Abstract
Purpose. Limited data are available to guide neoadjuvant treatment of borderline resectable (BRPC) and locally advanced (LAPC) pancreatic cancer.
Material and methods. We updated our institutional outcomes with a neoadjuvant chemotherapy and stereotactic body radiotherapy (SBRT) approach. An IRB-approved analysis was performed of all BRPC and LAPC patients treated with our departmental treatment protocol. After staging, medically fit patients underwent chemotherapy for 2–3 months, with regimen at the discretion of the treating medical oncologist. Patients then received SBRT delivered in five consecutive daily fractions with median total radiation doses of 30 Gy to tumor and 40 Gy dose painted to tumor-vessel interfaces. This was followed by restaging imaging for possible resection. Overall survival (OS), event free survival (EFS), and locoregional control (LRC) rates were estimated and compared by Kaplan-Meier and log-rank methods.
Results. We identified 159 patients, 110 BRPC and 49 LAPC, with 14.0 months median overall follow-up. The resection and margin negative (R0) rate for BRPC patients who completed neoadjuvant therapy was 51% and 96%, respectively. Estimated median OS was 19.2 months for BRPC patients and 15.0 months for LAPC patients (p = 0.402). Median OS was 34.2 months for surgically resected patients versus 14.0 months for unresected patients (p < 0.001). Five of 21 (24%) LAPC patients receiving FOLFIRINOX chemotherapy underwent R0 resection. In LAPC, FOLFIRINOX recipients underwent R0 resection more often than other chemotherapy recipients (5 of 21 vs. 0 of 28, p = 0.011). There was a trend for improved survival in those resected LAPC patients (p = 0.09). For those not undergoing resection, one year LRC was 78%. Any grade ≥ 3 potentially radiation-related toxicity rate was 7%.
Conclusions. These data underscore the feasibility, safety, and effectiveness of neoadjuvant SBRT and chemotherapy for BRPC and LAPC.
Pancreatic cancer is the fourth most common cause of cancer death in the US [Citation1]. For the most common exocrine cancers, patients who undergo tumor resection have the best chance of cure, while unresectable cancers are nearly uniformly fatal [Citation2]. Novel techniques are required to improve the survival and quality of life of patients.
One emerging technique for the treatment of pancreatic cancer is stereotactic body radiation therapy (SBRT). SBRT is used by specialized centers to deliver a high dose of precisely targeted radiation in a short course of therapy [Citation3]. Unresectable locally advanced pancreatic cancers (LAPC) cause significant pain, obstruction, and other morbidity due to direct extension of the primary tumor [Citation4]. Utilized to slow tumor progression, some early SBRT experiences in LAPC of up to 85 patients have demonstrated local control (LC) rates over 80% at one year [Citation5–8]. Borderline resectable pancreatic cancers (BRPC) involve nearby vasculature with elevated risk for margin positive (R1) resection and are frequently treated to improve resectability [Citation9,Citation10]. Neoadjuvant chemotherapy and sequential SBRT with high dose to the area of tumor-vessel interface have been employed in BRPC with high rates of margin negative (R0) resection [Citation11,Citation12].
Evolving combination chemotherapies are also improving outcomes in pancreatic cancer. Large randomized trials of the oxaliplatin, irinotecan, fluorouracil, and leucovorin (FOLFIRINOX) or the gemcitabine with nab-paclitaxel regimens have demonstrated improved survival and radiographic response compared to single agent gemcitabine [Citation13,Citation14]. The gemcitabine, docetaxel, and capecitabine (GTX) chemotherapy regimen has also been associated with promising radiographic responses [Citation15].
These multi-agent chemotherapy regimens are being applied to earlier stages of pancreatic cancer, often in sequential fashion with radiotherapy. Initial experiences with FOLFIRINOX and radiotherapy in BRPC and LAPC have demonstrated encouraging results [Citation16–19]. The ongoing ALLIANCE-A021101 trial seeks to evaluate in BRPC the safety and outcomes of neoadjuvant FOLFIRINOX followed by concurrent capecitabine and conventional radiation prior to resection with adjuvant gemcitabine.
