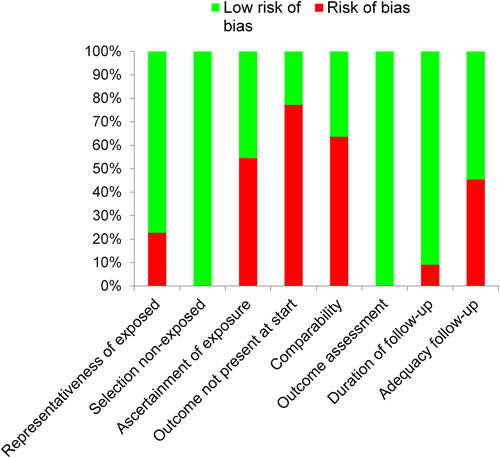
Out of a maximum of nine stars, we found that the median and mean number of stars awarded to a study was six. The highest score was eight stars (two studies [Citation25,Citation30]) and the lowest score was four stars (one study [Citation46]) (). provides the risk of bias indicators of the included studies. Most of the studies (77%; 17/22) were deemed to be representative of breast cancer populations, while five studies were thought to be unrepresentative, two of which included participants from a particular profession (Nurses [Citation39], Public School Teachers [Citation34]), another two studies included participants who were already physically active (i.e. runners and walkers [Citation45,Citation46]) and in one study only white and Hispanic participants were eligible for inclusion [Citation41]. All 22 studies recruited non-exposed participants from the same cohort as exposed participants. More than half of the studies (55%; 12/22) were at risk of bias due to the method used to ascertain exposure (i.e. physical activity levels were assessed via self-administered questionnaires). Only 23% (5/22) of the studies included participants that did not have breast cancer at the point of study enrollment. The majority of studies (64%; 14/22) did not control for known confounders adequately, and only nine (41%) controlled for stage/nodal status, age and treatment. All 22 studies assessed outcomes (i.e. all-cause and breast cancer-related death and recurrence) via record linkage. The median and mean follow-up period was eight years. Only two studies (9%) were deemed at risk of bias due to inadequate follow-up durations (i.e. < 5 years). Ten (45%) of the 22 studies were considered at risk due to the proportion of participants lost to follow-up (< 20%) and/or the completeness of vital statistics data (< 10%).
Table II. Risk of bias summary: review authors’ judgments about each risk of bias item for each included study using the Newcastle-Ottawa Scale.
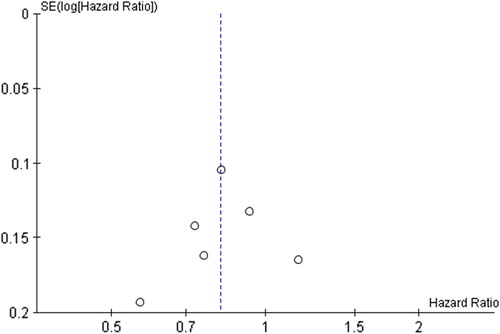
Publication bias was difficult to describe because of the relatively small number of studies included in the current meta-analysis. Visual inspection of the funnel plot did not suggest publication bias for lifetime recreational physical activity and both all-cause and breast cancer-related death as the studies were distributed symmetrically (i.e. inverted funnel shape) about the summary effect size (). There was potential publication bias across other comparisons in this current review (see Supplementary 3, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.998275, for additional funnel plots).
Outcome evaluation and meta-analysis
Full details of results from the epidemiological studies can be found in Supplementary 4, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.998275 and a summary of results is provided in . Forest plots for each comparison are shown between and .
Figure 4. Forest plot with random effects overall hazard ratio for association between lifetime recreational (pre-diagnosis) physical activity (highest vs. lowest physical activity categories) and all-cause death in breast cancer survivors. Red squares indicate hazard ratios (HRs), and solid horizontal lines represent 95% confidence intervals (CIs). The vertical solid line indicates point of no effect. Black diamond indicates overall effect.
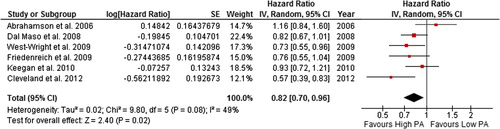
Figure 5. Forest plot with random effects overall hazard ratio for association between lifetime recreational (pre-diagnosis) physical activity (highest vs. lowest physical activity categories) and breast cancer-related death in breast cancer survivors.
