Abstract
Purpose. To investigate if testicular cancer survivors (TCSs) have a higher incidence of work loss compared with the population, accounting for stage, treatment and relapse.
Material and methods. A cohort of 2146 Swedish TCSs diagnosed 1995–2007 (seminoma n = 926, non-seminoma n = 1220) was identified in the SWENOTECA (Swedish-Norwegian Testicular Cancer Group) register, and matched 1:4 to population comparators. Prospectively recorded work loss data (both before and after diagnosis) were obtained from national registers through September 2013. Adjusted relative risks (RR) and 95% confidence intervals (CI) of sick leave and/or disability pension were calculated annually and overall with Poisson- and Cox regression, censoring at relapse. The mean number of annual work days lost was also estimated.
Results. TCSs were at a modestly increased annual risk of work loss up to the third year of follow-up (RR3rd year 1.25, 95% CI 1.08, 1.43), attributed to a more pronounced risk among extensively treated patients (4 chemotherapy courses: RR3rd year 1.60, 95% CI 1.19, 2.15; > 4 courses: RR3rd year 3.70, 95% CI 2.25, 6.11). Patients on surveillance or limited treatment (radiotherapy, 1–3 chemotherapy courses) did not have an increased risk of work loss beyond the first year. TCSs receiving > 4 chemotherapy courses had higher mean number of annual days of work loss up to the 10th year post-diagnosis, and a five-fold risk of disability pension (RR 5.16, 95% CI 2.00, 10.3).
Conclusion. Extensively treated TCSs, but not those on surveillance or limited treatment, are at increased risk of work loss long-term, not explained by relapse. These patients may benefit from early rehabilitation initiatives.
In the US and most European countries, testicular cancer is the most common malignancy in young men aged 20–40 years, with a variation in incidence worldwide from 3 to 15 per 100 000 males and per year [Citation1,Citation2]. Since the introduction of cisplatinum-based treatment in the 1980s, more than 95% of the patients are cured [Citation3]. The increasing incidence of testicular cancer [Citation4] and high survival rates, has led to a growing number of testicular cancer survivors (TCSs), underscoring the importance of monitoring long-term health in this group [Citation5,Citation6]. As the majority of the patients are in their working ages or just entering the labor market when diagnosed, the ability to work following cancer treatment is an important aspect of rehabilitation, both individually, and for the society.
Cancer survivors in general are more likely to suffer from impaired health, leading to loss of work ability in comparison to healthy individuals, especially during the year of diagnosis and the year after [Citation7,Citation8]. In many previous investigations, however, researchers have had limited ability to consider potential variation in work loss by cancer site and especially by treatment [Citation9–11]. Previous studies have mostly not reported any significantly increased risks of work loss among TCSs, but the studies have been limited in size (n ≤ 600) [Citation7,Citation9,Citation10,Citation12]. Also, few previous studies have investigated the short- or long-term effects of work loss for testicular cancer survivors in relation to treatment modality [Citation7,Citation10], or in comparison to the general population [Citation7,Citation12] and none have considered the impact of cancer recurrence. Therefore, we aimed to investigate short- and long-term incidence of sick leave and disability pension among testicular cancer patients compared with the general population, separating the patients by stage and treatment intensity (no or limited treatment vs. extensive treatment). In this study, 2146 Swedish testicular cancer patients and 8448 matched population comparators, were investigated with up to 19 years of follow-up.
Material and methods
Setting
In Sweden, sick leave and disability pension, part- or full time, are tax-funded and open to everyone. The first day of sick leave is not compensated and Day 2 until Day 14 is paid by the employer. The Swedish social insurance system compensates and the Swedish Social Insurance Office (Försäkringskassan) administrates the compensation from the 14th day and onwards (except between January 1997 to March 1998 when 28 days was the limit). Disability pension is compensated from Day 1. The number of lost work days is registered in the Social Insurance Agency in the Micro Data Analysis of Social Insurance database (MiDAS). Sick leave episodes < 14 days are not registered, but sick leave episodes > 14 days as well as all disability pension episodes are registered from the first day and thus included in the database. Usually retirement takes place at 65 years of age but can be offered from the age of 61 or later up to the age of 67 years.
