Abstract
Background. Group medical consultations (GMCs) provide individual medical visits in the presence of ≤ 7 peer- patients. This study evaluated the efficacy of GMCs in the yearly breast cancer surveillance of BRCA mutation carriers.
Material and methods. This randomized controlled trial compared GMCs (intervention group, n = 63) with individual medical visits (control group, n = 59). Between-group differences on the primary outcomes distress and empowerment, were analyzed one week and three months after the visit. Feasibility is evaluated in terms of demand, acceptability and practicability.
Results. No between-group differences were found on primary outcomes. More themes were discussed in GMCs. Seventy-five percent of GMC-participants experienced peer support. Carriers reported significantly higher satisfaction with individual visits. GMCs were less time-efficient.
Conclusion. This is the first GMC study which reports results in favor of individual visits. The hereditary nature of the condition differentiates our study population from earlier studied GMC groups. Even though most participants experienced peer support and received more information, the lower patient satisfaction may be explained by the lack of individual time with the clinician and disruption of normal surveillance routines. As the need for peer support and additional information is present in a substantial part of carriers, future research should study the process of peer support.
Female BRCA mutation carriers have a cumulative breast cancer risk of 60–80% to the age of 70 [Citation1]. They are offered the options of yearly breast cancer screening or prophylactic mastectomy. Although prophylactic mastectomy minimizes the risk of breast cancer to nearly 100%, comparable survival rates are seen for prophylactic mastectomy and yearly breast cancer surveillance [Citation2]. The physical and psychological consequences of DNA test results and a prophylactic mastectomy can be complex and remain for a long period [Citation3,Citation4]. Therefore, support in decision making may be one of the aspects of the yearly breast cancer surveillance visit.
Although mean distress levels of all BRCA mutation carriers are not increased one year after genetic testing [Citation3], around 25–34% of the carriers still experience moderately to severely increased levels of distress [Citation5,Citation6]. Predictors of long-term increased levels of distress are using passive and palliative coping styles, performing excessive breast self-examination, having lost a first-degree relative to breast cancer, overestimating one's breast cancer risk, or having high unmet needs [Citation5–7].
There is general agreement among health care professionals on the importance of providing psychosocial support to all carriers, however there is no consensus on providing this kind of services as part of standard care.
The effect of peer support in the form of standardized group educational or psychosocial interventions have been studied during genetic counseling of women with a family history of breast cancer [Citation8,Citation9]. Beneficial effects compared to individual counseling were found on anxiety, accessibility of genetic services, and time efficiency for providers [Citation8,Citation9]. Few uncontrolled studies focused on peer-support groups for tested BRCA carriers, e.g., in the form of supportive-expressive group therapy, family retreats, or educational support groups [Citation10–12]. These support groups seemed promising in decreasing levels of cancer worries, anxiety, depression, and unmet needs, while improving social support and decision making. A trial on telephone-based peer support resulted in decreased levels of distress and unmet needs in the intervention group [Citation13]. Most peer support programs involve multiple sessions, thus they are time consuming, costly and less accessible. Including peer support in the yearly medical breast cancer surveillance visits at the outpatient clinic is a new form of providing medical visits. These group medical consultations (GMCs) provide a series of individualized medical visits by a clinician in the presence of at most seven peer carriers. The other carriers are not merely present as spectators, they are also encouraged to participate with questions concerning issues that are discussed in a patient's visit. In the GMC the clinician is accompanied by a social worker, who directs the GMC and will also discuss psychosocial related issues. Additional information provision, sharing experiences and peer modeling are all well known techniques to increase self-management or patient empowerment in cancer care. As a GMC replaces one standard individual medical visit, it also includes physical breast examination, which is performed individually by the clinician, prior to the GMC. This medical component and the discussion of the personal (BRCA-related) complaints is what sets GMCs apart from other peer support groups.