This report updates the outcomes and toxicity using induction chemotherapy and SBRT for BRPC and LAPC in our institutional experience of 159 patients. Several LAPC patients were able to undergo surgical resection after treatment, and this outcome was correlated with receipt of FOLFIRINOX chemotherapy.
Material and methods
Initial staging and patients
After institutional review board approval, an internal database was queried to identify all patients who received at least one dose of induction chemotherapy and SBRT as part of our institutional protocol for the treatment of BRPC or LAPC. These patients were treated from 2009 to 2014 and had American Joint Committee on Cancer 7th edition stage IIA-III adenosquamous or adenocarcinoma. Definitions of anatomic resectability were applied from the 2009 expert consensus statement which were adopted by the National Comprehensive Cancer Network (NCCN) [Citation9].
Staging methods under this protocol have been previously described [Citation7]. In summary, patients typically undergo physical examination, standard blood chemistries including CA19-9, multidetector thin-slice pancreatic protocol computed tomography (CT) scan, positron emission tomography (PET) scan, endoscopic ultrasound (EUS), specialist pathology review, and presentation at gastrointestional tumor board. The highest stage identified by any imaging modality determined the clinical stage and treatment. Included BRPC patients were initially judged fit to medically tolerate resection without probable distant metastases. Three clinically T2-T3 classification patients judged unlikely to ever tolerate resection are included as LAPC patients.
Chemotherapy treatment
Patients first received induction chemotherapy at the discretion of the treating medical oncologist. For BRPC, patients most commonly received induction chemotherapy with three 21-day cycles of the GTX regimen as gemcitabine 750 mg/m2 and docetaxel 30 mg/m2 intravenous (IV) on Days 4 and 11, and capecitabine 750 mg/m2 orally twice daily on Days 1 through 14. For LAPC, the most common induction regimen was six 14-day cycles of FOLFIRINOX as oxaliplatin 85 mg/m2, folinic acid 400 mg/m2, irinotecan 180 mg/m2, fluorouracil, 400 mg/m2 IV bolus Day 1, and fluorouracil 1200 mg/m2/day IV continuous 46-hour infusion on Days 1–2. Gemcitabine alone was most commonly given in three four-week cycles at 1000 mg/m2 IV on Days 1, 8 and 15. Several patients received gemcitabine as above with the addition of nab-paclitaxel 125 mg/m2 IV.
Patients were identified as receiving “per protocol” for purposes of this study if they received chemotherapy for at least the number of cycles listed with less than one week of protocol interruption. Dose reductions were allowed for toxicities.
Radiation treatment and restaging
Under EUS guidance, 2–4 fiducial markers were placed into the tumor [Citation20]. A fluoroscopic study was then performed to gauge the amplitude of tumor motion by tracking the markers during respiration with an applied abdominal compression device. For tumor motion ≥ 1 cm, radiation treatment utilized respiratory gating by the Varian Real-Time Position Management system (Varian Medical Systems Inc., Palo Alto, CA, USA). Patients were immobilized by a BodyFix cradle (Elekta, Stockholm, Sweden). Isocenter was then determined on non-contrast CT simulation scan followed by repeat four-dimensional CT with IV and oral contrast.
SBRT began at least seven days after last chemotherapy administration (median 20 days). Five consecutive daily fractions were delivered by Varian Truebeam or Trilogy linear accelerator to gross tumor volume (GTV) within the pancreas plus motion with a 3–5mm planning tumor volume expansion. Intensity modulated radiation therapy (IMRT) was utilized to plan 28–30 Gy (median 30 Gy) to the planning tumor volume with simultaneous dose painting to up to 50 Gy (median 40 Gy) to areas of vessel involvement by tumor. Dose was limited by organs at risk as follows: for each of duodenum, small bowel, and stomach mean < 20 Gy, volume receiving 30 Gy (V30) < 2 cm3, V35 Gy < 0.5 cm3; each kidney mean < 10 Gy, spinal cord maximum 20 Gy.
