Abstract
Background: Obesity is a major risk factor of chronic-diseases, including cardiovascular-diseases (CVD). Increasing evidence is showing the association of heat-shock protein (HSP) with type-2 diabetes and CVD; however, there is little data on the relationship between the genetic-polymorphisms of HSP70-2 with obesity.
Aim: The present study has investigated the association between 1267HSP70-2 genetic polymorphism and obesity in an Iranian population with 317 subjects.
Subjects and methods: Anthropometric parameters and biochemical measurements were measured in all the samples, while genotypes were determined using PCR-RFLP. Univariate/multivariate analyses were conducted to explore the relationship between the genetic-polymorphisms and obesity.
Results: The data showed a significant association between 1267HSP70-2 polymorphism in obese subjects, compared to the non-obese group. Moreover, it was observed that this polymorphism was associated with obesity in the CAD + group, which had a high BMI compared to non-obese controls.
Conclusion: The 1267HSP70-2 polymorphism is associated with obesity in an Iranian population, supporting a possible potential genetic link between obesity and cardiovascular diseases.
Introduction
Obesity is one of the major risk factors of metabolic and chronic diseases and its prevalence is increasing worldwide (Jou & Techakehakij, Citation2012; Sakowicz et al., Citation2013). Several studies have identified a number of genetic variants associated with obesity (Yu et al., Citation2012). In the past, genetic studies of obesity were concentrated on the rare monogenic syndromes. However, the associations between genetic polymorphisms and obesity are frequently reported as heterogeneous (Bienertova-Vasku et al., Citation2008; Paracchini et al., Citation2005).
Heat shock proteins (HSPs) belong to a multi-gene family and their molecular weight varies from 8–150 kDa (Whitley et al., Citation1999). Heat and several other stimuli such as cold, UV radiation, alcohol, heavy metal ions, oxidation, low glucose, infection, inflammation and hypoxia can affect heat shock protein expression (Kiang & Tsokos, Citation1998). Heat shock proteins are typically named and classified according to their molecular size. HSP70, a 70 kDa protein, is coded by HSP70 gene (Whitley et al., Citation1999). Three genes that encode members of the HSP70 class are HSP70-1 (HSPA1), HSP70-2 (HSPA1B) and HSP70- hom (HSPA1L). All three of these genes are located in the region of MHC class III (Sargent et al., Citation1989). HSP70-1 and HSP70-2 code similar proteins with 641 amino acids (Milner & Campbell, Citation1990).
Some studies have shown that inflammation and high levels of serum lipids could result in increased accumulation of lipids in the artery wall. Accumulation of lipids induces the secretion of inflammatory cytokines such as tumour necrosis factor-alpha (TNF-α) from macrophages and adipocytes. TNF plays a role in activation of the serine threonine kinase, namely JNK (c-jun amino terminal kinase) and IKK (inhibitor of B kinase) in insulin-responsive (Watt et al., Citation2006). JNK and IKK can lead to insulin resistance by phosphorylation of the IRS-1 (Insulin Receptor Substrate 1) (Hotamisligil, Citation2006) and HSP70-2 expression can inhibit the activation of the JNK and IKK. Induction of HSP70-2 prevents insulin resistance and, thus, is regarded as a potential target in the prevention and treatment of obesity and type 2 diabetes (Chung et al., Citation2008; Hooper & Hooper, Citation2009). These potential properties suggest the HSP70 gene may be a candidate in the aetiology of obesity.
There is a growing body of evidence showing that this gene is associated with type 2 diabetes (Zouari Bouassida et al., Citation2004) and coronary artery disease (Hrira et al., Citation2012). Moreover, in our recent study we reported an association between the HSP70-2 gene +1267A > G polymorphism and cardiovascular disease in 628 Iranian patients (Mardan-Nik et al., Citation2014). In the present study we have investigated further the relationship between HSP70-2 gene polymorphisms and obesity in an Iranian population with 317 subjects.
Materials and methods
Study population
The study population consisted of 317 patients (57.4% male and 42.6% female, aged 35–78 years) who underwent coronary angiography in the Ghaem Hospital Medical Center, Mashhad, Iran (Zomorrodian et al., Citation2015). Of the total group of patients, 94 were obese (BMI ≥ 30). Control subjects (n = 233, 86.1% male and 13.9% female, aged 37–67 years) were healthy volunteers (93 obese and 130 non-obese). Informed consent was taken from all the participants. The study was approved by the Ethics Committee of the Mashhad University of Medical Sciences (MUMS).
