To the Editor,
The standard treatment for locally advanced rectal cancer is radio(chemo)therapy (RCT) followed by total mesorectal excision (TME) surgery [Citation1]. This preoperative treatment is associated with gastrointestinal morbidity, such as diarrhea, fecal incontinence, bowel dysfunction, obstruction and perforation [Citation2].
Radiation-induced gastrointestinal toxicity is primarily dependent on the small bowel volume receiving 15 Gy (V15) and 45 Gy (V45) [Citation3–5]. Highly conformal radiation techniques, such as intensity-modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT) effectively decrease the dose to the small bowel, thereby reducing the radiation-induced bowel toxicity [Citation6].
Mechanical small bowel displacement techniques, including prone positioning on a bellyboard, also reduce the dose to the bowel [Citation7–9]. However, bellyboards have been associated with patient discomfort and reduced position reproducibility [Citation10,Citation11].
There is a substantial inter- and intra-fractional rectal motion, especially in the anterior direction. Therefore, the precise irradiation with IMRT or VMAT increases the risk to miss the tumor. Larger set-up margins can compensate for this, but might abolish the dosimetrical benefit of prone position on a bellyboard.
The use of a bellyboard in VMAT for rectal cancer has not yet been reported before. We investigated whether prone position on a bellyboard for rectal cancer radiotherapy is still beneficial in terms of dosimetry when highly conformal VMAT is applied.
Material and methods
Eleven patients with stage II/III rectal cancer scheduled for preoperative RCT were prospectively enrolled. Each patient underwent a planning computed tomography (CT) in both prone on a bellyboard (MacroMedics® Pelvic Prone BoardTM) and supine position. The clinical target volume (CTV) was delineated according to the guidelines of Roels et al. [Citation12]. As a result of the anterior rectal motion and the steep dose fall-off of VMAT, the CTV was extended anteriorly with a planning target volume (PTV) margin of 1.5 cm (see Supplementary Appendix, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1064159). In the other directions, a 1 cm margin was applied.
A dose of 45 Gy in 25 fractions was prescribed to the PTV using two arcs of 358° (Eclipse® planning system). Average dose-volume histograms (DVHs) were created by calculating for each structure the mean relative volume in 0.1 Gy dose bins. The homogeneity index (HI) of the PTV was defined as [(D2-D98)/Dprescribed x 100] and the conformity index (CI) as V95/PTVvolume.
Treatment positions were compared using Wilcoxon non-parametric matched pairs tests, with statistical significance assumed for p ≤ 0.05 (Statistica version 12, Statsoft®).
Methodological details can be found in the Supplementary Appendix available online at http://informahealthcare.com/doi/abs/10.3109/0284186X. 2015.1064159.
Results
Dosimetric parameters are summarized in and illustrated in . There were no significant differences in the PTV volume, D2, D98 and Dmean, CI and HI between the prone and supine plans.
Figure 1. Average dose-volume histograms of small bowel, large bowel and bowel bag: VMAT prone with bellyboard versus VMAT supine. Doses are in Gy, volumes in cm3.
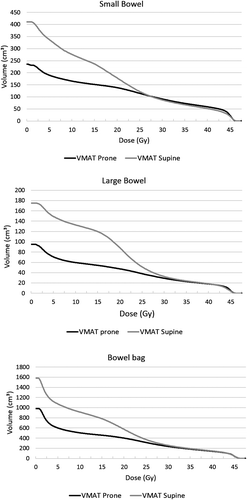
Table I. Dosimetrical comparison VMAT prone with bellyboard versus VMAT supine (median, IQR).
The bowel volumes were significantly lower in prone position on a bellyboard compared to supine position [median small bowel 154.8 cm3 vs. 348.0 cm3 (p = 0.010), median large bowel volume 66.3 cm3 vs. 117.7 cm3 (p = 0.013), median bowel bag volume 1029.0 cm3 vs. 1343.7 cm3 (p = 0.008), for the prone and supine scans, respectively]. Prone positioning significantly reduced the V15 of the small bowel (median 64.4 cm3 vs. 194.8 cm3; p = 0.008), the V15 of the large bowel (median 38.5 cm3 vs. 71.3 cm3; p = 0.006) and the V15 of the bowel bag (median 433.4 cm3 vs. 762.2 cm3; p = 0.003). The V45 of all bowel structures was also lower in prone position, although this was not statistically significant.
The bladder volume was significantly lower in prone position compared to supine position (92.7 cm3 vs. 111.0 cm3, respectively; p = 0.003), however this did not lead to a significant reduction in V40 of the bladder.