After completion of radiation treatment, patients were restaged by repeat examination, pancreas protocol CT, PET/CT, and multidisclipinary tumor board presentation. Patients were eligible for surgery if there continued to be no imaging evidence of distant metastases, if they met criteria for resectable or borderline resectability, and if they were judged to be able to tolerate resection medically.
Surgery and pathology
Surgery was usually performed 1–2 months after SBRT, and all patients except one underwent surgery within three months after SBRT. Patients with tumors of the pancreatic head underwent pancreaticoduodenectomy. Tumors of the pancreatic body or tail were removed by distal pancreatectomy and splenectomy. Rare patients required total pancreatectomy due to concerns of diffuse tumor infiltration or numerous intraductal mucinous papillary neoplasms. A vascular surgeon was available when necessary for repair or resection of the superior mesenteric vein (SMV) or portal vein (PV).
Pathology was reported by pathologists with expertise in gastrointestinal pathology. College of American Pathology protocols were used for examination, including tumor response grade used for response to neoadjuvant therapy [Citation9]. Margin negative was defined as no tumor cells at the edge of the specimen.
Follow-up and analysis
Patients were evaluated every 3–6 months for the first five years and yearly thereafter. All charts were reviewed to identify outcomes and potentially radiation-related toxicity. Acute side effects were defined as those occurring within 90 days of radiation treatment. Side effects were graded according to version 4.0 of the National Cancer Institute Common Terminology Criteria for Adverse Events.
Statistical testing was performed with IBM SPSS (IBM SPSS Statistics for Windows, Version 20.0; IBM Corp, Armonk, NY, USA). Overall survival (OS), event free survival (EFS), and locoregional control (LRC) were estimated by the Kaplan-Meier method and compared by the log-rank (Mantel-Cox) test, with time starting at the date of diagnosing biopsy. EFS was defined as freedom from disease progression or death from any cause.
Results
A total of 159 patients, 110 BRPC and 49 LAPC, have been treated with the induction chemotherapy and SBRT protocol. Their characteristics are listed in . GTX chemotherapy was given to 89 (81%) BRPC patients, while FOLFIRINOX was most common (21 patients, 43%) in the LAPC group. Gemcitabine alone was prescribed to 17 (35%) LAPC patients, usually due to concerns about the ability of individual patients to tolerate the FOLFIRINOX regimen. Of the 110 BRPC patients, 56 (51%) underwent curative intent resection, while 46 (42%) patients became unresectable during neoadjuvant therapy due to local or distant progression. Another eight (7%) patients suffered decline in performance status that precluded resection. Of the 49 LAPC patients, seven (14%) underwent surgical exploration based on performance status and imaging response suggestive of resectability, though two were found to be unresectable at exploration.
Table I. Patient characteristics and neoadjuvant treatments.
Five (10%) LAPC patients underwent R0 resection. It was observed that all five LAPC patients undergoing complete resection received FOLFIRINOX chemotherapy per protocol (at least six, two-week cycles received within 84 days). The five patients represent 24% of the 21 FOLFIRINOX recipients and 36% of the 14 per protocol FOLFIRINOX recipients. demonstrates that when compared to other chemotherapy protocols, receipt of FOLFIRINOX was statistically significant for resection in these LAPC patients (p = 0.011).
Table II. Induction chemotherapy regimens used with SBRT in LAPC patients with resection outcomes.
Surgical and pathologic outcomes are displayed in . Vascular repair was required in 22 (36%) patients, though positive margins were only obtained in two (3%) patients. Despite clear evidence of disease regression on imaging, LAPC patients who successfully underwent resection were found to either have moderate response (60%) or poor response (40%) by tumor response grade. There were four (7%) patients observed to have pathologic complete response (pCR). None of these patients have yet relapsed (EFS log-rank p = 0.066 for pCR vs. any other response).