As described in our study protocol paper, the concept of GMCs has been studied before in a wide range of patient populations, especially in patients with chronic diseases [Citation14–16]. Previous studies revealed beneficial effects of GMCs on patient and professional satisfaction, information provision, health behavior, self-efficacy and knowledge of the disease, quality of life, and quality of care [Citation14–16]. Recently, the interest in GMCs in cancer follow-up care is also growing [Citation17,Citation18].
This randomized controlled trial (RCT) will evaluate the effect of GMCs on psychological distress and empowerment in the breast cancer surveillance of BRCA mutation carriers compared to individual medical visits.
Material and methods
A comprehensive protocol of this study was published previously [Citation16]. Patients were eligible for participation if they were a female carrier of a BRCA1 or a BRCA2 mutation and had an age between 25 and 60. One year after the start of the inclusion, the original criterion for being an eligible carrier, having been diagnosed with a BRCA mutation maximally two years ago, was reconsidered since the number of eligible carriers was lower than expected. Carriers were excluded if they were currently involved in a diagnostic work-up because of a suspicion of breast cancer, if they had a history of prophylactic mastectomy, if they had current psychiatric disease precluding visits in a group or if they had insufficient command of the Dutch language to be able to follow a group discussion and fill out questionnaires in Dutch. Carriers who declined participation in the study were asked to fill out a one-time questionnaire. The Radboudumc Medical Review Ethics Committee granted permission to perform this study.
Study procedures
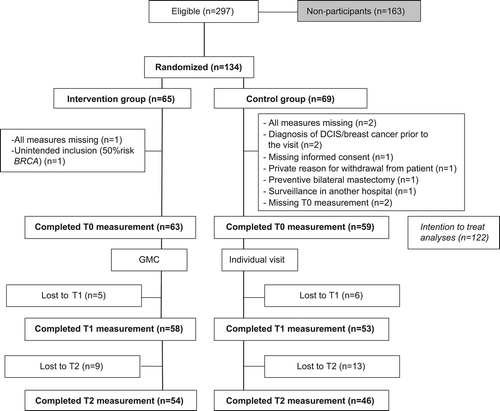
Carriers were randomized into one of the two arms after informed consent was obtained (). To minimize changes in the yearly surveillance appointment schedule of carriers, the period between randomization and the individual visit or GMC varied between one week and several months. This variation partly explained the high exclusion rate after randomization and before the baseline measurement. Block randomization per 16 carriers was performed for each GMC, to ensure every professional had the same number of carriers in a GMC (n = 8) and an individual visit (n = 8). To evaluate the GMCs, participants filled out questionnaires at three time points: at one week prior to the medical visit (T0), one week after (T1), and three months (T2) after the intervention. Professionals filled out a questionnaire after each GMC or individual visit.
Intervention
Participants in the control group received a standard individual breast cancer surveillance visit (± 15 min) with the clinician at the outpatient clinic for Hereditary Cancer. Around two weeks prior to this yearly visit, carriers had a breast magnetic resonance imaging (MRI) and in case of 30 years or more, also a mammography. The visit included discussion of scan results and clinical breast examination by the clinician. The content of discussion of medical and psychosocial issues was depending on the patient's needs. Some examples of common themes were benefits of breast self-examination, choice for yearly surveillance or preventive mastectomy, or preventive mastectomy and its side effects. For carriers in the intervention group, this standard individual breast cancer surveillance visit was replaced with a GMC. A GMC provided individual medical visits by a clinician conducted within a group of 4–8 peer carriers. A social worker was present to lead the discussion, to support the clinician in discussing psychosocial issues, and to ensure every carrier receives equal attention. The duration of the GMC was dependent on the number of participants and at most 90 minutes in case of eight participants. All participants received individual breast examination by the clinician, 30 minutes prior to the GMC. All participants were welcome to bring their partner or relative to the GMC. The concept of GMCs as described in the Dutch manual on GMCs was followed [Citation19]. In summary, after the clinical breast examinations, carriers gathered in a group meeting room at the outpatient clinic. The social worker shortly introduced the GMC and participants introduced themselves. Afterwards, the visits started. Themes that were relevant for several participants lead to a general discussion in which the other participants were involved. In total, one oncologist, one internist onco-geneticist, and one medical doctor, working for the Hereditary Cancer Clinic, were involved as medical professionals in the GMCs. One social worker chaired the GMCs. A researcher observed the GMC content in the background. The same professionals also performed the individual visits of patients in the control group. Further details were previously published [Citation16].