Patient cohort
TCSs were identified from the Swedish part of the clinical SWENOTECA (Swedish Norwegian Testicular Cancer Group) database. The SWENOTECA register holds information on clinical stage [CS I-IV and tumor marker positive disease (Mk+)] [Citation13], treatment modality and relapse for up to 10 years after diagnosis, for non-seminomas patients since 1995 and seminoma patients since 2000 [Citation3]. All Swedish cancer centers report to the SWENOTECA database, which is then cross-checked with the Swedish Cancer Register [Citation14] once a year through the personal national registration numbers assigned to each resident in Sweden at birth or permanent residency. The completeness of the registers is almost 100%. A total of 2292 TCSs with unilateral testicular cancer at ages 18–60 years were diagnosed from 1 July 1995 (seminomas from 1 July 2000) to 31 December 2007 (Supplementary Figure 1, available online at: http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1020967). TCS that died (n = 51) (Causes of Death Register) [Citation15] or emigrated (n = 26) (Statistics Sweden) [Citation16] within two years from diagnosis, or those with a previous cancer diagnosis according to the Swedish Cancer Register [Citation14] (except previous non-melanoma skin cancer) (n = 28), were excluded.
We classified the patients into two main groups and six subgroups based on treatment: 1) no or limited treatment including: 1a) surveillance, 1b) radiotherapy [for adjuvant (20 Gy/10 fractions or 25.2 Gy /14 fractions) or metastatic disease (27 Gy/15 fractions), 1c] chemotherapy one course (EP, BEP or carboplatin single), 1d) chemotherapy 2–3 courses (BEP, PEI or TIP) (SWENOTECA treatment Guidelines listed in Supplementary Table I and II available online at: http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1020967) extensive treatment: 2a) chemotherapy four courses and 2b) chemotherapy > 4 courses (with any of the above mentioned regimens and in some instances also high-dose chemotherapy with stem-cell support).
Comparison cohort
Four male cancer-free comparators for each TCS were randomly sampled from the National population register kept at Statistics Sweden [Citation16], matched on birth year and calendar year of diagnosis of the patient. Comparators who died or emigrated within the first two years of follow-up were excluded (Supplementary Figure 1 available online at: http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1020967).
Sick leave and disability pension
For all study participants, we retrieved exact dates of all periods of sick leave (> 14 days) and disability pension from two years prior to diagnosis until end of follow-up 30 September 2013, as well as the number of days per year of sick leave and/or disability pension, from the Social Insurance Agency database [Citation17]. “0” days thus represented individuals with no episodes longer than 14 days of sick leave absence per year (and no disability pension) since episodes shorter than 14 days are not registered in the Social Insurance Agency database. A new sick-leave episode occurring within five days of the previous episode was paid directly by the social security agent, and thus recorded. Disability pension is granted if work capacity is reduced by at least 25%. There are three levels of disability pension, 25%, 50% or 100%. Periods of sick leave can occur during the time not covered by the disability pension. In case of recovery or decline of work capacity, the degree of disability pension could be changed. The terminology disability pension was used 1992–2002, but was changed to sickness compensation (30–64 years of age) or activity compensation (19–29 years of age) in 2003. We used the term disability pension to describe disability pension, sickness compensation or activity compensation.
Covariates
From the LISA-database (database for health insurance and labor market studies) held at Statistics Sweden [Citation16], we gathered information for patients and comparators on educational level (≤ 9, 10–12, > 12 years) and unemployment status (unemployed > 50% of the time: yes/no) within the year before diagnosis (data available on all Swedish residents 16 years or older).