Table III. Surgical and pathologic outcomes.
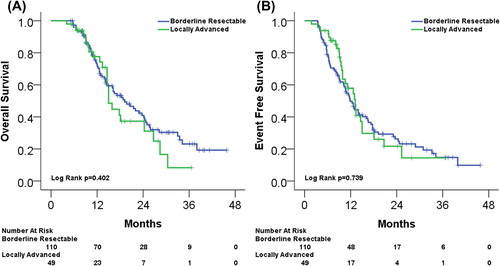
Median follow-up was 14.0 months (range 4–46 months) with median OS and EFS estimated at 18.1 months and 12.7 months, respectively. shows OS and EFS for BRPC and LAPC. Median OS and EFS were 19.2 months and 11.9 months in the BRPC group and 15.0 months and 13.2 months in the LAPC group (comparisons not significant).
Figure 1. (A) Kaplan-Meier estimated OS for BRPC and LAPC. (B) Kaplan-Meier estimated EFS for BRPC and LAPC.
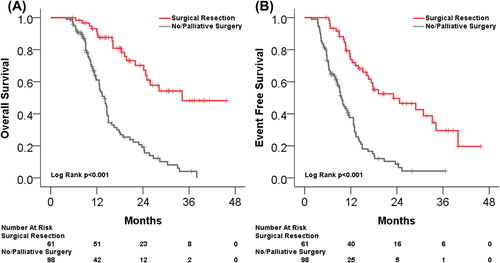
demonstrates the improved OS and EFS for patients undergoing resection (p < 0.001 for both). Median OS and EFS were 34.2 months and 23.1 months for the curative intent surgery group compared to median OS and EFS of 14.0 months and 9.5 months for patients not undergoing curative intent surgery. Median follow-up for patients undergoing surgery who have not died was 20.3 months (range 6–46 months). The two patients with R1 resections remain without evidence of disease at 6.8 and 27.3 months after surgery.
Figure 2. (A) Kaplan-Meier estimated OS for all patients undergoing curative-intent surgical resection compared to those without curative-intent surgery. (B) Kaplan-Meier estimated EFS for the same patients.
For patients not undergoing surgery, the 12 month LRC rate was 78% and the 18 month LRC rate was 63%. In the LAPC patients undergoing surgery, there have been no deaths and one locoregional failure. Follow-up is limited in these patients (range 2.1–15.4 months, median 5.6 months), however, a trend is observed towards improved survival for LAPC patients undergoing resection compared to no resection (log-rank p = 0.09).
Potentially radiation related grade ≥ 3 acute and/or late toxicity was observed in 11 (7%) patients. The most common grade 3 or higher toxicity was gastrointestinal bleeding from the duodenum or stomach (six patients), though in three patients bleeding was clearly associated with duodenal extension of tumor. This was controlled with endoscopic techniques in all but one patient who elected for hospice care only. Any acute grade 1–2 toxicity was reported in 83 (52%) patients. Fatigue was most common (31%). Epigastric pain was reported by 23 (14%) patients, though opiates were required by only 12 (8%) patients. All toxicities are listed in Supplementary Table I (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1004367) with further detail regarding observed toxicities.
Discussion
In the present study, we demonstrate outcomes of all 159 patients at our institution who received neoadjuvant chemotherapy and SBRT for the treatment of BRPC and LAPC pancreatic adenocarcinoma with median follow-up of 14 months. This represents the largest series of SBRT in pancreatic cancer to date. Of the 110 BRPC patients, 56 (51%) underwent curative-intent resection, with 96% R0 resection rate. Patients undergoing resection lived significantly longer than patients not undergoing resection. Out of 49 LAPC patients, five responded sufficiently on imaging to undergo negative margin resection after neoadjuvant treatment. These patients all received the FOLFIRINOX regimen, with superior resection rate compared to gemcitabine-based regimens. Patients who did not undergo resection had a 78% LRC rate at one year. Grade ≥ 3 toxicity potentially attributable to radiation therapy was observed in only 11 (7%) of patients.