Study outcomes
All outcomes were measured at T0, T1 and T2, or otherwise specified. The primary efficacy outcome measures were psychological distress (SCL-90) [Citation20] and patient empowerment (CEQ) [Citation21]. Secondary efficacy outcomes were cancer worry (Cancer Worry Scale) [Citation22], quality of life (EORTC-QLQ C30 and EORTC-BR23 [Citation23,Citation24]). Frequency of breast self-examination (BSE) (T0 and T2), decisions concerning breast cancer preventive options and certainty about these decisions were measured by multiple choice questions.
Feasibility was assessed in terms of demand, acceptability, and practicability [Citation25].
Demand for GMCs was expressed in the percentage of study participants from all eligible BRCA mutation carriers. Non-participants were asked to fill out a questionnaire including baseline measures and an additional question about the reason for non-participation.
Acceptability of the intervention was measured in terms of general carrier and professional satisfaction using a five-point scale (not satisfied at all – very satisfied) and a modified version of the QUOTE questionnaire [Citation19] at T1 concerning the face-to-face visit and at T2 concerning the video GMCs. The QUOTE questionnaire measures, for example satisfaction with available clinician's time, or being listened to by the clinician on a three-point scale (no – a little – yes). Other intervention-specific (multiple-choice) questions focused on the participants’ experienced support from peer-carriers, the discussion of personal themes in the group, the ability to ask all questions, and their choice for a GMC in the future. To evaluate whether the content of the visits fulfilled the needs for surveillance care, participants filled out a checklist at T0 to indicate the themes they wanted to discuss during the visit. At T1 participants indicated which themes were actually discussed. Professionals used a similar theme list directly after the visit to report the content of the individual visits or GMCs. Themes discussed during GMCs were also reported by an observer (researcher) using a checklist. Participant retention was measured by recording the numbers of and reasons for study and intervention dropouts.
Practicability was considered as the self-reported time investment for the professionals and duration of the GMCs and individual visits (including time for preparation), also reported by the professionals. In addition, we evaluated the experiences with practical or logistic aspects related to integration of GMCs in surveillance routines, e.g. planning of MRI and mammography.
Sample size and statistical analyses
The original sample size calculation was based on SCL-90 data of breast cancer survivors [Citation16], since data of BRCA mutations were not available at that time. A total of 160 participants (80 in each arm) was considered necessary to find a clinically relevant difference of 13.5 points on psychological distress, using a power of 80% and a two-tailed probability level for significance testing of 0.05. A study dropout percentage of 15% was accounted for. During inclusion we noticed that assumptions and expected effects in this calculation were overestimated. Baseline distress levels were lower than expected, within the normal distress range, which made it unlikely to achieve significant improvement on this outcome. As the dropout rates were lower than expected (9.0%) and due to financial constraints, we decided to stop the inclusion ahead of reaching the calculated sample size (n = 134).
Descriptive statistics were used to describe participants’ demographics at baseline and the feasibility outcomes. Independent t-tests and χ2-test were used to compare several baseline characteristics between both groups. χ2 and Pearson's correlations were performed to find factors related to carrier satisfaction. The statistical analyses on the efficacy outlining consisted of covariance analyses (ANCOVA) which compared the outcome measures at T1, corrected for baseline (T0), between the intervention group and control group. Covariates included in this analysis were the baseline scores on that particular outcome and SCL-90 and CEQ. Analyses were done according to modified intention-to-treat (mITT) methodology. mITT allows the exclusion of some randomized subjects in a justified way (i.e. patients who were deemed ineligible after randomization or certain patients of whom all measurements were missing) [Citation26]. All reasons for exclusions after randomization are shown in . As we were interested in whether patients improved or not, delta scores were calculated for categorical data concerning frequency of BSE, decisions concerning preventive options and the related certainty about these decisions of baseline and T1 or T2 measurements and compared between both groups using χ2-tests or independent t-tests.