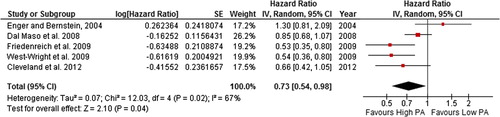
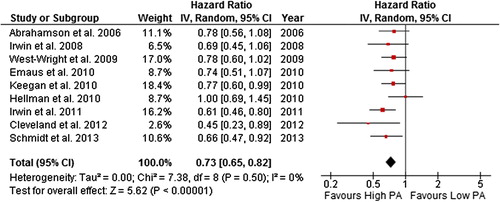
Figure 7. Forest plot with random effects overall hazard ratio for association between recent pre-diagnosis recreational physical activity (highest vs. lowest physical activity categories) and breast cancer-related death in breat cancer survivors.
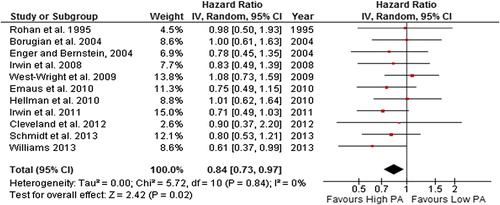
Figure 8. Forest plot with random effects overall hazard ratio for association between post-diagnosis recreational physical activity (highest vs. lowest physical activity categories) and all-cause death in breast cancer survivors.
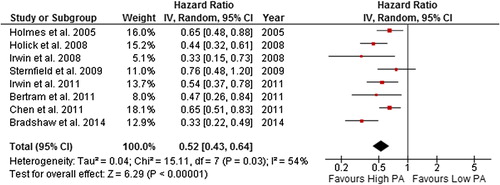
Figure 9. Forest plot with random effects overall hazard ratio for association between post-diagnosis recreational physical activity (highest vs. lowest physical activity categories) and breast cancer-related death in breast cancer survivors.
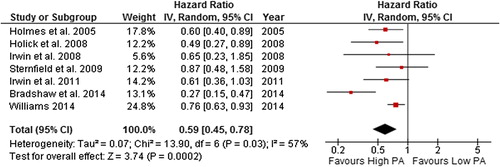
Figure 10. Forest plot with random effects overall hazard ratio for association between post-diagnosis meeting recommended physical activity guidelines (meeting vs. not meeting physical activity guidelines) and all-cause death in breast cancer survivors.
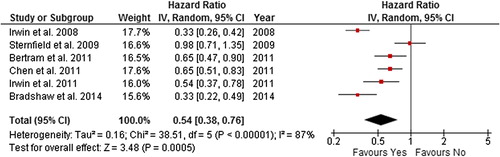
Figure 11. Forest plot with random effects overall hazard ratio for association between post-diagnosis meeting recommended physical activity guidelines (meeting vs. not meeting physical activity guidelines) and breast cancer-related death in breast cancer survivors.
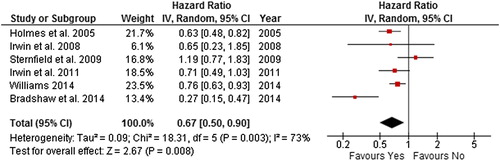
Figure 12. Forest plot with random effects overall hazard ratio for association between Pre-diagnosis physical activity (highest vs. lowest physical activity categories) and breast cancer events in breat cancer survivors.

Figure 13. plot with random effects overall hazard ratio for association between Post-diagnosis physical activity (highest vs. lowest physical activity categories) and breast cancer events in breast cancer survivors.

Table III. Summary of random effects overall hazard ratios (HR) and 95% confidence intervals (CI) for association between recreational physical activity (PA) variables and death and breast cancer (BC) events in breast cancer survivors.
Lifetime pre-diagnosis recreational physical activity
The summary HR (95% CI) was 0.82 (0.70–0.96) and 0.73 (0.54–0.98) in random effects models for all-cause death and breast cancer-related death, respectively, for breast cancer survivors with the highest levels of self-reported lifetime pre-diagnosis recreational physical activity compared to the lowest. However, there was moderate and potentially substantial heterogeneity in the all-cause death (49%) and breast cancer-related death (67%) comparisons, respectively. When studies with quality score of < 5 stars were excluded, heterogeneity was reduced to levels described as “might not be important”.