The data presented here compares similarly in some respects to our earlier series of 73 patients receiving induction chemotherapy and SBRT with 10.5 months of median follow-up, though there are several significant advancements [Citation7]. The median radiation doses to tumor and tumor-vessel abutment escalated from 25 Gy and 35 Gy, to 30 Gy and 40 Gy, respectively, with minimal change in significant toxicity rate. The prior series identified no LAPC patients undergoing resection after neoadjuvant treatment. Since that publication, the number of LAPC patients tripled, with a higher proportion receiving FOLFIRINOX. The median follow-up time is longer in this study, and particularly the maximum follow-up time in this study is nearly doubled to 46 months. This particularly impacts median OS estimates for the longest lived patients. Previously, the median OS in the surgically resected group was estimated at 19.2 months, however, very few patients had follow-up to that time, making the estimate inaccurate. Median OS for the surgically resected patients is now estimated to be 34.2 months, which still has the potential to be underestimated due to follow-up time.
This study is comparable in size and outcomes to the 2008 report of BRPC by Katz et al. which included 125 patients who completed neoadjuvant conventional chemoradiotherapy for BRPC, albeit with a different definition for borderline resectability than that used here [Citation21]. In their study, 66 (53%) patients underwent curative intent resection after neoadjuvant treatment, with 94% R0 resection rate and 6% pathologic complete response. Further, the median OS in their series was 40 months for resected patients, 13 months for patients not undergoing resection, and 18 months for all patients. This is similar to the results of the current study as follows: 34.2 months for resected patients, 11.3 months for BRPC and LAPC patients not undergoing resection, and 19.2 months for all BRPC patients.
Complete surgical resections of LAPC after neoadjuvant therapy with single agent chemotherapy and radiation have been reported in only 1–5% of LAPC patients in large series [Citation22,Citation23]. However, several recent publications have reported more frequent downstaging of LAPC with FOLFIRINOX. Hosein et al. recently published their series of 18 LAPC patients receiving FOLFIRINOX [Citation16]. At maximum response or tolerability, five underwent R0 resection. Of the remainder, three patients later underwent R0 resection after further chemoradiotherapy for an impressive 44% overall resection rate. Faris et al. also reported their initial experience of FOLFIRINOX followed by conventional chemoradiotherapy in 22 LAPC patients and resection rate of 23% was achieved. Here we demonstrate five (24%) patients resected after FOLFIRINOX and SBRT compared with no resected LAPC patients of 28 treated with gemcitabine-based regimens and SBRT. To our knowledge, this work is the first to suggest superiority of FOLFIRINOX compared to other chemotherapy regimens in converting LAPC to resectability. Given the trend towards improved survival for surgically resected LAPC patients, prospective study is warranted to evaluate the superiority of FOLFIRINOX compared to gemcitabine-based regimens in the neoadjuvant setting.
Conclusions
The induction chemotherapy and SBRT protocol was applied to the largest series to date (n = 159) of BRPC and LAPC patients with 51% resection rate for BRPC patients and 24% resection rate for LAPC patients who received FOLFIRINOX. The size and follow-up rivals the largest single institution series of BRPC patients receiving conventional neoadjuvant chemoradiotherapy. While patient numbers are limited in the current study, FOLFIRINOX chemotherapy was associated with a higher resection rate in LAPC than gemcitabine-based regimens. At 14.0 months of median follow-up, significant OS benefit was identified for patients undergoing surgery, and trend for OS was identified for the subgroup of LAPC patients undergoing surgery. One year LRC was 78% in those patients not undergoing surgery. Grade ≥ 3 potentially radiation-related toxicity rate was only 7%. This data underscores the feasibility, safety, and effectiveness of SBRT-based regimens for BRPC and LAPC.