Missing T1 and T2 measurements were solved by imputation using data from the most recent measurement (last observation carried forward). For one carrier baseline empowerment data was missing. As all other baseline and follow-up data of this participant was complete, we imputed T0, using the mean of the participant's empowerment scores at T1 and T2. Participants were compared with non-participants on age and baseline outcomes on distress and empowerment by using independent t-tests.
SPSS 20.0 was used to analyze the data.
Results
Carrier characteristics are shown in . Sixty-five carriers were randomized for the intervention group, 69 carriers for the control group. Reasons for exclusion after randomization are described in , leaving a total of 122 carriers.
Table I. Carrier and treatment characteristics.
Efficacy outcomes
Groups did not significantly differ on distress [F(1,118) = 0.001, p = 0.974] and empowerment [F(1,118) = 1.129, p = 0.290] levels at T1, when corrected for baseline (T0) levels. At T2, the effects of GMCs on distress and empowerment were neither significantly different compared to individual visits (). A significant time effect on distress was found [F(1,119) = 4.601, p = 0.017], with a significant difference between T0 and T1 (p = 0.006), showing that all carriers in the study improved irrespective of the type of visit (GMC or individual).
Table II. ANCOVA analysis on mean scores of primary and secondary outcomes in intervention and control group at T1 and T2 controlled for baseline scores (T0).
As shown in the secondary outcomes cancer worry and the quality of life subscales (global health status, sexual functioning, sexual enjoyment, and future perspective) were not significantly different between both groups, neither directly following the medical visit nor after three months.
Changes in frequency of breast self-examination between T0 and T2 were not significantly different between both groups. Neither changes in decisions concerning breast cancer preventive options (changed vs. no change), nor changes in certainty about these decisions were different between GMCs or individual visits at T1 (Supplementary Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049292).
Feasibility outcomes
Demand
Between April 2011 and April 2014 297 BRCA carriers were informed about the study. Forty-five percent (n = 134) of these eligible carriers agreed on participation and signed informed consent (). Forty-two percent (n = 69) of the non-participants filled out a questionnaire. Non-participants showed distress, empowerment and cancer worry levels equivalent to study participants at baseline. No significant differences in age, marital status, education level, employment status, years since DNA test results and choices for breast cancer preventive options were found between non-participants and participants (Supplementary Table II, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049292). However, study participants addressed more themes to discuss during the visit (GMC or individual) compared to non-participants. Compared to non-participants, more participants indicated that they wanted to discuss the following themes during the coming visit: preventive mastectomy (χ2 = 4.10, p = 0.043), factors involved in decision making (χ2 = 7.31, p = 0.007), breast reconstruction (χ2 = 7.13, p = 0.008) and contact with a social worker (χ2 = 6.001, p = 0.014). Mammography and MRI results were less often mentioned by participants (χ2 = 5.89, p = 0.015). Main reasons for non-participation were no need for contact with peer-carriers (46.4%), worry about other carriers’ medical backgrounds (27.5%), time constraints (27.5%), and personal questions, which they did not want to discuss in the presence of other carriers (24.6%).