More recent pre-diagnosis recreational physical activity
The random-effects model effect size for all-cause death was 0.73 (0.65–0.82) and 0.84 (0.73–0.97) for breast cancer-related death when the highest levels of self-reported more recent pre-diagnosis recreational physical activity was compared to the lowest. There was no evidence of heterogeneity in these comparisons (i.e. I2 = 0%). When studies that assessed physical activity in the five years before diagnosis were included, the result remained significant for both all-cause (HR = 0.71, 0.63–0.81; I2 = 0%) and breast cancer-related (HR = 0.84, 0.71–1.00; I2 = = 0%) death.
Pre-diagnosis recreational physical activity and breast cancer events
Due to insufficient data regarding recurrence we combined in the current analysis, one study which included recurrence as an outcome [Citation25] and another [Citation32] which included recurrence and breast cancer progression and new breast cancer primaries as one outcome. This combined outcome was referred to as breast cancer events. In order to obtain sufficient numbers of studies to analyse breast cancer events and pre-diagnosis physical activity, lifetime and more recent pre-diagnosis physical activity were combined (two studies were included in the analysis). The random effects model summary effect size for breast cancer events was 0.72 (0.56–0.91) for when the highest level of self-reported post-diagnosis recreational physical activity was compared to the lowest. No evidence of heterogeneity was found.
Post-diagnosis recreational physical activity
The random effects model summary effect size for all-cause death was 0.52 (0.43–0.64) and 0.59 (0.45–0.78) for breast cancer-related death when the highest level of self-reported post-diagnosis recreational physical activity was compared to the lowest. However, there was potentially substantial heterogeneity in the all-cause death and breast cancer-related death comparisons (54% and 57%, respectively). When studies with quality score of < 5 stars were excluded, both the summary effect and heterogeneity remained unchanged. However, when the most extreme study result was excluded from the analysis ([Citation44] in both comparisons), heterogeneity was reduced to 0%, and the summary effect remained significant in both all-cause and breast cancer- related death comparisons.
Meeting recommended physical activity guidelines post-diagnosis
The summary HR was 0.54 (0.38–0.76) and 0.67 (0.50–0.90) in a random effects model for all-cause death and breast cancer-related death, respectively, for breast cancer survivors meeting recommended physical activity guidelines post-diagnosis compared to those who were not. However, there was considerable heterogeneity in the all-cause death and breast cancer-related death comparison (87% and 73%, respectively). When studies with quality score of < 5 star were excluded, the summary effect and heterogeneity remained unchanged. The removal of the most extreme study results [Citation33,Citation36,Citation44] in both comparisons from the analysis, reduced heterogeneity to 0%, and the significance of the summary effect remained unchanged in both comparisons.
Post-diagnosis recreational physical activity and breast cancer events
Again, due to an insufficiency of data regarding recurrence we combined in the current analysis, two studies which included recurrence as an outcome [Citation33,Citation39] and one study [Citation43] which included recurrence and new breast cancer primaries as one outcome. This combined outcome was also referred to as breast cancer events. The random effects model summary effect size for breast cancer events was 0.79 (0.63–0.98) for when the highest level of self-reported post-diagnosis recreational physical activity was compared to the lowest. No evidence of heterogeneity was found.