Biometric and biochemical measurements
Anthropometric parameters of individuals including weight, height, BMI, waist circumference, hip circumference and waist/hip ratio as well as systolic and diastolic blood pressures were measured as previously described (Emamian et al., Citation2015; Ghayour-Mobarhan et al., Citation2008; Mirhafez et al., Citation2015a). Obesity was defined according to the World Health Organisation (WHO) (Farshidi et al., Citation2010).
Biochemical analysis
A full fasted lipid profile was determined for each subject. Serum lipids and fasting blood glucose (FBG) concentrations were measured by enzymatic methods as previously described (Ghayour-Mobarhan et al. 2008; Mirhafez et al. Citation2015b; Oladi et al., Citation2015).
DNA isolation and SNP selection and genotyping
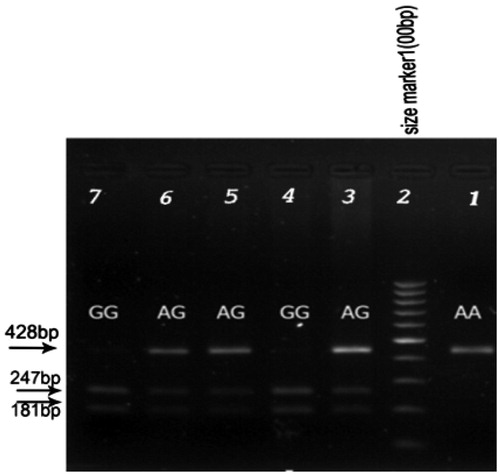
Genomic DNA was extracted from peripheral blood using a commercially available DNA isolation kit (Genet bio, Daejeon, Korea) according to the manufacturer’s protocol. The quality of the DNA (ng/μl) samples was assessed using agarose gel-electrophoresis and the concentration quantities by spectrophotometry (Nano Drop 1000, Thermo Scientific, Wilmington, NC). Genotyping for the rs1061581 polymorphism was carried out using polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) technique. Amplification of HSP70-2 gene was performed using forward and reverse primers as follows: sense 5′-CATCGACTTCTACACGTCC-3′ and antisense 5′-CGGAGTAGGTGGTGAAGATC-3′. The PCR reaction was performed in 25 ml final volume, using 100 ng of genomic DNA, 0.2 mM dNTPs, 2 mM MgCl2, 1 × Taq DNA polymerase buffer, 0.32 pmol each primer and 1 unit of Taq DNA polymerase (Genet bio, Korea). The polymerase chain reaction conditions were as follows: initial denaturation at 95°C for 5 minutes, followed by 30 cycles denaturation at 95°C for 30 seconds, annealing at 61°C for 30 seconds, DNA extension at 72°C for 1 minute and the final extension at 72°C for 7 minutes. All amplification cycles were performed in PCR system Verity 96 well thermocycler (Applied Biosystems, CA). PCR products were digested for 16 hours at 37°C with 1 μl of PstI (Fermentas, Vilnius, Lithuania). For separation of RFLP products, electrophoresis on 1.5% agarose gel with ethidium bromide staining and visualising by UV light was performed. DNA lacking polymorphic PstI site (adenine at 1267 nt) within the HSP70-2 gene produced a fragment of 428 bp (A allele), whereas the presence of 1267 G allele generated two fragments of 247 and 181 bp after PstI digestion (G allele). Finally, a direct sequencing approach (Company of Sequetech, CA) was used to confirm the genotypes obtained by PCR-RFLP for some of the subjects in different groups ().
Figure 1. PCR-RFLP of the amplified segment in 1267HSP70-2 gene. The genotype was labelled on corresponding sequences and the sites which were marked with black arrows were the SNP of HSP70-2 gene. Electrophoresed on 1.5% agarose, stained with ethidium bromide. Lane 2, 100bp DNA ladder. Lane 3, 5 and 6 heterozygote for HSP70-2 genotype, Lane 4 and 7 homozygote GG for HSP70-2 genotype. Lane 1 homozygote AA for HSP70-2 genotype.