Statistics
Three different methods were used to assess outcome. First, the relative risk (RR) and 95% confidence interval (CI) of having at least one period of sick leave (≥ 14days) and/or disability pension (≥ 1 day) per year of follow-up were calculated in a Poisson regression analysis. In multivariable analyses, based on a priori knowledge of possible confounding, we adjusted for education level (< 9, 10–12, > 12 years), sick leave 1–2 years prior to enrollment (yes/no), and unemployment (> 50% 0–1 year prior to enrollment, yes/no) and matching variables age (18–60) and calendar year of diagnosis (1995–2007). This model was also used to estimate RRs in patient subgroups stratified by tumor type, clinical stage and treatment. Here, we focused on risk in the third and fifth years of follow-up. Second, the mean number of days of sick leave and/or disability pension per year of follow-up was estimated and compared between groups [Citation18]. The distribution of number of annual days of work loss were assessed as exact number of days and also in categories “0”, 15–120, 121–240, > 240 days per year and the 75th and 90th percentile days. Non-parametric bootstrapping was used to compute 95% CIs of mean annual days of work loss. Third, the hazard ratio (HR) and 95% CI of disability pension were calculated in a Cox regression analysis. Patients with a previous episode of disability pension were excluded from this analysis. Study participants were followed until death, emigration, age 65 years, testicular cancer relapse or 30 September 2013, whichever occurred first. Multivariable adjustment was performed with the same variables as in the Poisson regression model described above. χ2-test was used for comparison between groups. A p-value of 0.05 was considered statistically significant and all tests were two-sided. We used SAS (version 9.2, SAS institute Inc., Cary, NC, USA) and STATA (StataCorp, release 12.0. College Station, TX, USA) statistical software for analyses.
Ethics
The study was ethically approved by the Regional Ethics Review Board, Stockholm, Sweden.
Results
The patient cohort included 2146 TCSs and the comparator cohort 8448 men with a median follow-up of 10 years (range 2–19 years). The majority of the TCSs were diagnosed with non-seminoma (57% N = 1220), and most were diagnosed with stage I disease (seminoma 88% N = 816, non- seminoma 57% N = 699) (). A total of 605 (29%) TCSs were followed with surveillance only. Treatment with radiotherapy only was restricted to patients with seminoma (32%). Patients with non-seminoma more often received > 4 courses of chemotherapy (5% vs. 0.3% in patients with seminoma). High-dose chemotherapy was given to 1% (N = 22) of the non-seminoma patients and to one of the seminoma patients. Relapse was diagnosed in 6% (N = 124) of all TCSs, and was more common among patients with non-seminoma (7%, N = 88) than seminoma (4%, N = 35).
Table I. Characteristics of testicular cancer survivors (TCSs) diagnosed in Sweden 2000–2007 (seminoma) or 1995–2007 (non-seminoma), and male population comparators matched to the patients by age and calendar year of diagnosis.
In the year following diagnosis, 64% of the TCSs had registrations of at least one episode of sick leave or disability pension (38% with 1–120 days, 26% with > 120 days) in comparison to 12% among the population comparators (7% with 1–120 days, 5% with > 120 days) (). During the second year after diagnosis and onwards, more than 80% of the TCSs no longer experienced work loss (). In analyses of risk of work loss in patient subgroups, patients with non-seminoma, but not seminoma, were at an increased risk of work loss in the third year of follow-up [RR3rd year 1.41, 95% (CI 1.18, 1.69)] (). By stage, an increased risk was restricted to patients with CS II-IV and Mk+ in comparison to the comparators [RR 3rd year 1.76, 95% CI (1.40, 2.21)]. The risk of work loss was not increased in any subgroup based on tumor type or stage during the fifth year ().
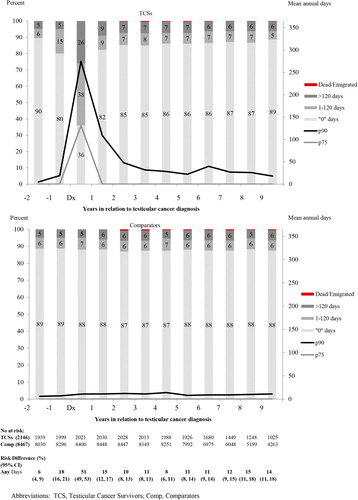
Table II. Relative risk (RR) and 95% confidence intervals (CI) of annual sick-leave or disability pension among TCSs compared with matched population comparators in the 3rd and 5th year after diagnosis. First, patients are grouped by tumor subtype and clinical stage (CS) and second by treatment modality. Follow-up is censored at relapse.