Acceptability
Carriers were significantly less satisfied with GMCs (3.7 ± 1.1) than individual visits (4.5 ± 0.8, p < 0.001). Professionals’ satisfaction showed a trend in favor of individual visits (GMC: 4.1 ± 0.5; individual: 4.4 ± 0.6, p = 0.067). Carrier satisfaction (low = 1–3 vs. high = 4,5) was positively related to the experience of peer-support and to the number of discussed topics (Supplementary Table III, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049292). Time since DNA test results, age, marital status, history of breast cancer or group sizes were not significantly related to carrier satisfaction (Supplementary Table III available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049292). Only 2% of carriers experienced barriers to discuss personal themes during the GMCs. Seventy-five percent experienced support from peer carriers, mainly by receiving practical advices (30%) or feelings of not being alone (49%). Thirty-seven percent would absolutely join a future GMC. Another 30% addressed some prerequisites for a future GMC, e.g. more homogenous groups concerning age, decision for preventive options, or breast cancer history (n = 11), more available time (n = 2), adjust planning to timing of scans (n = 2), exclude positive scan results from GMC (n = 2), more space for exchange of experiences and feelings (n = 2). Most carriers were able to address all their questions during GMCs (86%) as well as in individual visits (89%). Carriers in both groups were equally satisfied about the available time with the clinician (χ2 = 4.293, p = 0.117) and both equally felt that the clinician was listening to their stories (χ2 = 0.008, p = 0.996).
At T1, carriers and professionals reported more discussed themes in GMCs. Topics being discussed more often included the breast cancer risk, preventive mastectomy, factors involved in decision making, surveillance of the ovaries, risk reducing salpingo-oophorectomy (RRSO), pregnancy, osteoporosis, hot flushes, sad feelings, fear and contact with a social worker (). According to professionals also breast reconstruction, contraceptive pills, and the response of relatives on the test results were discussed more frequently in GMCs. Professionals reported more discussed themes compared to carriers (), while frequencies of discussed topics by the observer were mostly in between carriers’ and professionals’ reported frequencies. Comparing the observer's results with those from professionals or patients, patients were under-reporting, while professionals over-estimated the number of discussed topics (). Reasons for intervention drop-outs in the intervention group (n = 6) were a cancelled GMC (n = 3), too late arrival for GMC (n = 1) and illness (n = 2).
Practicability
Thirteen GMCs were performed by three different medical professionals and one social worker. On average 4.4 ± 1.4 (range 3–8) carriers participated per GMC. The duration of GMC was on average 20 ± 4 minutes per carrier, including physical examination, which is significantly higher compared to 15 ± 4 minutes per individual visit (t = 3.9; p < 0.001, 95% CI 2.24–6.94). Also, time per carrier for GMC preparation by the medical professional was significantly higher (8 ± 7 minutes) compared to preparation time for individual visits (5 ± 3 minutes) (t = 2.2; p = 0.031, 95% CI 0.30–6.11). During individual visits all carriers received results from MRI and mammography. In GMCs we were unable to reschedule imaging tests in line with the GMC for all participants. Consequently, only a minority of GMC participants received scan results during the GMC, which lead to additional medical outpatient visits or telephone appointments to inform carriers about their scan results (based on observations from the clinicians). Patients from different clinicians were mixed to increase the likelihood of finding enough participants to organize the GMC.
Discussion
GMCs in the surveillance of BRCA mutation carriers have led to an increase in information provision and peer support. Despite these beneficial findings for GMCs, carriers in individual visits were more satisfied and levels of distress and empowerment were not significantly different after a GMC compared to individual visits.
We should comment that by performing this trial we disrupted normal surveillance routines, i.e. many surveillance visits were not scheduled in line with MRI and/or mammography, which lead to additional medical outpatient visits or telephone appointments to inform carriers about their scan results. Also, the number of carriers per GMC was smaller than intended and participants were not always scheduled with their own clinician. These factors have potentially limited the positive impact of our intervention.
