Abstract
This report summarises the proceedings of a conference organised by the Italian Association of Hospital Cardiologists. The aim was to consider the process by which dietary guidelines (DG) are developed and the quality of evidence underpinning these guidelines, as well as debating whether or not this has resulted in DG that are effective in terms of health improvement. Key points were a caution about false positives in research, the importance of holistic DG rather than single nutrient targets, the need for appropriate disease endpoints in studies and control of confounders, a plea for less reliance on observational studies which cannot address cause-and-effect relationships and a need to bear in mind unintended consequences. Options for improving the system and the quality of evidence were discussed.
Introduction
Dietary guidelines (DG), defined here as population-based nutrition targets, are an important aspect of public health nutrition and have been determined by expert bodies worldwide (World Health Organisation, Citation2003), at European level (European Food Safety Authority, Citation2010a) and in individual countries (Department of Health, Citation1991; US Department of Agriculture & Department of Health and Human Services, Citation2010). Macronutrient-specific guidelines, in particular, those relating to fat and sugar, are typically used to underpin policy or communications tools such as food labelling, food-based DG, nutrient profiling and health marks (European Food Safety Authority, Citation2010b). In addition, benchmarking of population dietary intakes against DG is often a spur for government intervention when progress appears to be slow. An example is the UK’s ban on advertising to children for foods classified as high in fat, sugar or salt (Office of Communications, Citation2007).
Given the importance of DG to health promotion and policy development, it would seem essential to base these on the best available evidence and to update them at regular intervals as the evidence evolves. However, in practise, this ideal may be limited by the large and complex evidence base, a lack of controlled, human trials, use of disease markers rather than actual endpoints, non-comparable methodologies and difficulties in achieving expert consensus. However, these limitations can be addressed by setting principles for DG development and giving more weight to better quality evidence (European Food Safety Authority, Citation2010b).
Recent public debates have focussed on the respective roles of dietary fat and sugar in chronic disease aetiology with calls for the blanket promotion of low fat or low saturated fat diets to be re-evaluated (German & Dillard, Citation2004) in the light of evidence suggesting a lack of impact on total and cardiovascular disease (CVD) mortality (Hooper et al., Citation2011). Concern has also been directed at the inference in DG that fat reduction should occur in tandem with an increase in the proportion of energy derived from carbohydrates as some types of these are implicated in dyslipidaemia and the development of type 2 diabetes (Astrup et al., Citation2011; Lichtenstein et al., Citation1998; Siri-Tarino et al., Citation2010).
In particular, sugar has attracted controversy, in part due to differences in expert opinion about its role in chronic disease development and whether or not an optimal upper level of intake can be defined. Widely different conclusions have been drawn by the World Health Organisation (WHO), which proposed a 5% energy population target for added sugars based on ecological studies on dental caries (World Health Organisation, Citation2015a) and the European Food Safety Authority, which declined to set an upper limit for sugars due to a lack of consistent, high-quality evidence (European Food Safety Authority, Citation2010c). From a dental perspective, the frequency of sugar consumption may be as important as the amount (European Food Safety Authority, Citation2010c) highlighting the challenge of setting one nutrient-specific DG to address several health concerns.
At the least, compliance with DG should not cause harm and should preferably confer health benefits on a population. This may not be the case for obesity prevalence which has risen from 42% to 66% of the US adult population between 1971 and 2011 despite a reduction in fat consumption from 45% to 34% total energy with a corresponding increase in carbohydrate consumption from 39% to 51% total energy (Cohen et al., Citation2015). In Australia, DG adherence in children was not found to be associated with lipid profiles (Golley et al., Citation2015) nor adiposity (Golley et al., Citation2011). Greater concordance with the US DASH diet was not associated with a lower risk of hypertension or CVD mortality in a large cohort of women (Folsom et al., Citation2007) while evidence linking fruit and vegetable consumption with the prevention of primary CVD is lacking (Hartley et al., Citation2013). In contrast, higher diet quality, represented by better compliance with DG, was consistently associated with an 11–28% reduced risk of death due to all causes, CVD, and cancer in an analysis of several large US cohorts (Liese et al., Citation2015). Similar associations have been noted in Chinese (Yu et al., Citation2014) and European populations (Merino et al., Citation2015). A systematic review of compliance with a Mediterranean-type diet found some benefits (Rees et al., Citation2013).