Stratified by treatment, TCSs with surveillance or limited treatment had no increased risk of work loss in the third or fifth year of follow-up compared with the population comparators (). This pattern of no association was consistent in all subgroups by age, educational level and calendar period as well as by the presence or absence of previous work loss or unemployment during the third and fifth year after diagnosis (). In line with the results for the entire group of patients with limited treatment, further subdivision by specific adjuvant treatment modalities (adjuvant radiotherapy, 1 course of carboplatin, 1 course of BEP), did not reveal increased risks for work loss following the year of diagnosis in any group (Supplementary Figures 2 and 3, available online at: http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1020967).
Table III. Mean days of sick-leave or disability pension among TCSs and population comparators (Comp) in the 3rd and 5th years after diagnosis. Patients are stratified by no or limited treatment versus extensive treatment, and then further by age, calendar period, educational level and previous sick-leave or disability pension. Follow-up is censored at relapse.
TCSs who had received extensive treatment were at increased risk of work loss during the third year in comparison to comparators [4 courses: RR3rd year 1.60, 95% CI (1.19, 2.15); > 4 courses RR3rd year 3.70, 95% CI (2.25, 6.11)] (). This risk was still detectable the fifth year after diagnosis among those TCSs who had received > 4 courses of chemotherapy [RR5th year 2.71, 95% CI (1.61, 4.54)] (). After excluding TCSs, who had received high-dose chemotherapy (N = 22), the risk was similarly increased [> 4 courses excl. high-dose treatment: RR5th year 2.85, 95% CI (1.78, 5.36)]. The risk also remained increased after excluding patients who underwent metastatic surgery [> 4 courses excl. surgery: RR5th year 3.04, 95% CI (1.22, 7.55)]. Further analyses of risks in the eighth and 10th years of follow-up did not reveal any increased risk of work loss in any of the treatment subgroups but the numbers were low (data not shown).
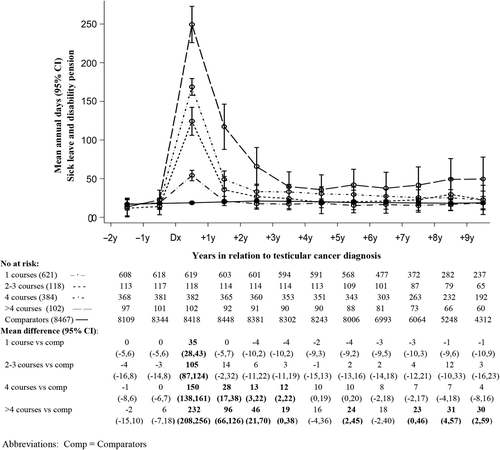
In analyses of mean lost work days the third and fifth year after diagnosis () among extensively treated TCSs compared to comparators, significant differences were observed in all age groups, but did not reach statistical significance during the period 2000–2007 (). Notably, significant differences compared to comparators were also observed among TCSs with no previous sick leave and/or disability pension (). During the first 10 years of follow-up (), TCSs who received > 4 courses of chemotherapy had significantly more mean days of sick leave and/or disability pension than comparators almost every year up to the 10th year [difference 30 days the 10th year (95% CI 2, 59)] and those TCSs receiving four courses of chemotherapy until the fifth year after diagnosis (). No differences were observed in any other group versus comparators beyond the first year (, Supplementary Figure 2 available online at: http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1020967).
In analysis of risk of disability pension, registered from Day 1, a similar pattern in risk by treatment was observed. Thus, extensively treated patients were at increased risk of disability pension [4 courses: HR 1.93 (95% CI 1.01, 3.71); > 4 courses HR 5.16 (95% CI 2.00, 10.3)], while patients on surveillance or with limited treatment were not at risk ().
Table IV. Hazard ratio (HR) and 95% confidence intervals (CI) of disability pension among testicular cancer survivors (TCSs) compared with matched population comparators. First, patients are grouped by tumor subtype and clinical stage (CS) and second by treatment modality. Follow-up is censored at relapse.