Discussion
This study shows that even with highly conformal VMAT and enlarged PTV margins, prone position on a bellyboard remains beneficial in reducing the dose to the organs at risk in rectal cancer treatment. Although the feasibility of VMAT for rectal cancer has been investigated before, to the best of our knowledge, this is the first study reporting on the role of a bellyboard in VMAT for rectal cancer [Citation13–15]. In a study of 20 patients who underwent preoperative three-dimensional conformal radiation therapy (3D-CRT), Kim et al. showed that a bellyboard reduced the irradiated small bowel volume [Citation8]. The same group confirmed these findings in the IMRT-setting [Citation16]. Nijkamp et al. investigated the bowel exposure using prone, supine and two different bellyboards when IMRT was applied [Citation9]. Eleven healthy volunteers underwent four magnetic resonance imaging (MRI) scans (prone, supine and prone on two different bellyboards). Similar to our findings in VMAT setting, the authors found that a bellyboard still attributes to a significant bowel dose reduction in the low and intermediate dose regions when IMRT was used.
The bladder volume was significantly larger in the supine scans compared to the prone scans. This might be explained by the fact that eight of the 11 patients were first installed in prone position. We did not use bladder protocol as from our experience this does not increase reproducibility and on the contrary, might be associated with patient distress and discomfort. The benefit of using a bladder protocol has also been questioned by others [Citation8,Citation9].
Some clinicians prefer not to use bellyboards for pelvic radiotherapy because they may cause patient discomfort (e.g. shortness of breath, pressure on the central venous catheter, on the stoma or on the pelvic bones in frail patients). Bellyboards have also been associated with reduced patient position reproducibility. Italia et al. reported that patients who were treated in prone position using a bellyboard and an immobilization cast were more likely to have an average setup error > 5 mm (23% vs. 11%) and a larger random setup error (1 SD = 4.2 mm vs. 1.8 mm supine) in the superior-inferior direction as compared to patients treated in supine position [Citation10]. Similarly, Allal et al. found larger average positioning errors for patients treated prone on a bellyboard compared to prone without a bellyboard, particularly in the antero-posterior direction (mean antero- posterior displacement of 4.5 mm vs. 1.8 mm, respectively) [Citation11]. Data from our own center however show that it is unnecessary to enlarge margins when setup imaging protocols are used [Citation17].
A potential concern of using a bellyboard in VMAT is that the beam might be attenuated when it passes through the bellyboard. However, in-house dose experiments showed that the attenuation of this type of bellyboard resulted in a negligible 0.6% dose deviation at the isocenter in a phantom for arc delivery (data not published). Therefore, we did not take the bellyboard-related attenuation into account, although the attenuation of the couch top was accounted for.
From a theoretical point of view, the better sparing of the organs at risk with VMAT facilitates dose escalation. In an additional analysis, we found that moderate dose escalation with VMAT up to 50 Gy did not increase the V15 and V45 of the bowel structures as compared to 3D-CRT up to 45 Gy (see Supplementary Appendix available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1064159).
Increasing the dose from 45 Gy in fractions of 1.8 Gy to 50 Gy in fractions of 2 Gy is beneficial for both the primary tumor and the subclinical tumoral deposits. The sigmoidal dose response relationship in rectal cancer implies that a small increase in dose has a large effect on the tumoral response [Citation18]. Therefore, the increase in dose from 45 Gy in 1.8 Gy fractions (EQD2 = 44.25 Gy; α/β = 10) to 50 Gy is expected to enhance the response to RCT.
Prolonging radiotherapy over several days or weeks might increase the average cell burden by tumor repopulation. Suwinski et al. demonstrated that an increase in dose of 0.54 (± 0.09) Gy/day is required to achieve a constant reduction in the rate of pelvic recurrences when the overall treatment is extended [Citation18]. This increase in dose is consistent with the rapid growth of tumor clonogens in subclinical foci, as demonstrated by the Gompertzian model of spontaneous growth and repopulation of tumors during radiotherapy [Citation19]. Increasing the dose per fraction from 1.8 Gy to 2 Gy might partially compensate for the rapid growth of subclinical tumor deposits and can therefore reduce pelvic recurrences that arise from tumor clonogens residual beyond the surgical margins [Citation18].
We were not able to investigate whether the dosimetrical benefit of prone position on a bellyboard translated into a lower radiation-induced toxicity. Due to the substantial motion of the rectum in the anterior direction, we applied a 1.5 cm anterior PTV margins which was adapted from the interfractional CTV motion as assessed by regularly performed cone beam computed tomography (CBCT) in an independent patient group (see Supplementary Appendix available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1064159). We acknowledge that this approach is not ideal and we believe that the implementation of highly CRT techniques should be accompanied by image-guided radiotherapy. Daily image guidance with CBCT will allow us to optimize – and potentially individualize – PTV margins in the future.