The success of current DG and the scientific basis on which they are developed was the topic of a recent conference held in Florence under the auspices of the Italian Association of Hospital Cardiologists. The present paper summarises the proceedings of this meeting.
Prof. Carlo La Vecchia (Italy) described the adverse impact of false positive results on the body of evidence used to inform DG. False positives are statistically significant associations between the risk of disease and nutrients or foods which are due to chance or bias rather than real cause-and-effect relationships. False positives have the potential to skew evidence leading to wasted time and resources attempting to confirm the findings through additional research. False positives may also lead to alarmist media stories as was the case when studies linked coffee consumption with myocardial infarction and pancreatic cancer (Grioni et al., Citation2015; Kuper et al., Citation2000). As Prof. La Vecchia explained, the problem had arisen because coffee consumption is strongly correlated with cigarette smoking and this had not been properly accounted for in the statistical analysis. Later meta-analyses disputed a cause-and-effect relationship between coffee and myocardial infarction (Crippa et al., Citation2014; Malerba et al., Citation2013). Finally, public confidence in health professionals may be challenged when false positives result in ineffective health messages which then change several months or years after publication.
Prof. La Vecchia described several reasons for false positives in nutrition research. First, the margin for error in observational studies is far wider than for randomised controlled trials (RCT). Thus, while a 10% change in risk in a RCT can be both statistically and clinically significant, this is not the case for a 10–20% change in risk in an observational study. A relative risk of 0.80–0.90 or 1.1–1.2 may be statistically significant due to a large sample size but, as a result of bias and confounders may not, in fact, represent a real relationship. This suggests that statistically significant changes in relative risk should be treated with caution unless actual differences are large. Second, publication bias enhances the likelihood of false positives as statistically significant results are more likely to be published than null results or those which go against the prevailing trend. Third, an accumulation of false positives, particularly from early small studies, and a dearth of null or opposing results may influence the results of meta-analyses in the wrong direction. A review of the findings of 55 meta-analyses on genetics found that associations reported by earlier studies were replicated, without evidence of heterogeneity and bias, in only 16% of cases (Ioannidis et al., Citation2003). Thus, evidence which appears to be consistent may actually be skewed leading to incorrect or ineffective DG. Fourth, the dietary methodologies used in observational studies, i.e. food frequency questionnaires or diet diaries, may encourage false positives due to the sheer number of variables they can produce; hundreds of individual foodstuffs plus 20–30 different nutrients (Decarli et al., Citation1996).
According to Prof. La Vecchia, the scientific community needs to remain vigilant about the unhelpful effect that false positives may have on public health advice and DG. Actions that could help include registering advance dissemination plans for all nutrition studies, improving the control of confounding variables, raising the statistical bar for observational study results to account for multiple comparisons and being cautious in the clinical interpretation of statistically significant associations unless supported by additional lines of evidence.
Prof. Dennis Bier (USA) discussed the evolution of dietary fat recommendations and questioned their scientific basis. In the 1970s, the Seven Countries Study (Keys, Citation1970) prompted fairly universal messages in developed countries to lower total fat, saturated fat and dietary cholesterol. Yet, the data from the Seven Countries Study were not straightforward – in most countries, individuals with a similar serum cholesterol level had widely different CVD outcomes (Ravnskov, Citation1995) indicating that the apparent relationship between serum cholesterol and CVD morbidity was influenced by other lifestyle factors.
This has been confirmed in subsequent studies such as the Minnesota Coronary Survey (Frantz et al., Citation1989) which tested the efficacy of a reduced saturated fat/reduced cholesterol diet on a randomised sample of 4393 institutionalised adults over a 4.5-year period. Despite a fall in serum cholesterol in the reduced fat group, there were no significant changes in the incidence of myocardial infarctions, sudden deaths or all-cause mortality. A meta-analysis found contradictory evidence for the apparent CVD benefits of dietary fat reduction, except when polyunsaturated fat was increased at the expense of saturated fat (Mozaffarian et al., Citation2010). This may be because all dietary fats increase total cholesterol and high-density lipoprotein cholesterol (HDL-c) but only saturated fats and trans fats increase low-density lipoprotein cholesterol (LDL-c) (Micha & Mozaffarian, Citation2010) (). More recently, a Cochrane review concluded that, while saturated fat reduction reduced the risk of cardiovascular events by 17%, the impact on total and cardiovascular mortality was less clear and statistically non-significant in many cases (Hooper et al., Citation2015).