Discussion
In this large nationwide population-based study, we observed an increased annual risk of sick leave and disability pension up to the third year after diagnosis among all TCSs in comparison to a matched general population sample. Specifically, TCSs who had received four or more courses of chemotherapy had more annual days of sick leave and/or disability pension persisting for several years after diagnosis, and the increase was not explained by high-dose chemotherapy, metastatic surgery or relapse. Importantly however, most TCSs treated with no or limited treatment, including radiotherapy and 1–3 chemotherapy courses, were not at increased risk of work loss beyond the first year following diagnosis.
We are unaware of any previous nationwide studies of work loss encompassing virtually all TCSs with detailed information on stage and treatment modality and long follow-up. In a previous Norwegian study, including 2008 cancer survivors of whom 155 were TCSs [Citation12], most cancer survivors were on sick leave the first year after diagnosis. Sick leave-levels then decreased within the next four years but stayed significantly higher than for comparators. However, TCSs had fewer sick leave episodes than their comparators five years post-diagnosis [Citation12]. Similarly, in another Nordic study by Lindbohm et al., no difference in work ability was detected in a cross-sectional study of 380 TCSs with a follow-up of between one and eight years after diagnosis in comparison to age-matched reference individuals [Citation10]. That study was restricted to TCSs with low-stage disease, and no obvious differences were observed for TCSs who had received chemotherapy compared to those who had not, although the number of chemotherapy courses was not considered. Hence, these two studies are broadly in line with our results of no increased risk of work loss among low-stage patients treated with no or limited treatment.
Among cancer patients overall, treatment modality, stage of disease, recurrence of cancer and other chronic conditions have been reported to affect employment and work loss although few studies have assessed risks long term [Citation8,Citation10,Citation19,Citation20]. Also, several studies on work loss in cancer survivors have been based on self-reported questionnaires, and could therefore be affected by misclassification as well as non-response [Citation10,Citation19,Citation21]. Swedish population-based health care and labor market registries with prospectively recorded lost work days with high coverage and minimal loss to follow-up provide a unique opportunity for longitudinal investigation of work loss following a cancer diagnosis. Another strength of the present study is the access to detailed register-based information on clinical stage, treatment and relapse on all TCSs nationwide. We were able to identify subgroups of TCSs who are at high risk of work loss, but also to reliably confirm the lack of an increase among patients receiving limited treatment.
Among the most extensively treated patients, our results showed an increased risk of sick leave and/or disability pension up to the fifth year after diagnosis, with more mean days of sick leave and/or disability pension up to the 10th year. This was not fully explained by high-dose chemotherapy or relapse. One weakness of this study was that we did not have information on pre-existing comorbidity either in TCSs or among the comparators, although we did have information about prior sick leave and disability pension. Taskila et al. reported that comorbidity was a risk factor for impaired work ability in cancer survivors [Citation19,Citation20]. Our results support this observation in showing that patients with prior work loss experienced a much greater mean number of lost work days following cancer diagnosis than those without such history. Importantly however, in our study, heavily treated patients lost significantly more days than comparators also in the absence of a history of work loss before diagnosis.
We found that TCSs had more days with work loss the year before diagnosis in comparison to comparators. This is likely explained by the delay between the initiation of examinations and orchiectomy/date of diagnosis.
Possible explanations for the increased risk in the most extensively treated TCSs are long-standing treatment side effects [Citation5,Citation6,Citation22,Citation23]. Most of the severe long-term effects, such as secondary cancer and cardiovascular disease after TC treatment have been reported to occur more than 10 years after treatment [Citation22,Citation24] and therefore could not readily explain the observed risks up to 10 years. In patients with other cancer types, chemotherapy has been associated with reduced cognitive function [Citation25]. This has also been reported in TCSs [Citation26], with possible effects on work ability. TCSs may also experience somatic complications, such as gonadal dysfunction [Citation5,Citation23,Citation27], oto- and neurotoxicity [Citation28] and pulmonary toxicity [Citation6], but it remains unclear to what extent these complications interfere with work ability.
