Abstract
Background. Drug-induced skin toxicity may correlate with treatment efficacy in cancer patients receiving chemotherapy or biological agents. The correlation of the capecitabine-associated hand-foot skin reaction (HFS) on outcome parameters in pancreatic cancer (PC) has not yet been investigated.
Methods. Within the multicentre phase III AIO-PK0104 trial, patients with confirmed advanced PC were randomly assigned to first-line treatment with either capecitabine plus erlotinib (150 mg/day, arm A) or gemcitabine plus erlotinib (150 mg/day, arm B). A cross-over to either gemcitabine (arm A) or capecitabine (arm B) was performed after failure of the first-line regimen. Data on skin toxicity were correlated with efficacy study endpoints using uni- and multivariate analyses. To control for guarantee-time bias (GTB), we focused on subgroup analyses of patients who had completed two and three or more treatment cycles.
Results. Of 281 randomised patients, skin toxicity data were available for 255 patients. Median time to capecitabine-attributed HFS was two cycles, 36 of 47 (77%) HFS events had been observed by the end of treatment cycle three. Considering HFS during first-line treatment in 101 patients treated with capecitabine for at least two cycles within the capecitabine plus erlotinib arm, time to treatment failure after first- and second-line therapy (TTF2) and overall survival (OS) both were significantly prolonged for the 44 patients (44%) with HFS compared to 57 patients without HFS (56%) (TTF2: 7.8 vs. 3.8 months, HR 0.50, p = 0.001; OS: 10.4 vs. 5.9 months, HR 0.55, p = 0.005). A subgroup analysis of 70 patients on treatment with capecitabine for at least three cycles showed similar results (TTF2: 8.3 vs. 4.4 months, HR 0.53, p = 0.010; OS: 10.4 vs. 6.7 months, HR 0.62, p = 0.056).
Conclusion. The present subgroup analysis from AIO-PK0104 suggests that HFS may serve as an independent clinical predictor for treatment outcome in capecitabine-treated patients with advanced PC.
Capecitabine (Cap) is an oral fluoropyrimidine that is selectively activated to 5-fluorouracil (5-FU) in tumour tissues by a three-step enzyme cascade [Citation1]. It is used as single agent chemotherapy and in combination regimens for treatment of different cancer entities, such as colorectal, breast and gastric cancer [Citation2–6]. In several pancreatic cancer (PC) clinical trials, Cap was combined with standard gemcitabine (Gem) for the treatment of patients with locally advanced or metastatic disease: three independent randomised trials comparing combination therapy with Gem/Cap versus single agent Gem in advanced PC found a trend for an improvement in overall survival (OS) with the combination regimen but each study failed to reach a level of statistical significance [Citation7–10]. Two meta-analyses, however, reported a statistically significant survival benefit for the combination of Gem/Cap when compared to single-agent Gem in patients with PC [Citation7–10]. Recently published data from the AIO-PK0104 trial suggests that Cap plus erlotinib followed by Gem or the reverse sequence with Gem plus erlotinib followed by Cap are equally effective with regard to time to treatment failure (TTF) and OS in advanced PC [Citation11]. To date, no predictive biomarker for treatment response to Cap has been identified in patients with PC.
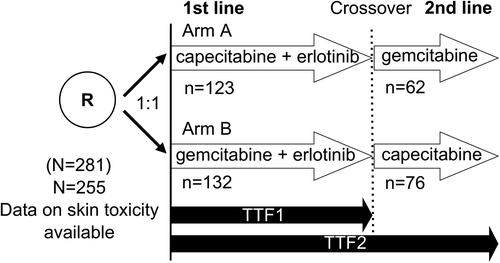
Hand-foot skin reaction (HFS) is a common toxicity observed in patients treated with Cap [Citation12]. In breast and colorectal cancer HFS has been reported to be an independent predictor for treatment response to Cap [Citation13–16]. In the present study the authors thus sought to examine whether HFS might also serve as a predictor for treatment efficacy in PC patients treated with Cap. We therefore analysed data from a previously published prospective multicentre study (AIO-PK0104) of the ”Arbeitsgemeinschaft Internistische Onkologie” (AIO) [Citation11]. In this randomised, cross-over phase III trial, a reference sequence of Gem plus erlotinib followed by second-line Cap was compared to a reverse experimental sequence of Cap plus erlotinib followed by Gem in patients with advanced PC ().