Figure 1. Differing impact of fatty acids on low-density lipoprotein cholesterol. Source: Based on Micha & Mozaffarian (Citation2010). Key: LDL-c, low-density lipoprotein cholesterol; PUFA, polyunsaturated fatty acids; MUFA, monounsaturated fatty acids; SFA, saturated fatty acids; CHO, carbohydrate.
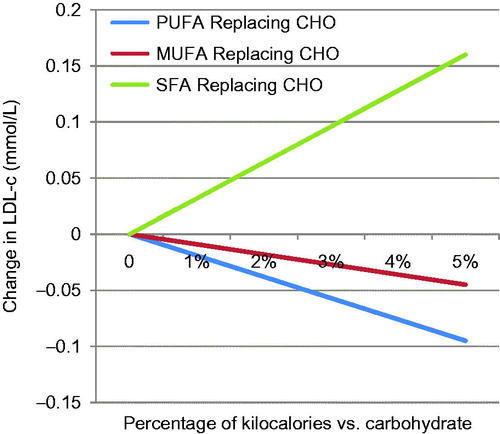
As Prof. Bier explained, carbohydrates are now known to increase small dense LDL-c explaining why reduced fat, high carbohydrate diets often do not lead to improvements in CVD mortality risk (Mozaffarian et al., Citation2010). Recent studies have tended to show a smaller effect size in CVD mortality following dietary interventions, e.g. increased intakes of long-chain n3 fatty acids (Rizos et al., Citation2012), possibly due to the influence of reduced rates of smoking and better medical care, including statins. Thus, it is clear from the example of DG for fats that a focus on one nutrient, or a single food as in the case of eggs which were limited due to concerns about dietary cholesterol, has not resulted in an effective strategy for dealing with CVD mortality. Indeed, both dietary cholesterol and eggs have now been rehabilitated in the UK (Gray & Griffin, Citation2009) and US DG (Federal Panel on Dietary Guidelines for Americans 2015 Committee, Citation2015).
Dietary interventions are much more complex than pharmaceutical interventions as diets are individual and represent an array of nutrients and bioactive substances which act individually or in combination to influence disease risk. Thus, adding or removing one nutrient or food group affects not just what is eaten, but what is left out of the diet. For this reason, Prof. Bier suggested that DG and their resulting public health messages should take a holistic whole-diet approach, rather than demonising individual nutrients or foods which could lead to imbalanced diets.
Prof. Luc Tappy (Switzerland) raised concerns about the choice of disease markers in studies and interrelationships between obesity and chronic disease which may act as confounders when considering which dietary factors contribute to CVD aetiology.
Risk factors for CVD, as identified by WHO, include unhealthy diets, low levels of physical activity, tobacco and excessive alcohol intake (World Health Organisation, Citation2015b). Yet, obesity is associated with an “unhealthy diet”, but also represents an independent risk factor for CVD (Chobanian et al., Citation2003) as well as a contributing factor to other CVD risk factors, such as type 2 diabetes, hypertension and dyslipidaemia (Fox et al., Citation2007). Within these correlations, Prof. Tappy suggested that visceral fat estimated by waist-to-hip ratio is a much better predictor of disease risk than body mass index (BMI) (Ohlson et al., Citation1985) (). This is because visceral fat releases fatty acids and hormones into the portal system and promotes an inflammatory state. Obesity also represents a risk factor for the development of fatty liver, leading in some cases to non-alcoholic fatty liver disease and cirrhosis. Here, intrahepatic fat is a better marker of metabolic complications than visceral fat, with strong correlations reported between intrahepatic fat and glucose tolerance (Fabbrini et al., Citation2009).
Figure 2. The importance of abdominal fat as a risk factor for type 2 diabetes. Source: Based on Ohlson et al. (Citation1985). Key: BMI, body mass index with tertile I representing lower BMI; WHR, the waist-to-hip ratio with tertile I representing lower WHR.