Statistical analyses
All data were analysed using the SPSS for Windows, version 22 software package (SPSS Inc, Chicago, IL). The Kolmogorov–Smirnov test was used to test the normality of the variables in each group. Data were expressed as median and interquartile range (IQ3–IQ1) for data with non-normal distribution. χ2 test was used for categorical data. The statistical difference in genotype distribution and allele frequencies between groups was assessed by the χ2 test. Compliance of genotypes with the Hardy-Weinberg equilibrium in each group was also assessed by χ2 test. Binary logistic regression was used to adjust for confounders. A 2-sided p < 0.05 was considered significant.
Results
Demographics and clinical characteristics of population
The demographic and metabolic characteristics of study subjects with and without obesity are presented in . The proportion of individuals who had coronary artery disease and obesity was 57.4% and 42.6% in males and females, respectively. The frequency of obesity observed in male patients was significantly higher compared to the female individuals (p < 0.001). Total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), hip circumference, waist/hip ratio, hs-CRP and FBS were significantly different between patients and control subjects with obesity. There was also no significant difference in the weight, waist circumference, BMI and TG: triglyceride concentrations (p > 0.05). The prevalence of diabetes mellitus and hypertension was significantly higher in the obese case group compared to the obese control group ().
There was a statistically significant difference for anthropometric and biochemical parameters between obese cases and non-obese control subjects except DBP. As would be expected, there were statistically significant differences for hypertension and diabetes between non-obese controls and obese cases ().
Table 1. Demographic and clinical characteristics of the subjects.
Association between 1267HSP70-2 polymorphism and obesity
The distribution of 1267Hsp70-2 genotypes and the allelic frequencies in study subjects are shown in . There was a deviation from the Hardy-Weinberg equilibrium in both obese cases and controls with obesity (p < 0.05). To rule out any genotyping errors, 10% of samples were genotyped. The frequencies of AA, AG and GG genotypes were 35.4%, 53.8% and 10.8% in the non-obese control and 14.9, 68.1% and 17% in obese cases, respectively. There was a significant association in genotype distribution between the two groups (p = 0.002). Compared with the GG genotype of HSP70-2 gene, subjects carrying the AA genotype showed a decreased risk in non-obese controls (p = 0.005, OR = 0.26, 95% CI = 0.105–0.678). Similar values were obtained after adjusting for individuals older than 60 years, sex, LDL-C, FBG, HDL and waist/hip ratio. There was a significant difference in frequency of the A allele between two groups (p = 0.005, OR = 0.580, 95% CI = 0.396–0.848) (). The frequencies of the AA, AG and GG genotypes were also 14.9%, 68.1% and 18.3% in the obese cases and 18.3%, 63.4% and 18.3% in the obese controls, respectively. In a dominant analysis model of the HSP70-2 gene +1267A/G position (AA vs AG + GG), the percentage of subjects who were either homo- or heterozygous for the G allele (1267AG and 1267GG) was significantly higher in obese cases than non-obese controls (85.1% vs 64.1%) (adj. p = 0.002, adj. OR = 4.898; 95% CI = 1.763–13.604) ().
Table 2. Genotype distribution and allele frequencies of 1267HSP70-2 polymorphism in non-obese and obese control groups.
Table 3. Dominant analysis model of HSP70-2 gene + 1267A > G polymorphism in non-obese control and obese control groups.
Table 4. Genotype distribution and allele frequencies of 1267HSP70-2 polymorphism in non-obese control and obese case group.
Table 5. Dominant analysis model of HSP70-2 gene + 1267A > G polymorphism non-obese control and obese case group.
Association between HSP70-2 rs1061583 polymorphism and clinical-biochemical parameters
We also examined the baseline characteristics between the genotype groups in overall groups, but there was no significant difference except SBP and DBP in non-obese controls (data not shown).
Discussion
The complex aetiology of obesity reflects effects of genes and environment as well as their interactions (Bienertova-Vasku et al., Citation2008). The HSP70-2 gene encodes a protein involved in the pathophysiology of obesity and diabetes (Bouchard, Citation2008; Chouchane et al. Citation2001; Chung et al., Citation2008). In this study it was hypothesised that it might be associated with the prevalence of obesity in Iranian population patients with CAD.