Patients and methods
Study design and treatment
Of 281 patients originally included in the randomised AIO-PK0104 phase III trial, data on skin toxicity was available for 255 patients. A detailed description of the patient population, the treatment protocol and the study design has already been published [Citation11]. Briefly patients with confirmed, treatment-naïve locally advanced or metastatic PC received first-line chemotherapy with Gem (1000 mg/m² intravenously over 30 minutes weekly × 7 followed by one week rest, then weekly × 3 every four weeks, according to the Burris regimen [Citation17]) in combination with erlotinib (150 mg daily) within the reference arm; in case of treatment failure, second-line therapy with single-agent Cap (1000 mg/m² twice daily for two weeks, followed by one week rest) was initiated. In the experimental arm, first-line therapy consisted of oral Cap (1000 mg/m² twice daily for two weeks, followed by one week rest) and erlotinib (150 mg daily); in case of treatment failure, second-line therapy with single-agent Gem (according to the Burris protocol described above) was recommended to the participating patients. Treatment continued until disease progression or unacceptable toxicity. Response evaluations according to RECIST (version 1.0) were performed using computed tomography (CT) imaging. Treatment response in Gem arms was evaluated eight weeks after treatment initiation (after the first cycle) and subsequently after every other treatment cycle (eight-week interval). For Cap arms, the first staging scan was performed after nine weeks (after the first three cycles), and subsequently after every other treatment cycle (six-week interval). The primary study endpoint was the time to treatment failure after first- and second-line chemotherapy (TTF2), with OS, time to treatment failure after first-line chemotherapy (TTF1) and safety being secondary study endpoints (). AIO-PK0104 was conducted according to GCP/ICH guidelines and according to the Declaration of Helsinki; it was registered at ClinicalTrials.gov, number NCT00440167.
Evaluation of hand-foot syndrome
Patients were evaluated for HFS on Day 1 of each treatment cycle by the respective study physicians at each participating study site using the National Cancer Institute Common Toxicity Criteria of Adverse Events (NCI-CTCAE) version 2.0.
Statistical analyses
Occurrence of HFS grade 1–3 during first-line chemotherapy, occurrence of HFS during the subsequent, protocol pre-defined second-line chemotherapy and HFS occurrence at any point during study treatment were analysed separately for each treatment regimen and correlated with TTF1, TTF2 and OS using the Kaplan-Meier method and log-rank test. For a multivariate analysis using an “adjustment for all” model, the three clinical parameters gender, stage of disease (locally advanced vs. metastatic) and Karnofsky performance status (KPS) were included. To control for a potential guarantee-time bias (GTB), additional subgroup analyses of patients who had completed two or more and three or more treatment cycles were performed. Multivariate Cox models as well as univariate models used likelihood ratio tests on a significance level of 0.05 (two-sided). SPSS PASW 18.0 (SPSS Inc., Chicago, IL, USA) software was used for statistical analyses.
Results
Patient characteristics
Within the AIO-PK0104 trial, 281 patients were included. Seven patients had to be withdrawn from the study before treatment initiation due to missing histological confirmation (n = 4), additional malignancy (n = 2) or bilirubin elevation (n = 1). For the current analysis, data on skin toxicity was available from 255 patients. Of those, 123 patients were treated in arm A with Cap plus erlotinib followed by second-line Gem (n = 62) in eligible patients, while the remaining 132 patients were allocated to treatment arm B, receiving Gem plus erlotinib followed by second-line Cap (n = 76) (see ). Median age of the study participants was 63 years, and most of them had metastatic PC (83%, n = 212); 63% (n = 159) of patients had a KPS of 90% or higher. Detailed patient characteristics like age, gender, performance status and stage of disease were comparable in both analysed treatment arms (as summarised in ).
Table I. Patient characteristics.
Outcome of AIO-PK0104
The primary study objective of AIO-PK0104 was a non-inferiority comparison of the two treatment arms with regard to TTF2, which was estimated to be 4.2 months in both treatment arms [hazard ratio (HR) 1.00; p = 1.0]. While OS also was comparable in both treatment arms (6.2 months with Gem plus erlotinib followed by Cap and 6.9 months with Cap plus erlotinib followed by Gem; HR 1.02, p = 0.90), first-line TTF1 was significantly prolonged in the Gem plus erlotinib group compared to Cap plus erlotinib (3.2 vs. 2.2 months; HR 0.69, p = 0.0034). In a pre-planned subgroup analysis the occurrence of skin rash was significantly correlated with improvements in both TTF2 and OS: patients without skin rash had a significantly worse outcome than patients with skin rash of grade 2 or above with regard to TTF2 (2.9 vs. 6.7 months) and OS (3.4 vs. 9.6 months) [Citation11].