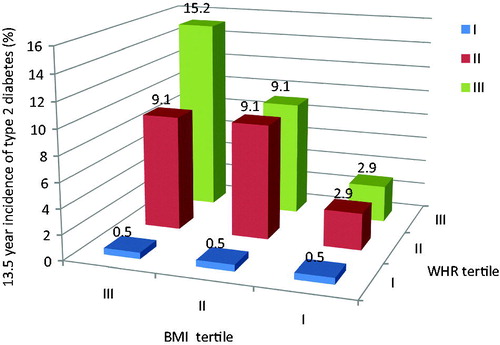
Turning to dietary recommendations, there has been interest in the role of fructose in the development of both obesity and non-alcoholic fatty liver disease. Prof. Tappy explained that some commentators claim that fructose stimulates de novo lipogenesis which increases the risk of intrahepatic fat (Perito et al., Citation2013). However, such views are based mainly on observational and animal studies as few human RCT are available. In a recent RCT (Lecoultre et al., Citation2013), overfeeding with fructose, glucose and fat all prompted significant increases in intrahepatic fat compared with a weight-maintenance diet suggesting that excess energy, not fructose per se, is the issue. This was confirmed by another RCT (Johnston et al., Citation2013) which found that excess energy intake as glucose or fructose let to increased intrahepatic fat stores, while the same amounts of glucose or fructose in weight-maintenance diets did not.
Prof. Tappy suggested that researchers should ensure that relationships between diet and chronic disease are real rather than modulated by obesity or excess energy intakes. Using the right markers in studies is important, e.g. visceral fat instead of BMI, and ensuring that study methodologies are designed to address the hypothesis rather than data being subjected to repeated secondary analyses to answer different research questions.
Ms Sigrid Gibson (UK) expanded on the theme of using the correct markers of disease by pointing out that BMI can misclassify individuals as around a quarter of adults with a normal BMI are at risk of CVD due to high visceral fat (Ashwell et al., Citation2012,Citation2014). A focus on weight or BMI also ignores the impact of exercise and sedentary behaviour on mortality (Ashwell & Gibson, Citation2014) and disease risk (Ekelund et al., Citation2015).
In terms of obesity, Ms. Gibson opined that the basic energy balance equation remains valid: that all sources of energy contribute to risk. Energy-dense diets are a key driver of overconsumption and weight gain, but there are important differences between macronutrients. Protein offers a short-term advantage as it may enhance satiety (Johnstone et al., Citation2008) while fat and alcohol are more energy dense than protein and carbohydrates. Over the long-term, both high protein and high carbohydrate energy-restricted diets produce similar weight loss (Naude et al., Citation2014) although high carbohydrate diets may offer a gut health advantage as they tend to be higher in fibre which stimulates butyrate-producing bacteria (Duncan et al., Citation2007).
Ms. Gibson suggested that dietary energy restriction remains the cornerstone of weight management but has limitations in severe obesity as the dramatic energy deficit needed to make an impact is difficult to sustain and requires a concurrent increase in micronutrient density to ensure that wider DG are met. This is where physical activity can play a valuable role.
Turning to general DG, the recent attention given to sugar has deflected attention from dietary patterns and may have unintended consequences such as avoidance of fruit or baked beans. Ms. Gibson noted that WHO’s recommendation that mean population sugar intakes remain <10% total energy was based on observational studies on dental caries, not obesity, while the <5% recommendation was based on ecological studies only (World Health Organisation, Citation2015a). Sugar-sweetened soft drinks have been associated with BMI and type 2 diabetes risk (Scientific Advisory Committee on Nutrition, Citation2014) but evidence for a similar association with other sources of sugars is lacking suggesting that overconsumption may be easier with liquids. A meta-analysis (Te Morenga et al., Citation2013), commissioned by WHO, concluded that the impact of added sugars on weight gain appeared to be mediated by changes in energy intakes, rather than an effect of sugars per se. In addition, the overall mean weight loss associated with sugar restriction, quoted in the meta-analysis, amounted to 0.75 kg which is unlikely to be clinically significant given that a successful intervention would be expected to deliver a weight loss of >10% baseline weight (National Heart, Lung and Blood Institute, Citation1998).
Therefore, Ms. Gibson concluded by saying that public health experts should guard against setting DG based on poor quality evidence, using insensitive markers such as BMI, and where conclusions have been extrapolated from one health outcome to another. These actions may reduce the efficacy, and thus credibility, of DG.
Prof. Aldo Maggioni (Italy) used examples of pharmacological trials to highlight the challenges of designing studies to prevent major chronic diseases. Despite impressive reductions in CVD mortality risk in developed countries, heart disease and stroke still account for around one-third of deaths worldwide (Unal et al., Citation2004). Reasons for the decline in incidence include dietary improvements, smoking cessation and medical interventions to target hypertension, clotting disorders and dyslipidaemia.