Subgroup analyses results
and show the different subgroup analyses of studies on all-cause and breast cancer mortality and forest plots for these analyses can be found in Supplementary 5, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.998275. There were insufficient studies available to perform subanalyses on breast cancer events. There were few included studies in each of the subgroup analyses and evidence of heterogeneity across the subanalyses. The associations between lifetime pre-diagnosis, more recent pre- and post-diagnosis physical activity and risk of all-cause mortality did not differ substantially by BMI category. Conversely, those with a BMI ≥ 25 kg/m2 had a greater reduction in breast cancer-related death. However, the risk reductions in breast cancer-related death with both lifetime pre-diagnosis and post-diagnosis physical activity were stronger for those BMI ≥ 25 kg/m2 compared to those < 25 kg/m2. The associations between both lifetime pre-diagnosis physical activity and risk of both all-cause and breast cancer-related death was stronger for postmenopausal breast cancer survivors compared to those who were premenopausal at diagnosis/study enrolment. Menopausal status did not alter the association between both more recent pre- or post-diagnosis physical activity and breast cancer-related death. However, post-diagnosis physical activity was associated with a greater risk reduction in all-cause death in postmenopausal compared to premenopausal breast cancer survivors. The associations between more recent pre-diagnosis and risk of all-cause mortality did not differ substantially by ER status. Conflicting associations were found between post-diagnosis physical activity and all-cause and breast cancer-related death by ER and ER/progesterone receptor (PR) status was found. Post-diagnosis physical activity was associated with greater risk reductions in all-cause death for ER+ and ER-/PR- compared to ER- and ER+/PR+ status, respectively, and greater risk reductions in breast cancer-related death for ER+/PR+ versus ER-/PR- status. The association between more recent pre-diagnosis physical activity and risk of all-cause death was stronger for local disease compared to regional disease breast cancer survivors. While the association between post-diagnosis physical activity and all-cause death did not differ substantially by breast cancer stage.
Table IV. Summary of BMI and menopausal subgroup analyses by recreational physical activity (PA) variable.
Table V. Summary of HR-status and stage subgroup analyses by recreational physical activity (PA) variable.
Discussion
Higher lifetime and more recent pre- and post-diagnosis recreational physical activity were significantly associated with a lower risk of all-cause and breast cancer-related death and breast cancer events. However, evidence of heterogeneity was found for associations between risk of all-cause and breast cancer-related death for lifetime pre-diagnosis and post-diagnosis recreational physical activity and meeting recommended physical activity guidelines.
Previous meta-analyses [Citation47–49], which consist of many of the prospective cohort studies included in this current study, support the positive role of physical activity on breast cancer outcomes and are in agreement with the findings of the current review. A meta-analysis of six studies found an 18% reduction in the risk of all-cause but no reduction in risk of breast cancer-related death with pre-diagnosis physical activity, while post-diagnosis physical activity was associated with 41% and 34% reductions in all-cause death and breast cancer-related death, respectively, and a reduction in breast cancer recurrence of 24% [Citation48]. A pooled analysis of four studies, which were all part of the current study, found that post-diagnosis physical activity of at least 10 MET-h/wk was associated with similar reductions in the risk of all-cause and breast cancer-related death (27% and 25%, respectively), but found no reduction in recurrence risk [Citation47].
The strong risk reductions in all-cause death associated with physical activity was unsurprising, given the evidence regarding the role of physical activity in reducing the risk of CVD [Citation50–52], which is the major cause of death in women worldwide (WHO, 2013). Furthermore, a recent cohort study reported a significant association between poor physical health (characterised by obesity and physical inactivity) and all-cause mortality and shorter time to additional breast cancer events [Citation53].
From visual inspection of the funnel plots, there was a suggestion of publication bias across all comparisons with the exception of perhaps lifetime recreational physical activity and both all-cause and breast cancer-related death. Publication bias could have influenced the results of the current review and analysis. The inclusion of only published studies in this review and meta-analysis could have increased the risk of publication bias. In addition to this, we searched only one electronic database. Unpublished studies, such as abstract or reports, often report smaller treatment effects [Citation54], however, these types of studies are often of poor quality and may not provide sufficient data for pooled or meta-analysis. To minimise the risk of missing relevant studies we carefully examined all relevant meta-analysis and systematic reviews, including three recent reviews [Citation47–49]. We were not aware of any relevant unpublished studies when this current study was conducted. Only studies published in the English language were included, which could have led to an increased risk of language bias. Although the exclusion of non-English language studies might result in smaller effect estimates, language bias is generally small [Citation55]. Researchers who abstracted and reviewed the data in this current study were not blinded to authors, institutions and journals. However, this may only introduce a small risk of bias, and requires a large amount of administration time [Citation56]. Another possible limitation of this review is that a protocol was not registered prior to study commencement, and therefore, there is a potential risk of bias in the review process. Knowledge of the findings of the studies included in a review could influence decisions made regarding eligibility criteria, comparisons made and outcomes assessed [Citation19].