Occurrence of HFS
In the overall population, HFS of any grade during first-line treatment was diagnosed in 60 patients (23%). As expected, HFS was more frequent in patients receiving Cap containing first-line therapy (38%, n = 47) than in those receiving the Gem-based front-line regimen (10%, n = 13) (). The majority of all HFS observed were mild to moderate (grade 1: n = 33; grade 2: n = 19). Only eight patients were impaired in their daily functions by HFS (grade 3). As indicated in , HFS occurred early in patients treated with Cap plus erlotinib. The majority of patients (77%, n = 36) had developed HFS by the end of treatment cycle three.
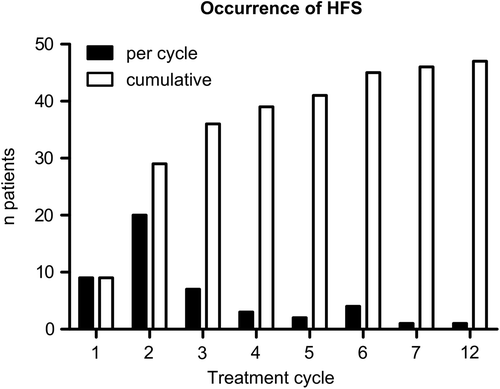
Table II. Frequency of hand-foot skin reaction during first-line therapy.
Treatment delay and dose reductions
Delays in study treatment application and dose reductions were observed more frequently in patients receiving Gem-based first-line treatment than in patients receiving Cap-based front-line therapy (50% vs. 38%, respectively, for all cause treatment delays and 53% vs. 24%, respectively, for all cause dose reductions). A Cap-induced HFS was responsible for a reduction of dose or treatment delay in less than 10% of all patients treated with first-line Cap (see ).
Table III. Delay of chemotherapy and dose reductions due to hand-foot skin reaction during first-line therapy.
Correlation of HFS with efficacy study endpoints
To evaluate whether HFS might serve as a predictive indicator for treatment efficacy of Cap, occurrence of HFS and its possible correlation to efficacy were analysed separately for patients treated with first-line Cap plus erlotinib versus patients treated with first-line Gem plus erlotinib: TTF1, TTF2 and OS were all significantly superior in patients who developed HFS during first-line therapy in the Cap plus erlotinib arm (4.0 vs. 2.0 months, 7.6 vs. 3.2 months and 10.2 vs. 4.4 months, respectively) upon univariate analysis. No significant correlation was observed in patients receiving Gem plus erlotinib first-line therapy (Supplementary Table I and Supplementary Figure 1, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1034877).
While these results suggest that HFS may serve as an indicator for treatment response to Cap-based chemotherapy, it remains unclear whether HFS serves as an independent predictor or solely as an indicator for treatment duration in patients responding well to Cap (as patients responding to therapy might have more time to develop HFS). We therefore performed subgroup analyses for patients who were on first-line treatment with Cap plus erlotinib for at least two or more and three or more treatment cycles. Of 123 patients initially eligible for analysis in the Cap plus erlotinib arm, 101 patients completed at least two treatment cycles; treatment cycle three was completed by 70 patients. The efficacy parameters TTF1, TTF2 and OS were all significantly superior in patients with HFS who had completed at least two treatment cycles (4.3 vs. 2.2 months, 7.8 vs. 3.8 months and 10.4 vs. 5.9 months for patients with or without HFS, respectively) (see and ). Within multivariate analysis TTF1, TTF2 and OS remained significantly correlated to HFS (data not shown). Similar results were observed for patients on treatment for three or more treatment cycles: despite the relatively smaller number of patients eligible for this analysis, the primary study endpoint (TTF2) as well as TTF1 remained significantly correlated to development of HFS with a similar trend for OS (TTF2 8.3 vs. 4.4 months, TTF1 4.5 vs. 3.0 months and OS 10.4 vs. 6.7 months for patients with or without HFS, respectively (see Supplementary Table II, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1034877).
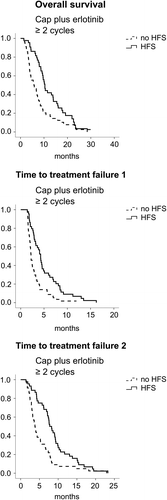
Table IV. Correlation of HFS during first-line therapy with efficacy study endpoints: patients on study for ≥ 2 cycles only.