Low HDL-c and high LDL-c remain the most important metabolic risk factors for CVD. However, as RCT show, targets for modulating these lipoproteins are still not met even when patients are given statins (Gitt et al., Citation2012). This is due to poor compliance in patients, 30% of whom may cease statin use after 1 year according to a review of medical records (Maggioni et al., Citation2014). Trials also show that some patients do not respond to statins and may benefit from other combinations of drugs (Robinson et al., Citation2015) including those that target type 2 diabetes (White et al., Citation2013) and obesity (James et al., Citation2010). In the case of obesity, pharmacological research has lagged well behind other conditions and there are few approved drugs to manage obesity.
To sum up, Prof. Maggioni suggested that the example of pharmacological trials highlights the issues of non-compliance and poor clinical response which could have implications for DG.
Prof. Furio Brighenti (Italy) began with the key point that nutrition research differs considerably from drug trials. This is because blinding is often impossible, diets are complex and contain multiple active components, intervention foods can displace other foods which may be exerting a health effect, there is a unique behavioural element of food choice, exposure cannot be fully determined due to differences in bioavailability, and dosage can be based on body weight, percentage energy intake or absolute amounts, making interpretation and wider applicability of findings difficult.
While many aspects of dietary studies may seem flawed, in particular, dietary assessment and nutritional composition of foods, these tools are still the best available. Therefore, the focus should be on making these tools as accurate and reproducible as possible, and by effectively translating nutrition research findings into DG. This can be done in a number of ways as described by Prof. Brighenti.
First, good evidence is required on the function of nutrients and how different exposures are likely to impact on health. Second, the quality of the available evidence needs to be considered as epidemiological surveys produce weaker and often less consistent evidence than RCT (Tai et al., Citation2014). Third, it is important to be certain, using supporting evidence, that associations revealed in observational studies represent cause-and-effect relationships (Mente et al., Citation2009). Fourth, information is needed on the effective dose or intake required by different populations. Fifth, the risk-benefit needs to be addressed, typically by health economists. The key steps in determining a causal link between food/nutrients and health are summarised in .
Table 1. Key steps in determining a causal link between a food/constituent and health.
The cost of carrying out nutrition studies often limits the scope and depth of investigations. For example, cost and time limitations determine that short-term or intermediate markers of health, such as blood pressure, serum lipids and faecal toxins are used instead of actual disease endpoints. Lack of expertise on ethics committees can also limit the scope of food studies as few members have direct nutrition experience and food trials represent less than 1% of proposals considered by ethics committees (Brighenti, unpublished results). In terms of dissemination, so-called “White hat bias” may result in the suppression of nutrition evidence which is contrary to previous findings or to prevailing beliefs. This can limit the efficacy of DG.
Yet, as Prof. Brighenti explained, these barriers must be overcome as nutrition evidence remains vitally important in many areas of health such as evaluating the effectiveness of diet therapies for diseases, justifying health claims on foodstuffs, creating evidence-based health messages for populations, establishing dietary reference values and helping at risk populations to lower their chances of developing disease.
Prof. Attilio Maseri (Italy) used the example of outliers in studies to highlight some limitations in using intervention trials to underpin DG. Consistent findings in RCT should not be expected every time as populations selected for a characteristic and believed to be homogenous may actually differ considerably in other areas which could impact on health outcomes. Thus, unexpected findings may not mean that the intervention was ineffective, but that another factor, perhaps unmeasured, influenced the finding.
An example given by Prof. Maseri was the FAMI study (Cristell et al., Citation2011) which collected data on 1047 individuals from three ethnically different countries. Attempts were made to relate C-reactive protein (CRP) to the risk of myocardial infarction. However, it was discovered that patients and controls had similar CRP levels and there was more variation within groups than between groups. This was also the case when looking at ethnic variations in CRP.
Whether RCTs are on nutrition or drug interventions, some patients will benefit while others do not. While DG should reflect benefits likely to be experienced by the majority within a population, interventions that may benefit sub-groups of people can still be relevant for individual dietary prescriptions.
Conclusion
The conference speakers made several important points:
As far as possible, DG should be based on cause-and-effect relationships determined by several lines of enquiry, not just observational studies.
Unexpected results, or those which conflict with existing views or evidence, should not be ignored or suppressed.
Markers of disease risk should be appropriate.
There should be increased awareness of potential confounders and false positives.