Substantial heterogeneity was found in several comparisons, including lifetime pre-diagnosis and post-diagnosis recreational physical activity, meeting recommended physical activity guidelines and risk of all-cause and breast cancer-related death. Therefore, the results of these comparisons should be treated with caution. In the case of lifetime pre-diagnosis recreational physical activity, when studies with Newcastle-Ottawa score of < 5 stars were excluded, heterogeneity was reduced to acceptable levels, while the removal of the most extreme study results reduced heterogeneity to an acceptable level in post-diagnosis recreational physical activity and meeting recommended physical activity guidelines analyses. Heterogeneity may have resulted from the variation in the methods of the included studies. For each comparison made in the current study, we used the highest and the lowest physical activity categories. However, there was a variation in how each study categorised the highest and lowest physical activity categories. Therefore, the strength of the association between the physical activity variables and breast cancer outcomes may be dependent on these cut-off points. Furthermore, physical activity outcomes were assessed differently across studies, which make comparisons between studies difficult. For lifetime physical activity some of the studies asked participants to recall physical activity performed over their entire lifetime up to the time of breast cancer diagnosis or one-year pre-diagnosis, while others collected physical activity data from specific age ranges pre-diagnosis. Recent pre-diagnosis recreational physical activity was assessed from one to three years prior to diagnosis, while post-diagnosis physical activity data was taken from six months to six years after diagnosis and participants were required to recall physical activity during different duration's post-diagnosis.
All studies assessed physical activity using either questionnaires or interviews. Both of these methods are known to be prone to biases, due mainly to recall errors and social desirability. Validation of these questionnaires is disputed and is difficult to establish due to a lack of a current gold standard criterion method for measuring habitual physical activity [Citation57]. Often physical activity questionnaires are validated against accelerometer data which itself has questionable validity. In addition to this, most of the physical activity data is related to moderate and vigorous- intensity physical activity, but not low-intensity physical activity. As a result, only a small fraction of an individual's physical activity may be measured in a given study, possibly leading to misclassification of some participants within studies. Moreover, studies varied in the information on types of activities requested from participants.
Observational studies provide insights into aetiology and allow us to make associations between certain factors and disease. However, these types of studies are not able to establish direct, casual links between physical activity and breast cancer outcomes [Citation58]. There are currently no randomised controlled trials (RCTs) available that have examined physical activity and all-cause death, breast cancer-related death and recurrence in breast cancer survivors. The on-going DIANA (Diet and Androgens)-5 is a RCT of the effectiveness of a Mediterranean diet and moderate physical activity in reducing additional breast cancer events in women with early stage invasive breast cancer at high risk of recurrence because of metabolic or endocrine milieu. This trial may provide important information regarding the effects of diet and exercise on breast cancer outcomes [Citation59]. However, RCTs are needed that can isolate the effects of physical activity on breast cancer outcomes.
Conclusion
There were significant associations between lifetime and recent pre-diagnosis recreational physical activity and risk of all-cause death; recent pre-diagnosis recreational physical activity was also found to be associated with the risk of breast cancer-related death. Post-diagnosis physical activity was found to significantly reduce the risk of both all-cause death and breast cancer-related death. However, effect estimates for these associations should be treated with caution due to evidence of heterogeneity. Future studies are needed to elucidate the mechanisms by which physical activity may increase survival among breast cancer survivors. This current review provides evidence to support the role of physical activity in potentially improving and extending overall survival after a diagnosis of breast cancer. There is a need for RCTs investigating the role of physical activity on all-cause death and breast cancer outcomes.
Supplementary material available online
Supplementary materials 1–5 to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.998275.
ionc_a_998275_sm6557.pdf
Download PDF (4.1 MB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. Cancer J Clin 2011;61:69–90.
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–403.
- Office for National Statistics. Cancer incidence and mortality 2007–09; 2012. Newport: Office for National Statistics; 2012.
- Moller H, Fairley L, Coupland V, Okello C, Green M, Forman D, et al. The future burden of cancer in England: Incidence and numbers of new patients in 2020. Br J Cancer 2007;96:1484–8.
- Lynch BM, Ne ilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Phys Activ Cancer 2011;186:13–42.
- Monninkhof EM, Velthuis MJ, Peeters PH, Twisk JW, Schuit AJ. Effect of exercise on postmenopausal sex hormone levels and role of body fat: A randomized controlled trial. J Clin Oncol 2009;27:4492–9.