Discussion
The present subgroup analysis from the prospective AIO-PK0104 trial investigated HFS as an indicator for Cap treatment efficacy in advanced PC. In our patient population, Cap-treated patients who developed HFS at any time during first-line chemotherapy had a significantly better treatment outcome with regard to TTF1, TTF2 and OS. However, retrospective and explorative subgroup analyses are prone to different types of bias: studies correlating efficacy parameters like TTF or OS with an event occurring during follow-up (such as HFS) might be confounded by GTB also referred to as immortal time bias [Citation18]. HFS is an event that can occur anytime during treatment. Choosing the right method to control for GTB in studies evaluating the significance of HFS as predictive factor is therefore challenging [Citation19]. In previous studies correlating HFS with treatment efficacy in colorectal and breast cancer no corrections for GTB were described [Citation13–15]. In our study HFS occurred early after a median treatment time of two cycles. To exclude that HFS merely indicates treatment duration, we focused on patients who were on treatment for at least two or three cycles (61% and 77% of all HFS had been observed by the end of treatment cycle two and three, respectively). Despite the relatively small number of patients available for these analyses, TTF2 – the primary endpoint of the AIO-PK0104 study – as well as TTF1 were significantly correlated with HFS. OS was significantly correlated with HFS in patients who had received a least two cycles of Cap-based chemotherapy, with a similar trend for patients on treatment for three or more cycles.
In our analysis, grading of HFS in individual patients was left to the discretion of the physician on-site. All grade HFS was reported in 38% of patients that received Cap-based first-line chemotherapy. Severe HFS (grade 3) was observed in only 6.5% of patients. This appears to be comparable to the range of 30–60% HFS reported in Cap-treated patients with different cancer entities as well as a previous trial investigating Cap plus erlotinib as second-line therapy in Gem refractory metastatic PC patients that reported an HFS incidence of 47% for any grade HFS (grade 3: 13%) [Citation14,Citation20–22].
The main methodological limitations of the present study arise from its retrospective design and the explorative nature of the statistical analyses. Prospective trials with pre-defined endpoints are necessary to confirm HFS as a predictive indicator of Cap efficacy in PC patients. Besides confirming our observation, these studies should also try to elucidate the underlying molecular link between Cap-induced HFS and treatment efficacy. A correlation between HFS and treatment efficacy of Cap has previously also been witnessed in patients with breast and colorectal cancer. For colorectal cancer HFS, but no other Cap-induced haematological or non-haematological toxicities, was reported to correlate with treatment efficacy [Citation14]. A possible explanation for this phenomenon can be derived from the intracellular metabolism and activation of Cap. As mentioned above, Cap is activated to the active metabolite 5-FU through a three step-enzyme cascade. The third step is mediated by thymidine phosphorylase (TP), an enzyme preferentially expressed in tumour tissues [Citation1]. TP is also present in the skin with significantly elevated concentrations in the palmar region [Citation23]. Hence, Cap-induced HFS might be indicative of differences in individual (tumoural) TP expression. Future trials on HFS and treatment efficacy of Cap should thus investigate the potential of TP as a predictive biomarker in advanced PC.
To our knowledge, this is the first study reporting a correlation between Cap-induced HFS and treatment efficacy outcomes in PC. Our findings thus might have important implications for further trials aiming on improving the dire prognosis of PC. Recently, advances have been made in patients with good performance status by using an intensive treatment regimen consisting of 5-FU, folinic acid, irinotecan and oxaliplatin (FOLFIRINOX) or by adding new agents like nab-paclitaxel to standard-of-care Gem [Citation24,Citation25]. The efficacy of XELOXIRI, a combinational chemotherapy that uses Cap (brand name: Xeloda) instead of infusional 5-FU (as in FOLFIRINOX) is currently being investigated by an open-label, single-arm phase II study in patients with advanced PC (NCT01558869). For patients with resectable disease, the addition of Cap to standard of care Gem in the adjuvant setting is investigated by a large randomised phase III trial (ESPAC-4 study; ISRCTN 96397434). HFS as an indicator for Cap efficacy should be validated prospectively in these settings and might help to identify patients who benefit most from those novel and innovative treatment strategies.
Supplementary material available online
Supplementary Figure 1 and Tables I–II to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1034877
ionc_a_1034877_sm5566.zip
Download Zip (143.2 KB)Acknowledgements
The authors would like to express our deep gratitude to all participating patients and their families as well as all study nurses, study coordinators and investigators for their active participation in this German multicentre AIO study. AIO-PK0104 was supported by an unrestricted research grant from Roche Pharma AG, Germany.