Secondary analyses of large datasets should be intelligently designed ensuring that the methods, outcome variables and statistics are appropriate for addressing the new hypothesis.
Better methodologies, more knowledgeable ethical committee input and reduced publication bias could improve the quality of nutrition research published.
DG should take a holistic view, rather than focussing on single nutrients or food groups, and should fit with wider lifestyle advice such as promoting physical activity.
Declaration of interest
The author received funding from SOREMARTEC (Italy) to attend the conference and write the report with the permission of the organisers, the Italian Association of Hospital Cardiologists.
References
- Ashwell M, Gibson S. 2014. A proposal for a primary screening tool: ‘keep your waist circumference to less than half your height’. BMC Med 12:207.
- Ashwell M, Gunn P, Gibson S. 2012. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev 13:275–286.
- Ashwell M, Mayhew L, Richardson J, Rickayzen B. 2014. Waist-to-height ratio is more predictive of years of life lost than body mass index. PLoS One 9:e103483.
- Astrup A, Dyerberg J, Elwood P, Hermansen K, Hu FB, Jakobsen MU, Kok FJ, et al. 2011. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: where does the evidence stand in 2010? Am J Clin Nutr 93:684–688.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, et al. 2003. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289:2560–2572.
- Cohen E, Cragg M, deFonseka J, Hite A, Rosenberg M, Zhou B. 2015. Statistical review of US macronutrient consumption data, 1965–2011: Americans have been following dietary guidelines, coincident with the rise in obesity. Nutrition 31:727–732.
- Crippa A, Discacciati A, Larsson SC, Wolk A, Orsini N. 2014. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol 180:763–775.
- Cristell N, Cianflone D, Durante A, Ammirati E, Vanuzzo D, Banfi M, Calori G, et al. 2011. High-sensitivity C-reactive protein is within normal levels at the very onset of first ST-segment elevation acute myocardial infarction in 41% of cases: a multiethnic case-control study. J Am Coll Cardiol 58:2654–2661.
- Decarli A, Franceschi S, Ferraroni M, Gnagnarella P, Parpinel MT, La Vecchia C, Negri E, et al. 1996. Validation of a food-frequency questionnaire to assess dietary intakes in cancer studies in Italy. Results for specific nutrients. Ann Epidemiol 6:110–118.
- Department of Health. (1991) Dietary reference values for food energy and nutrients for the United Kingdom. Report of the panel on dietary reference values of the Committee on Medical Aspects of Food Policy. London: HMSO.
- Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. 2007. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol 73:1073–1078.
- Ekelund U, Ward HA, Norat T, Luan J, May AM, Weiderpass E, Sharp SJ, et al. 2015. Physical activity and all-cause mortality across levels of overall and abdominal adiposity in European men and women: the European Prospective Investigation into Cancer and Nutrition Study (EPIC). Am J Clin Nutr 101:613–621.
- European Food Safety Authority (EFSA). 2010a. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J 8:1461.
- European Food Safety Authority (EFSA). 2010b. Scientific Opinion on principles for deriving and applying Dietary Reference Values. EFSA J 8:1458.
- European Food Safety Authority (EFSA). 2010c. Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J 8:1462.
- European Food Safety Authority (EFSA). 2011. Scientific and technical guidance for the preparation and presentation of an application for authorisation of a health claim. EFSA J 9:2170.
- Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, et al. 2009. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA 106:15430–15435.
- Federal Panel on Dietary Guidelines for Americans 2015 Committee. (2015) Draft report. [Online]. Available at: www.health.gov/dietaryguidelines/2015-BINDER/meeting7/docs/DGAC-Meeting-7-SC-1.pdf. Accessed on 21 May 2015.
- Folsom AR, Parker ED, Harnack LJ. 2007. Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. Am J Hypertens 20:225–232.
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, et al. 2007. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116:39–48.
- Frantz Jr ID, Dawson EA, Ashman PL, Gatewood LC, Bartsch GE, Kuba K, Brewer ER. 1989. Test of effect of lipid lowering by diet on cardiovascular risk. The Minnesota Coronary Survey. Arteriosclerosis 9:129–135.
- German JB, Dillard CJ. 2004. Saturated fats: what dietary intake? Am J Clin Nutr 80:550–559.
- Gitt AK, Drexel H, Feely J, Ferrières J, Gonzalez-Juanatey JR, Thomsen KK, Leiter LA, et al. 2012. Persistent lipid abnormalities in statin-treated patients and predictors of LDL-cholesterol goal achievement in clinical practice in Europe and Canada. Eur J Prev Cardiol 19:221–230.