Compared to other GMC studies, this is the first to report results favoring individual visits in terms of patient and professional satisfaction and time efficiency. It should be noted, however, that earlier reported effects of GMCs were not very strong, were based on multiple sessions or had limitations in their research design, as some were lacking control groups [Citation14,Citation17,Citation27]. In addition, BRCA carriers are an exceptional patient population in several ways. Carriers have only one surveillance visit per year. Therefore, participating in a GMC implies that a woman has no individual contact with her clinician for two years. As the BRCA mutation is inherited most of the carriers have family members carrying the same mutation, with whom they can share experiences and information.
Keeping these comments into our minds, we should note that baseline distress levels were low, even equivalent to distress levels in a healthy population, with only eight percent (n = 10) reporting clinically increased levels of distress (≥ 160). Other studies reported increased distress levels in 25–34% of the BRCA mutation carriers [Citation6]. The small number of distressed carriers in our study population probably caused a floor effect. These results could imply a selection bias of non-distressed carriers being more willing to participate in GMCs. However, comparison of distress levels between study participants and non-participants did not reveal any differences regarding distress or empowerment. Besides, baseline cancer worry levels are comparable to other populations with increased hereditary cancer risks [Citation28]. The time effect on distress between T1 and T0 can probably be attributed to the visit (GMC or individual), as the period between both measures was only two weeks, including the GMC or individual visit in between. A decrease in distress after a yearly surveillance visit is a well known trend [Citation7]. During individual visits all carriers received results from MRI and mammography, which will have reassured most carriers, while only part of the carriers received these results during a GMC. Due to logistic and planning issues, not all GMC participants received results during this visit, which may have influenced their change in distress levels. We should also notice the use of the conservative, though widely accepted LOCF method for handling missing data, which may have decreased a possible effect of the intervention.
Our results suggested a higher need for decision related information regarding preventive breast cancer options among study participants compared to non-participants. GMCs are able to fulfill these needs by the provision of more information, both decision related as well as psychosocial information. Majority of participants received answers to all their questions. The importance of social support, from relatives, friends, peers and health professionals in relation to lower levels of distress, coping with the DNA test results and fewer unmet needs have been shown [Citation3,Citation5–7]. Needs for social support are being addressed by GMCs and were positively evaluated by carriers as well. These findings are in line with results from a study on GMCs during follow-up of breast cancer [Citation18].
Nevertheless, information provision and social support were obviously not the only factors influencing satisfaction. However, our quantitative data did not provide any explanations for the lower satisfaction rates in GMCs. Based on qualitative data from several open ended questions, we hypothesize that limited individual time with the clinician could be a possible explanation. During the GMC, on average participants received an equal amount of individual time from the clinician compared to an individual visit, but in fact they seemed to experience a lack of personal attention from the clinician. According to the experience of the clinicians carriers considered the GMC as something additional, besides the individual visit. After a GMC, professionals observed that many participants returned within several months for an individual visit prior to the originally scheduled yearly appointment. Unfortunately, we cannot confirm this observation with the available data since we only collected health care utilization up to three months after the visit. The qualitative data also suggests a preference for receiving mammography or MRI results during an individual contact with the clinician rather than during a GMC. Professionals experienced barriers to discuss alarming results in the GMC.
Finally, also the professional satisfaction was in favor of individual visits compared to GMCs, which is partly explained by the previous mentioned observations. Professionals experienced the GMC as very time consuming and intense to perform. These experiences were confirmed by results of the time measures: time for preparation as well as time per carrier during the GMC were significantly higher in GMCs. The small group numbers can partly explain these inefficiency results. We expect larger number of participants in a non-research setting, considering the lack of a control group. Furthermore, positive experiences by professionals were also described, such as the reduced need for repeating the same information every visit during a day of outpatient clinics, which is accompanied by the possibility to have more diverse and elaborate discussions in a GMC.
Practical implications
We can conclude that the demand for GMCs is high, around 45% of the eligible carriers was willing to participate. Also the need for BRCA-specific information (e.g. preventive mastectomy) and psychosocial support is high among participants. GMCs partly fulfill these needs by the provision of additional information and psychosocial and peer support. However, participants missed the individual attention during GMCs, which seemed to negatively influence their satisfaction about the visit. Also, most of the non-participants (i.e. approximately half of the target population in clinical practice) expressed a strong preference for no contact with peers, indicating a selection bias in our trial.