Declaration of interest: The following authors have no conflict of interest: Stephan Kruger, Ruediger P. Laubender, Erika Kettner, Angela Märten, Cornelia Winkelmann, Stefan Klein, Georgi Kojouharoff, Ludwig Fischer von Weikersthal, Michael Geissler, Tim F. Greten, Susanna Hegewisch-Becker and Sebastian Stintzing. Stefan Boeck has been a consultant and received honoraria for scientific presentations, research funding, consultant, travel grants from Celgene; research funding from Clovis Oncology; and honoraria for scientific presentations, research funding, and travel grants from Roche. Volker Heinemann has been a consultant and received honoraria for scientific presentations and research funding from Roche. Ursula Vehling-Kaiser has been on the advisory board for Roche. Dirk Waldschmidt has been a consultant and received honoraria for scientific presentations, research funding and travel grants from Bayer, Celgene and Roche. Thomas C. Gauler has received honoraria for scientific presentations, advisory boards, and travel grants from Lilly and Roche. Michael R. Clemens has received honoraria for scientific presentations from Vifor Pharma Deutschland and a travel grant from Amgen. Dominik P. Modest has received honoraria for scientific presentations, research funding, and travel grants from Roche. Michael Haas has received travel grants and research funding from Boehringer-Ingelheim, has been a consultant and received travel grants from Celgene, and travel grants from Roche.
References
- Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 1998;34:1274–81.
- Twelves C, Wong A, Nowacki MP, Abt M, Burris H, 3rd, Carrato A, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704.
- Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: Results of a large phase III study. J Clin Oncol 2001;19:4097–106.
- Bajetta E, Procopio G, Celio L, Gattinoni L, Della Torre S, Mariani L, et al. Safety and efficacy of two different doses of capecitabine in the treatment of advanced breast cancer in older women. J Clin Oncol 2005;23:2155–61.
- O’Shaughnessy J, Miles D, Vukelja S, Moiseyenko V, Ayoub JP, Cervantes G, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: Phase III trial results. J Clin Oncol 2002;20:2812–23.
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36–46.
- Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta-analysis of randomized trials: Evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 2008;8:82.
- Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 2009;27: 5513–8.
- Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schuller J, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: A randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 2007;25:2212–7.
- Scheithauer W, Schull B, Ulrich-Pur H, Schmid K, Raderer M, Haider K, et al. Biweekly high-dose gemcitabine alone or in combination with capecitabine in patients with metastatic pancreatic adenocarcinoma: A randomized phase II trial. Ann Oncol 2003;14:97–104.
- Heinemann V, Vehling-Kaiser U, Waldschmidt D, Kettner E, Marten A, Winkelmann C, et al. Gemcitabine plus erlotinib followed by capecitabine versus capecitabine plus erlotinib followed by gemcitabine in advanced pancreatic cancer: Final results of a randomised phase 3 trial of the ‘Arbeitsgemeinschaft Internistische Onkologie’ (AIO-PK0104). Gut 2013;62:751–9.
- Gressett SM, Stanford BL, Hardwicke F. Management of hand-foot syndrome induced by capecitabine. J Oncol Pharm Pract 2006;12:131–41.
- Taguchi T, Nakayama T, Masuda N, Yoshidome K, Akagi K, Nishida Y, et al. Study of low-dose capecitabine monotherapy for metastatic breast cancer. Chemotherapy 2010;56:166–70.
- Hofheinz RD, Heinemann V, von Weikersthal LF, Laubender RP, Gencer D, Burkholder I, et al. Capecitabine-associated hand-foot-skin reaction is an independent clinical predictor of improved survival in patients with colorectal cancer. Br J Cancer 2012;107:1678–83.
- Stintzing S, Fischer von Weikersthal L, Vehling-Kaiser U, Stauch M, Hass HG, Dietzfelbinger H, et al. Correlation of capecitabine-induced skin toxicity with treatment efficacy in patients with metastatic colorectal cancer: Results from the German AIO KRK-0104 trial. Br J Cancer 2011;105: 206–11.
- Twelves C, Scheithauer W, McKendrick J, Seitz JF, Van Hazel G, Wong A, et al. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: Final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol 2012;23:1190–7.
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol 1997;15:2403–13.
- Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol 2013;31:2963–9.
- Buyse M, Piedbois P. On the relationship between response to treatment and survival time. Stat Med 1996; 15:2797–812.
- Kulke MH, Blaszkowsky LS, Ryan DP, Clark JW, Meyerhardt JA, Zhu AX, et al. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol 2007;25:4787–92.
- Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 2005;23:792–9.
- Cassidy J, Twelves C, Van Cutsem E, Hoff P, Bajetta E, Boyer M, et al. First-line oral capecitabine therapy in metastatic colorectal cancer: A favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol 2002;13:566–75.
- Milano G, Etienne-Grimaldi MC, Mari M, Lassalle S, Formento JL, Francoual M, et al. Candidate mechanisms for capecitabine-related hand-foot syndrome. Br J Clin Pharmacol 2008;66:88–95.
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364: 1817–25.
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369:1691–703.