A weakness of our study is that we only had records of sick-leave episodes registered by the Social Insurance Agency, beyond 14 days (if not repeated within a short time frame). This has led to an underestimation of the total work loss due to sickness among TCSs. However, this also applies to the population comparators and hence should not have affected the comparison between the two groups. If patients have had more frequent short (< 14 days) periods of sick leave than comparators (starting later than 5 days of a previous sick-leave episode), the ratios may also be somewhat underestimated. Reassuringly, in analysis of disability pension only (recorded from the first day), similar risk patterns were observed, strengthening the overall results. The welfare system in Sweden differs to those in other countries which could make this study less applicable internationally, but the terms and conditions for sick leave and disability pension were also equal to both TCSs and comparators. On a different note, the fact that TC treatment is relatively uniform worldwide increases the value of our findings.
In conclusion, we have shown that most TCSs, receiving no or limited treatment, do not have an increased risk of work loss in comparison to the general population beyond the first year after diagnosis. However, extensively treated TCSs have a prolonged increased risk of sick leave and/or disability pension, especially those who have received more than four cycles of chemotherapy, which cannot be explained by cancer relapse. Further studies are needed to investigate underlying reasons for the increased risk of work loss among the most heavily treated patients. Physicians should be aware of this risk in order to provide optimized support and early work-related rehabilitation.
Supplementary material available online
Supplementary Figures 1–3 and Table I available online at: http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1020967
ionc_a_1020967_sm1445.pdf
Download PDF (302.9 KB)Acknowledgments
The authors would like to thank the members of SWENOTECA for providing clinical data. Strategic Research Program in Epidemiology at Karolinska Institute, Cancerfonden, Landstinget Kronoberg, Cancerstiftelsen Kronoberg and Lund University Faculty of Medicine and University Hospital Research Grants. Jerkeman M has received clinical trials support from Celgene, Janssen and Mundipharma. Glimelius I was supported by the Swedish Society of Medicine and the Swedish Society for Medical Research. All authors declare that they have no competing interests.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bray F, Richiardi L, Ekbom A, Pukkala E, Cuninkova M, Moller H. Trends in testicular cancer incidence and mortality in 22 European countries: Continuing increases in incidence and declines in mortality. Int J Cancer 2006; 118:3099–111.
- McGlynn KA, Devesa SS, Sigurdson AJ, Brown LM, Tsao L, Tarone RE. Trends in the incidence of testicular germ cell tumors in the United States. Cancer 2003;97: 63–70.
- SWENOTECA. Available from: http://skane.se/sv/Webbplatser/tumorregistret/Patientprocessarbete/Testikelcancer/Vardprogram/.
- Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: A review. J Urol 2003;170: 5–11.
- Nord C, Bjoro T, Ellingsen D, Mykletun A, Dahl O, Klepp O, et al. Gonadal hormones in long-term survivors 10 years after treatment for unilateral testicular cancer. Eur Urol 2003;44:322–8.
- Haugnes HS, Bosl GJ, Boer H, Gietema JA, Brydoy M, Oldenburg J, et al. Long-term and late effects of germ cell testicular cancer treatment and implications for follow-up. J Clin Oncol 2012;30:3752–63.
- Torp S, Nielsen RA, Fossa SD, Gudbergsson SB, Dahl AA. Change in employment status of 5-year cancer survivors. Eur J Public Health 2013;23:116–22.
- Marino P, Luis Sagaon T, Laetitia M, Anne-Gaelle le CS. Sex differences in the return-to-work process of cancer survivors 2 years after diagnosis: Results from a large French population-based sample. J Clin Oncol 2013;31:1277–84.
- Gudbergsson SB, Torp S, Flotten T, Fossa SD, Nielsen R, Dahl AA. A comparative study of cancer patients with short and long sick-leave after primary treatment. Acta Oncol 2011;50:381–9.
- Lindbohm ML, Taskila T, Kuosma E, Hietanen P, Carlsen K, Gudbergsson S, et al. Work ability of survivors of breast, prostate, and testicular cancer in Nordic countries: A NOCWO study. J Cancer Surviv 2012;6:72–81.