Supplementary material available online
Supplementary Table I available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1004367
ionc_a_1004367_sm6309.docx
Download MS Word (22.4 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29.
- Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: Report from the National Cancer Database. Cancer 2007;110:738–44.
- Potters L, Kavanagh B, Galvin JM, Hevezi JM, Janjan NA, Larson DA, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010;76:326–32.
- Brescia FJ. Palliative care in pancreatic cancer. Cancer Control 2004;11:39–45.
- Chang DT, Schellenberg D, Shen J, Kim J, Goodman KA, Fisher GA, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009;115: 665–72.
- Mahadevan A, Miksad R, Goldstein M, Sullivan R, Bullock A, Buchbinder E, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys 2011; 81:e615–22.
- Chuong MD, Springett GM, Freilich JM, Park CK, Weber JM, Mellon EA, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys 2013;86:516–22.
- Schellenberg D, Kim J, Christman-Skieller C, Chun CL, Columbo LA, Ford JM, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2011;81:181–8.
- Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: Expert consensus statement. Ann Surg Oncol 2009;16:1727–33.
- Springett GM, Hoffe SE. Borderline resectable pancreatic cancer: On the edge of survival. Cancer Control 2008;15: 295–307.
- Chuong MD, Springett GM, Weber J, Klapman J, Vignesh S, Hodul PJ, et al. Induction gemcitabine-based chemotherapy and neoadjuvant stereotactic body radiation therapy achieve high margin-negative resection rates for borderline resectable pancreatic cancer. J Radiat Oncol 2012;1:273–81.
- Rajagopalan MS, Heron DE, Wegner RE, Zeh HJ, Bahary N, Krasinskas AM, et al. Pathologic response with neoadjuvant chemotherapy and stereotactic body radiotherapy for borderline resectable and locally-advanced pancreatic cancer. Radiat Oncol 2013;8:254.
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25.
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369:1691–703.
- Fine RL, Fogelman DR, Schreibman SM, Desai M, Sherman W, Strauss J, et al. The gemcitabine, docetaxel, and capecitabine (GTX) regimen for metastatic pancreatic cancer: A retrospective analysis. Cancer Chemother Pharmacol 2008;61:167–75.
- Hosein PJ, Macintyre J, Kawamura C, Maldonado JC, Ernani V, Loaiza-Bonilla A, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer 2012;12:199.
- Faris JE, Blaszkowsky LS, McDermott S, Guimaraes AR, Szymonifka J, Huynh MA, et al. FOLFIRINOX in locally advanced pancreatic cancer: The Massachusetts General Hospital Cancer Center experience. Oncologist 2013;18:543–8.
- Boone BA, Steve J, Krasinskas AM, Zureikat AH, Lembersky BC, Gibson MK, et al. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol 2013;108:236–41.
- Tinchon C, Hubmann E, Pichler A, Keil F, Pichler M, Rabl H, et al. Safety and efficacy of neoadjuvant FOLFIRINOX treatment in a series of patients with borderline resectable pancreatic ductal adenocarcinoma. Acta Oncol 2013;52:1231–3.
- Vignesh S, Hoffe SE, Saif MW. EUS-guided pancreatic diagnosis and beyond. Highlights from the “2011 ASCO gastrointestinal cancers symposium”. San Francisco, CA, USA. January 20–22, 2011. JOP 2011;12:86–91.
- Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. J Am Coll Surg 2008;206:833–46; discussion 46–8.
- Crane CH, Abbruzzese JL, Evans DB, Wolff RA, Ballo MT, Delclos M, et al. Is the therapeutic index better with gemcitabine-based chemoradiation than with 5-fluorouracil-based chemoradiation in locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys 2002;52:1293–302.
- Kim HJ, Czischke K, Brennan MF, Conlon KC. Does neoadjuvant chemoradiation downstage locally advanced pancreatic cancer? J Gastrointest Surg 2002;6:763–9.