- Kaaks R. Nutrition, hormones, and breast cancer: Is insulin the missing link? Cancer Causes Control 1996;7:605–25.
- Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J Natl Cancer Inst 2002;94:606–16.
- McTiernan A, Tworoger SS, Rajan KB, Yasui Y, Sorenson B, Ulrich CM, et al. Effect of exercise on serum androgens in postmenopausal women: A 12-month randomized clinical trial. Cancer Epidemiol Biomarkers Prev 2004;13:1099–105.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Br Med J 2009;339:b2535.
- Spruance SL, Reid JE, Grace M, Samore M. Hazard ratio in clinical trials. Antimicrob Agents Chemother 2004;48: 2787–92.
- Wells GA, Shea B, O’Connell D, Peterson J, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of Nonrandomised studies in meta-analyses; 2011.
- Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. Br Med J 2014;349:g4490.
- Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Child 2008;93:458–61.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997;315:629–34.
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006;295:676–80.
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Br Med J 2011;343:d4002.
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J 2003; 327:557–60.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88.
- Thomson CA, McCullough ML, Wertheim BC, Chlebowski RT, Martinez ME, Stefanick ML, et al. Nutrition and physical activity cancer prevention guidelines, cancer risk, and mortality in the women's health initiative. Cancer Prev Res (Phila) 2014;7:42–53.
- Kwan ML, Weltzien E, Kushi LH, Castillo A, Slattery ML, et al. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J Clin Oncol 2009;27:919–26.
- George SM, Irwin ML, Smith AW, Neuhouser ML, Reedy J, McTiernan A, et al. Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early-stage breast cancer. Cancer Causes Control 2011;22:589–98.
- Pierce JP, Stefanick ML, Flatt SW, Natarajan L, Sternfeld B, Madlensky L, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol 2007;25:2345–51.
- Schmidt ME, Chang-Claude J, Vrieling A, Seibold P, Heinz J, Obi N, et al. Association of pre-diagnosis physical activity with recurrence and mortality among women with breast cancer. Int J Cancer 2013;133:1431–40.
- Cleveland RJ, Eng SM, Stevens J, Bradshaw PT, Teitelbaum SL, Neugut AI, et al. Influence of prediagnostic recreational physical activity on survival from breast cancer. Eur J Cancer Prev 2012;21:46–54.
- Chen X, Lu W, Zheng W, Gu K, Matthews CE, Chen Z, et al. Exercise after diagnosis of breast cancer in association with survival. Cancer Prev Res (Phila) 2011;4:1409–18.
- Irwin ML, McTiernan A, Manson JE, Thomson CA, Sternfeld B, Stefanick ML, et al. Physical activity and survival in postmenopausal women with breast cancer: Results from the women's health initiative. Cancer Prev Res (Phila) 2011;4:522–9.
- Emaus A, Veierod MB, Tretli S, Finstad SE, Selmer R, Furberg AS, et al. Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Res Treat 2010;121:651–60.
- Hellmann SS, Thygesen LC, Tolstrup JS, Gronbaek M. Modifiable risk factors and survival in women diagnosed with primary breast cancer: Results from a prospective cohort study. Eur J Cancer Prev 2010;19:366–73.
- Keegan TH, Milne RL, Andrulis IL, Chang ET, Sangaramoorthy M, Phillips KA, et al. Past recreational physical activity, body size, and all-cause mortality following breast cancer diagnosis: Results from the Breast Cancer Family Registry. Breast Cancer Res Treat 2010;123:531–42.
- Friedenreich CM, Gregory J, Kopciuk KA, Mackey JR, Courneya KS. Prospective cohort study of lifetime physical activity and breast cancer survival. Int J Cancer 2009;124:1954–62.
- Sternfeld B, Weltzien E, Quesenberry CP Jr., Castillo AL, Kwan M, Slattery ML, et al. Physical activity and risk of recurrence and mortality in breast cancer survivors: Findings from the LACE study. Cancer Epidemiol Biomarkers Prev 2009;18:87–95.
- West-Wright CN, Henderson KD, Sullivan-Halley J, Ursin G, Deapen D, Neuhausen S, et al. Long-term and recent recreational physical activity and survival after breast cancer: The California Teachers Study. Cancer Epidemiol Biomarkers Prev 2009;18:2851–9.