- Smedby KE. Cancer survivorship and work loss – what are the risks and determinants? Acta Oncol 2014;53: 721–3.
- Torp S, Nielsen RA, Gudbergsson SB, Fossa SD, Dahl AA. Sick leave patterns among 5-year cancer survivors: A registry-based retrospective cohort study. J Cancer Surviv 2012;6:315–23.
- Peckham MJ, Bagshaw KD, Blandy JP. Prognostic factors in advanced non-seminatous germ-cell testicular tumours: Results of a multicenter study. Report from the Medical Research Council Working Party on Testicular Tumours. Lancet 1985;1:8.
- Register SC. Available from: http://www.socialstyrelsen.se/register/halsodataregister/cancerregistret/inenglish.
- The Causes of Death Register S. Available from: http://www.socialstyrelsen.se/register/dodsorsaksregistret.
- Sweden S. Available from: www.scb.se.
- Agency TSI. Available from: http://www.forsakringskassan.se.
- Neovius M, Simard JF, Askling J. How large are the productivity losses in contemporary patients with RA, and how soon in relation to diagnosis do they develop? Ann Rheum Dis 2011;70:1010–5.
- Taskila T, Martikainen R, Hietanen P, Lindbohm ML. Comparative study of work ability between cancer survivors and their referents. Eur J Cancer 2007;43:914–20.
- Taskila T, Lindbohm ML. Factors affecting cancer survivors’ employment and work ability. Acta Oncol 2007;46: 446–51.
- Bottcher HM, Steimann M, Ullrich A, Rotsch M, Zurborn KH, Koch U, et al. Work-related predictors of not returning to work after inpatient rehabilitation in cancer patients. Acta Oncol 2013;52:1067–75.
- Haugnes HS, Wethal T, Aass N, Dahl O, Klepp O, Langberg CW, et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: A 20-year follow-up study. J Clin Oncol 2010;28:4649–57.
- Joly F, Heron JF, Kalusinski L, Bottet P, Brune D, Allouache N, et al. Quality of life in long-term survivors of testicular cancer: A population-based case-control study. J Clin Oncol 2002;20:73–80.
- Travis LB, Fossa SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst 2005;97:1354–65.
- Wefel JS, Witgert ME, Meyers CA. Neuropsychological sequelae of non-central nervous system cancer and cancer therapy. Neuropsychol Rev 2008;18:121–31.
- Skoogh J, Steineck G, Stierner U, Cavallin-Stahl E, Wilderang U, Wallin A, et al. Testicular-cancer survivors experience compromised language following chemotherapy: Findings in a Swedish population-based study 3–26 years after treatment. Acta Oncol 2012;51:185–97.
- Sprauten M, Brydoy M, Haugnes HS, Cvancarova M, Bjoro T, Bjerner J, et al. Longitudinal serum testosterone, luteinizing hormone, and follicle-stimulating hormone levels in a population-based sample of long-term testicular cancer survivors. J Clin Oncol 2014;32:571–8.
- Sprauten M, Darrah TH, Peterson DR, Campbell ME, Hannigan RE, Cvancarova M, et al. Impact of long-term serum platinum concentrations on neuro- and ototoxicity in Cisplatin-treated survivors of testicular cancer. J Clin Oncol 2012;30:300–7.
- Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, et al. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J Clin Oncol 1989;7:1748–56.
- Ibrahim A, Zambon E, Bourhis JH, Ostronoff M, Beaujean F, Viens P, et al. High-dose chemotherapy with etoposide, cyclophosphamide and escalating dose of carboplatin followed by autologous bone marrow transplantation in cancer patients. A pilot study. Eur J Cancer 1993;29A:1398–403.
- Rodenhuis S, Baars JW, Schornagel JH, Vlasveld LT, Mandjes I, Pinedo HM, et al. Feasibility and toxicity study of a high-dose chemotherapy regimen for autotransplantation incorporating carboplatin, cyclophosphamide and thiotepa. Ann Oncol 1992;3:855–60.