Concerning the practicability of GMCs, individual visits were more time efficient. GMCs and individual visits were equally effective in reducing distress after the visit. Based on these results we might consider a different form of GMCs, which is additionally to the yearly surveillance visit. However, peer support seemed to be differently received by BRCA mutation carriers compared to other patient populations participating in GMCs. Before further implementation or development of peer support programs, future studies should focus on the process of peer support in this group. Of note, we should stay aware of the possible pitfalls of peer support, e.g. peers influencing decision making towards prophylactic mastectomy. Previous information-support groups for BRCA mutation carriers in our center seemed to shorten the time to decide for prophylactic mastectomy [Citation12]. GMCs did not change decisions concerning breast cancer preventive options nor changed certainty about these decisions. Possible explanations are the single session structure of GMCs, which limits the amount of bonding and influence between participants, or the absence of role models who have completed prophylactic mastectomy, who were excluded from our study.
Our results indicate that especially BRCA mutation carriers with higher needs for information will participate in GMCs. Other carrier characteristics were non-related to participation or satisfaction. GMCs seemed not suitable for each carrier and it is difficult to predict, based on carrier characteristics, which carriers will participate. Therefore, future peer support programs should be integrated in standard care for only those carriers who report needs for additional information and peer contact. Future research should focus on alternative ways of guided peer support groups, which are more efficient and separated from medical visits. Peer support has been offered in a variety of forms, which all resulted in some beneficial findings, although results are inconclusive [Citation10–13]. More research is needed to identify effective components of guided peer support groups for mutation carriers.
Supplementary material available online
Supplementary Table I–III available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049292
ionc_a_1049292_sm5971.pdf
Download PDF (103.2 KB)Acknowledgments
The study is funded by the Pink Ribbon foundation, the Netherlands. We thank all study participants and Martine van Koolwijk, social worker and Wilmy Bos, clinical nurse specialist, for their contributions to the GMCs.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- King MC, Marks JH, Mandell JB, New York Breast Cancer Study G. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003;302: 643–6.
- Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol 2010;28:222–31.
- Beran TM, Stanton AL, Kwan L, Seldon J, Bower JE, Vodermaier A, et al. The trajectory of psychological impact in BRCA1/2 genetic testing: Does time heal? Ann Behav Med 2008;36:107–16.
- Fuller S, Anderson RC. Adjustment issues related to bilateral prophylactic mastectomy in women at elevated risk of developing breast cancer. Plast Surg Nurs 2009;29:33–8.
- Farrelly A, White V, Meiser B, Jefford M, Young MA, Ieropoli S, et al. Unmet support needs and distress among women with a BRCA1/2 mutation. Fam Cancer 2013;12:509–18.
- den Heijer M, Seynaeve C, Vanheusden K, Timman R, Duivenvoorden HJ, Tilanus-Linthorst M, et al. Long-term psychological distress in women at risk for hereditary breast cancer adhering to regular surveillance: A risk profile. Psychooncology 2013;22:598–604.
- van Dooren S, Seynaeve C, Rijnsburger AJ, Duivenvoorden HJ, Essink-Bot ML, Tilanus-Linthorst MM, et al. Exploring the course of psychological distress around two successive control visits in women at hereditary risk of breast cancer. Eur J Cancer 2005;41:1416–25.
- Bowen DJ, Burke W, McTiernan A, Yasui Y, Andersen MR. Breast cancer risk counseling improves women’s functioning. Patient Educ Couns 2004;53:79–86.
- Calzone KA, Prindiville SA, Jourkiv O, Jenkins J, DeCarvalho M, Wallerstedt DB, et al. Randomized comparison of group versus individual genetic education and counseling for familial breast and/or ovarian cancer. J Clin Oncol 2005;23:3455–64.