- Dal Maso L, Zucchetto A, Talamini R, Serraino D, Stocco CF, Vercelli M, et al.,Prospective Analysis of Case-control studies on Environmental factors and health (PACE) study group: Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer 2008;123: 2188–94.
- Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: The health, eating, activity, and lifestyle study. J Clin Oncol 2008;26:3958–64.
- Holick CN, Newcomb PA, Trentham-Dietz A, Titus- Ernstoff L, Bersch AJ, Stampfer MJ, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev 2008;17:379–86.
- Abrahamson PE, Gammon MD, Lund MJ, Britton JA, Marshall SW, Flagg EW, et al. Recreational physical activity and survival among young women with breast cancer. Cancer 2006;107:1777–85.
- Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA 2005;293:2479–86.
- Borugian MJ, Sheps SB, Kim-Sing C, Van Patten C, Potter JD, Dunn B, et al. Insulin, macronutrient intake, and physical activity: Are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol Biomarkers Prev 2004;13:1163–72.
- Enger SM, Bernstein L. Exercise activity, body size and premenopausal breast cancer survival. Br J Cancer 2004; 90:2138–41.
- Rohan TE, Fu W, Hiller JE. Physical activity and survival from breast cancer. Eur J Cancer Prev 1995;4:419–24.
- Bertram LA, Stefanick ML, Saquib N, Natarajan L, Patterson RE, Bardwell W, et al. Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: Findings from the WHEL Study. Cancer Causes Control 2011;22:427–35.
- Bradshaw PT, Ibrahim JG, Khankari N, Cleveland RJ, Abrahamson PE, Stevens J, et al. Post-diagnosis physical activity and survival after breast cancer diagnosis: The Long Island Breast Cancer Study. Breast Cancer Res Treat 2014; 145:735–42.
- Williams PT. Breast cancer mortality vs. exercise and breast size in runners and walkers. PLoS One 2013; 8:e80616.
- Williams PT. Significantly greater reduction in breast cancer mortality from post-diagnosis running than walking. Int J Cancer 2014;135:1195–202.
- Beasley JM, Kwan ML, Chen WY, Weltzien EK, Kroenke CH, Lu W, et al. Meeting the physical activity guidelines and survival after breast cancer: Findings from the after breast cancer pooling project. Breast Cancer Res Treat 2012;131:637–43.
- Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med Oncol 2011;28:753–65.
- Ellsworth RE, Valente AL, Shriver CD, Bittman B, Ellsworth DL. Impact of lifestyle factors on prognosis among breast cancer survivors in the USA. Expert Rev Pharmacoecon Outcomes Res 2012;12:451–64.
- Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 2011;378:1244–53.
- Kodama S, Tanaka S, Heianza Y, Fujihara K, Horikawa C, Shimano H, et al. Association between physical activity and risk of all-cause mortality and cardiovascular disease in patients with diabetes: A meta-analysis. Diabetes Care 2013;36:471–9.
- Myers J. Cardiology patient pages. Exercise and cardiovascular health. Circulation 2003;107:e2–5.
- Saquib N, Pierce JP, Saquib J, Flatt SW, Natarajan L, Bardwell WA, et al. Poor physical health predicts time to additional breast cancer events and mortality in breast cancer survivors. Psychooncology 2011;20:252–9.
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev 2009;(1): MR000006.
- Juni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta-analyses of controlled trials: Empirical study. Int J Epidemiol 2002;31:115–23.
- Berlin JA. Does blinding of readers affect the results of meta-analyses? University of Pennsylvania Meta-analysis Blinding Study Group. Lancet 1997;350:185–6.
- Helmerhorst HJ, Brage S, Warren J, Besson H, Ekelund U. A systematic review of reliability and objective criterion- related validity of physical activity questionnaires. Int J Behav Nutr Phys Act 2012;9:103.
- Carlson MD, Morrison RS. Study design, precision, and validity in observational studies. J Palliat Med 2009;12: 77–82.
- Villarini A, Pasanisi P, Traina A, Mano MP, Bonanni B, Panico S, et al. Lifestyle and breast cancer recurrences: The DIANA-5 trial. Tumori 2012;98:1–18.