To the best of our knowledge this is the first study evaluating the association of 1267HSP70-2 polymorphism with the prevalence of obesity in Iranian patients with CAD. Our results showed a significant decrease of AA genotype in the obese case group when compared with the G allele carriers (GG and AG) (p = 0.005, OR = 0.26, 95% CI = 0105–0.678), suggesting that this genotype may be considered as a protective marker in the non-obese control group. This observation is consistent with previous results that indicated GG genotype in Tunisians was correlated with obesity (Chung et al., Citation2008). A recent report by Zouari Bouassida et al. (Citation2004) demonstrated that the 1267 HSP70-2G > A variant could convey an increased risk for obesity and type 2 diabetes. In addition to the single locus analysis our study indicated that there was no association between 1267HSP70-2 polymorphism in both cases and controls with obesity (p = 0.77). However, allelic and genotypic frequencies for the polymorphism of HAP70-2 + 1267A/G were significantly different between obese and non-obese in the control group (p = 0.013; p = 0.006).
Pociot et al. (Citation1993) also reported that mRNA expression in insulin-dependent diabetes mellitus (IDDM) patients with homozygous GG genotype was decreased compared to the heterozygotes (AG) and hemozygotes (AA).
A major strength of this study is that it was performed in a well-characterised cohort of individuals, with or without obesity; however, the main limitation of this study is the cross-sectional study design and modest sample size. In addition, subjects with Angio − had a significantly different mean age compared to the Angio + group; however, this variable was adjusted for in the logistic regression model. Also, it is possible that other lifestyle characteristics, e.g. diet, have an influence on the outcome.
In conclusion, we demonstrate the significant association of HSP70-2 gene +1267 HSP70-2G > A polymorphisms with obesity and show that subjects with GG genotype or those who carried the G allele were associated with obesity, supporting further studies on evaluating the role and expression level of this emerging marker.
Acknowledgements
This study was supported by a grant from the Research Council of the Mashhad University of Medical Sciences.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bienertova-Vasku J, Bienert P, Tomandl J, Forejt M, Vavrina M, Kudelkova J, Vasku A. 2008. No association of defined variability in leptin, leptin receptor, adiponectin, proopiomelanocortin and ghrelin gene with food preferences in the Czech population. Nutr Neurosci 11:2–8
- Bouchard C. 2008. Gene-environment interactions in the etiology of obesity: defining the fundamentals. Obesity 16:S5–S10
- Chouchane L, Danguir J, Beji C, Bouassida K, Camoin L, Sfar H, Gabbouj S, Strosberg AD. 2001. Genetic variation in the stress protein hsp70-2 gene is highly associated with obesity. Int J Obes Relat Metab Disord 25:462–466
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, et al. 2008. HSP72 protects against obesity-induced insulin resistance. PNAS 105:1739–1744
- Emamian M, Avan A, Pasdar A, Mirhafez SR, Sadeghzadeh M, Moghadam MS, Parizadeh SM, Ferns GA, Ghayour-Mobarhan M. 2015. The lipoprotein lipase S447X and cholesteryl ester transfer protein rs5882 polymorphisms and their relationship with lipid profile in human serum of obese individuals. Gene 558:195–199
- Farshidi H, Nikparvar M, Zare S, Bushehri E, Eghbal Eftekhaari T. 2010. Obesity pattern in south of Iran: 2002-2006. ARYA Atheroscler 4:1
- Ghayour-Mobarhan M, Sahebkar A, Parizadeh SM, Moohebati M, Tavallaie S, RezaKazemi-Bajestani SM, Esmaeili HA, Ferns G. 2008. Antibody titres to heat shock protein 27 are elevated in patients with acute coronary syndrome. Int J Exp Pathol 89:209–215