- McKinnon W, Naud S, Ashikaga T, Colletti R, Wood M. Results of an intervention for individuals and families with BRCA mutations: A model for providing medical updates and psychosocial support following genetic testing. J Genet Couns 2007;16:433–56.
- Esplen MJ, Hunter J, Leszcz M, Warner E, Narod S, Metcalfe K, et al. A multicenter study of supportive-expressive group therapy for women with BRCA1/BRCA2 mutations. Cancer 2004;101:2327–40.
- Landsbergen KM, Prins JB, Kamm YJ, Brunner HG, Hoogerbrugge N. Female BRCA mutation carriers with a preference for prophylactic mastectomy are more likely to participate an educational-support group and to proceed with the preferred intervention within 2 years. Fam Cancer 2010;9:213–20.
- White VM, Young MA, Farrelly A, Meiser B, Jefford M, Williamson E, et al. Randomized controlled trial of a telephone-based peer-support program for women carrying a BRCA1 or BRCA2 mutation: Impact on psychological distress. J Clin Oncol Epub 2014 Nov 17.
- Edelman D, McDuffie JR, Oddone E, Gierisch JM, Nagi A, Williams JW. Shared medical appointments for chronic medical conditions: A systematic review. VA Evidence-based Synthesis Program Reports. Washington, DC: Department of Veteran Affairs; 2012.
- Slyer JT, Ferrara LR. The effectiveness of group visits for patients with heart failure on knowledge, quality of life, self-care, and readmissions: A systematic review. JBI Database System Rev Implement Reports 2013;11:58–81.
- Visser A, Prins JB, Hoogerbrugge N, van Laarhoven HW. Group medical visits in the follow-up of women with a BRCA mutation: Design of a randomized controlled trial. BMC Womens Health 2011;11:39.
- Trotter K, Schneider SM, Turner BS. Group appointments in a breast cancer survivorship clinic. J Adv Pract Oncol 2013;4:423–31.
- Visser A, van Laarhoven HW, Govaert PH, Schlooz MS, Jansen L, van Dalen T, et al. Group medical consultations in the follow-up of breast cancer: A randomized feasibility study. J Cancer Surviv Epub 2015 Jan 13.
- Seesing FM, Raats I. Gezamenlijk Medisch Consult. Een praktische handleiding. Houten: Bohn Stafleu; 2009.
- Arrindell WA, Ettema JHM. Symptom Checklist-90; Handleiding bij een multidimensionele psychopathologie-indicator. Lisse: Swets & Zeitlinger B.V.; 2003.
- van den Berg SW, van Amstel FK, Ottevanger PB, Gielissen MF, Prins JB. The cancer empowerment questionnaire: Psychological empowerment in breast cancer survivors. J Psychosoc Oncol 2013;31:565–83.
- Custers JA, van den Berg SW, van Laarhoven HW, Bleiker EM, Gielissen MF, Prins JB. The Cancer Worry Scale: Detecting fear of recurrence in breast cancer survivors. Cancer Nurs 2014;37:E44–50.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Sprangers MA, Groenvold M, Arraras JI, Franklin J, te Velde A, Muller M, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. J Clin Oncol 1996;14:2756–68.
- Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, et al. How we design feasibility studies. Am J Prev Med 2009;36:452–7.
- Abraha I, Montedori A. Modified intention to treat reporting in randomised controlled trials: Systematic review. Br Med J 2010;340:c2697.
- Seesing FM, Drost G, Groenewoud J, van der Wilt GJ, van Engelen BG. Shared medical appointments improve QOL in neuromuscular patients: A randomized controlled trial. Neurology 2014;83:240–6.
- Lammens CR, Aaronson NK, Wagner A, Sijmons RH, Ausems MG, Vriends AH, et al. Genetic testing in Li-Fraumeni syndrome: Uptake and psychosocial consequences. J Clin Oncol 2010;28:3008–14.