In conclusion, we demonstrated that prone position on a bellyboard is associated with a reduced bowel dose in the VMAT setting. Moderate dose escalation with VMAT up to 50 Gy in 2 Gy fractions does not increase the dose to the organs at risk compared to 3D-CRT up to 45 Gy in 1.8 Gy fractions. Future research should focus on ways to further improve the response to RCT without increasing treatment-related toxicity.
Supplementary material available online
Supplementary Appendix and Supplementary Figures 1, 2 Supplementary Table I, II available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1064159.
ionc_a_1064159_sm1169.pdf
Download PDF (190 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355: 1114–23.
- Swellengrebel HA, Marijnen CA, Verwaal VJ, Vincent A, Heuff G, Gerhards MF, et al. Toxicity and complications of preoperative chemoradiotherapy for locally advanced rectal cancer. Br J Surg 2011;98:418–26.
- Baglan KL, Frazier RC, Yan D, Huang RR, Martinez AA, Robertston JM. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys 2002;52:176–83.
- Kavanagh BD, Pan CC, Dawson LA, Das SK, Li XA, Ten Haken RK, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys 2010;76:S101–7.
- Gunnlaugsson A, Kjellén E, Nilsson P, Bendahl PO, Willner J, Johnsson A. Dose-volume relationships between enteritis and irradiated bowel volumes during 5-fluorouracil and oxaliplatin based chemoradiotherapy in locally advanced rectal cancer. Acta Oncol 2007;46:937–44.
- Samuelian JM, Callister MD, Ashman JB, Young-Fadok TM, Borad MJ, Gunderson LL. Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2012;82: 1981–7.
- Wiesendanger-Wittmer EM, Sijtsema NM, Muijs CT, Beukema JC. Systematic review of the role of a belly board device in radiotherapy delivery in patients with pelvic malignancies. Radiother Oncol 2012;102:325–34.
- Kim TH, Chie EK, Kim DY, Park SY, Cho KH, Jung KH, et al. Comparison of the belly board device method and the distended bladder method for reducing irradiated small bowel volumes in preoperative radiotherapy of rectal cancer patients. Int J Radiat Oncol Biol Phys 2005;62:769–75.
- Nijkamp J, Doodeman B, Marijnen C, Vincent A, van Vliet-Vroegindeweij C. Bowel exposure in rectal cancer IMRT using prone, supine, or a belly board. Radiother Oncol 2012;102:22–9.
- Italia C, Fiorino C, Ciocca M, Cattaneo GM, Montanaro P, Bolognesi A, et al. Quality control by portal film analysis in radiotherapy for prostate cancer: A comparison between two different institutions and treatment techniques. Tumori 1998;84:640–8.
- Allal AS, Bischof S, Nouet P. Impact of the “belly board” device on treatment reproducibility in preoperative radiotherapy for rectal cancer. Strahlenther Onkol 2002;178: 259–62.
- Roels S, Duthoy W, Haustermans K, Penninckx F, Vandecaveye V, Boterberg T, et al. Definition and delineation of the clinical target volume for rectal cancer. Int J Radiat Oncol Biol Phys 2006;65:1129–42.
- Cilla S, Deodato F, Digesù C, Macchia G, Picardi V, Ferro M, et al. Assessing the feasibility of volumetric-modulated arc therapy using simultaneous integrated boost (SIB-VMAT): An analysis for complex head-neck, high-risk prostate and rectal cancer cases. Med Dosim 2014;39:108–16.
- Cilla S, Caravatta L, Picardi V, Sabatino D, Macchia G, Digesù C, et al. Volumetric modulated arc therapy with simultaneous integrated boost for locally advanced rectal cancer. Clin Oncol 2012;24:261–8.
- Lobefalo F, Bignardi M, Reggiori G, Tozzi A, Tomatis S, Alongi F, et al. Dosimetric impact of inter-observer variability for 3D conformal radiotherapy and volumetric modulated arc therapy: The rectal tumor target definition case. Radiat Oncol 2013;8:176.
- Kim JY, Kim DY, Kim TH, Park SY, Lee SB, Shin KH, et al. Intensity-modulated radiotherapy with a belly board for rectal cancer. Int J Colorectal Dis 2007;22:373–9.
- Roels S, Verstraete J, Haustermans K. Set-up verification on a belly-board device using electronic portal imaging. J Radiother Pract 2007;6:73–82.
- Suwinski R, Taylor JM, Withers HR. Rapid growth of microscopic rectal cancer as a determinant of response to preoperative radiation therapy. Int J Radiat Oncol Biol Phys 1998;42:943–51.
- O’Donoghue JA. The response of tumours with Gompertzian growth characteristics to fractionated radiotherapy. Int J Radiat Biol 1997;72:325–39.
- Gay HA, Barthold HJ, O’Meara E, Bosch WR, El Naga I, Al-Lozi R, et al. Pelvic normal tissue contouring guidelines for radiation therapy: A Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys 2012; 83:e353–62.