- Hooper PL, Hooper PL. 2009. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperon 14:113–115
- Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature 444:860–867
- Hrira MY, Chkioua L, Slimani A, Chahed H, Mosbah H, Khaldoun HB, Ferchichi S, et al. 2012. Hsp70-2 gene polymorphism: susceptibility implication in Tunisian patients with coronary artery disease. Diagn Pathol 7:1596–1597
- Jou J, Techakehakij W. 2012. International application of sugar-sweetened beverage (SSB) taxation in obesity reduction: factors that may influence policy effectiveness in country-specific contexts. Health Policy 107:83–90
- Kiang JG, Tsokos GC. 1998. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther 80:183–201
- Mardan-Nik M, Pasdar A, Jamialahmadi K, Biabangard-Zak A, Mirhafez SR, Ghalandari M, Tajfard M, et al. 2014. Association of heat shock protein70-2 gene polymorphism with coronary artery disease in an Iranian population. Gene 550:180–184
- Milner CM, Campbell RD. 1990. Structure and expression of the three MHC-linked HSP70 genes. Immunogenetics 32:242–251
- Mirhafez SR, Avan A, Pasdar A, Kazemi E, Ghasemi F, Tajbakhsh A, Tabaee S, Ferns GA, Ghayour-Mobarhan M. 2015a. Association of tumor necrosis factor-α promoter G-308A gene polymorphism with increased triglyceride level of subjects with metabolic syndrome. Gene 568:81–84
- Mirhafez SR, Pasdar A, Avan A, Esmaily H, Moezzi A, Mohebati M, Meshkat Z, Mehrad-Majd H, Eslami S, Rahimi HR, Ghazavi H, Ferns GA, Ghayour-Mobarhan M. 2015b. Cytokine and growth factor profiling in patients with the metabolic syndrome. Br J Nutr 113:1911–1919
- Oladi M, Nohtani M, Avan A, Mirhafez SR, Tajbakhsh A, Ghasemi F, Asadi A, Elahdadi Salmani M, Mohammadi A, Hoseinzadeh L, Ferns GA, Ghayour Mobarhan M. 2015. Impact of the C1431T Polymorphism of the Peroxisome Proliferator Activated Receptor-Gamma (PPAR-γ) Gene on fasted serum lipid levels in patients with coronary artery disease. Ann Nutr Metab 66:149–154
- Paracchini V, Pedotti P, Taioli E. 2005. Genetics of leptin and obesity: a HuGE review. AJE 162:101–114
- Pociot F, Rønningen K, Nerup J. 1993. Polymorphic Analysis of the Human MHC-Linked Heat Shock Protein 70 (HSP70-2) and HSP70-Hom Genes in Insulin-Dependent Diabetes Mellitus (IDDM). Scand J Immunol 38:491–495
- Sakowicz A, Stępniak D, Hejduk P, Pietrucha T. 2013. The genetics of metabolic syndrome. Med Sci Technol 54:48–53
- Sargent CA, Dunham I, Trowsdale J, Campbell RD. 1989. Human major histocompatibility complex contains genes for the major heat shock protein HSP70. PNAS 86:1968–1972
- Watt MJ, Hevener A, Lancaster GI, Febbraio MA. 2006. Ciliary neurotrophic factor prevents acute lipid-induced insulin resistance by attenuating ceramide accumulation and phosphorylation of c-Jun N-terminal kinase in peripheral tissues. Endocrinology 147:2077–2085
- Whitley D, Goldberg SP, Jordan WD. 1999. Heat shock proteins: a review of the molecular chaperones. JVS 29:748–751
- Yu Z, Han S, Cao X, Zhu C, Wang X, Guo X. 2012. Genetic polymorphisms in adipokine genes and the risk of obesity: a systematic review and meta-analysis. Obesity 20:396–406
- Zomorrodian D, Khajavi-Rad A, Avan A, Ebrahimi M, Nematy M, Azarpazhooh MR, Emamian M, Sadeghzade M, Mirhafez SR, Mohammadi M, Mousavi M, Esmaeili H, Moohebati M, Parizadeh MR, Ferns GA, Ghayour-Mobarhan M. 2015. Metabolic syndrome components as markers to prognosticate the risk of developing chronic kidney disease: evidence-based study with 6492 individuals. J Epidemiol Community Health 69:594–598
- Zouari Bouassida K, Chouchane L, Jellouli K, Chérif S, Haddad S, Gabbouj S, Danguir J. 2004. Polymorphism of stress protein HSP70-2 gene in Tunisians: susceptibility implications in type 2 diabetes and obesity. DMJ 30:175–180