- Golley RK, Hendrie GA, McNaughton SA. 2011. Scores on the dietary guideline index for children and adolescents are associated with nutrient intake and socio-economic position but not adiposity. J Nutr 141:1340–1347.
- Golley RK, McNaughton SA, Hendrie GA. 2015. A dietary guideline adherence score is positively associated with dietary biomarkers but not lipid profile in healthy children. J Nutr 145:128–133.
- Gray J, Griffin B. 2009. Eggs and dietary cholesterol – dispelling the myth. Nutr Bull 34:66–70.
- Grioni S, Agnoli C, Sieri S, Pala V, Ricceri F, Masala G, Saieva C, et al. 2015. Espresso coffee consumption and risk of coronary heart disease in a large Italian cohort. PLoS One 10:e0126550.
- Hartley L, Igbinedion E, Holmes J, Flowers N, Thorogood M, Clarke A, Stranges S, et al. 2013. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev 6:CD009874.
- Hooper L, Martin N, Abdelhamid A, Davey Smith G. 2015. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev 6:CD011737.
- Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, Moore H, Davey Smith G. 2011. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev 7:CD002137.
- Ioannidis JP, Trikalinos TA, Ntzani EE, Contopoulos-Ioannidis DG. 2003. Genetic associations in large versus small studies: an empirical assessment. Lancet 361:567–571.
- James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, Torp-Pedersen C, et al. 2010. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 363:905–917.
- Johnston RD, Stephenson MC, Crossland H, Cordon SM, Palcidi E, Cox EF, Taylor MA, et al. 2013. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology 145:1016.e2–1025.e2.
- Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. 2008. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr 87:44–55.
- Keys A (ed.). 1970. Coronary heart disease in seven countries. Circulation 41:1–200.
- Kuper HE, Mucci LA, Trichopoulos D. 2000. Coffee, pancreatic cancer and the question of causation. J Epidemiol Community Health 54:650–651.
- Lecoultre V, Egli L, Carrel G, Theytaz F, Kreis R, Schneiter P, Boss A, et al. 2013. Effects of fructose and glucose overfeeding on hepatic insulin sensitivity and intrahepatic lipids in healthy humans. Obesity (Silver Spring) 21: 782–785.
- Lichtenstein AH, Kennedy E, Barrier P, Danford D, Ernst ND, Grundy SM, Leveille GA, et al. 1998. Dietary fat consumption and health. Nutr Rev 56:S3–S19.
- Liese AD, Krebs-Smith SM, Subar AF, George SM, Harmon BE, Neuhouser ML, Boushey CJ, et al. 2015. The Dietary Patterns Methods Project: synthesis of findings across cohorts and relevance to dietary guidance. J Nutr 145:393–402.
- Maggioni AP, Rossi E, Cinconze E, De Rosa M, ARNO Cardiovascular Observatory. 2014. Use and misuse of statins after ACS: analysis of a prescription database of a community setting of 2,042,968 subjects. Eur J Prev Cardiol 21: 1109–1116.
- Malerba S, Turati F, Galeone C, Pelucchi C, Verga F, La Vecchia C, Tavani A. 2013. A meta-analysis of prospective studies of coffee consumption and mortality for all causes, cancers and cardiovascular diseases. Eur J Epidemiol 28:527–539.
- Mente A, de Koning L, Shannon HS, Anand SS. 2009. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med 169:659–669.
- Merino J, Guasch-Ferré M, Martínez-González MA, Corella D, Estruch R, Fitó M, Ros E, et al. 2015. Is complying with the recommendations of sodium intake beneficial for health in individuals at high cardiovascular risk? Findings from the PREDIMED study. Am J Clin Nutr 101:440–448.
- Micha R, Mozaffarian D. 2010. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids 45:893–905.
- Mozaffarian D, Micha R, Wallace S. 2010. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 7:e1000252.
- National Heart, Lung, and Blood Institute. 1998. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res. 6:51S–210S.
- Naude CE, Schoonees A, Senekal M, Young T, Garner P, Volmink J. 2014. Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: a systematic review and meta-analysis. PLoS One 9:e100652.
- Office of Communications (Ofcom). (2007). Television advertising of food and drink products to children. Final statement. London: Ofcom.
- Ohlson LO, Larsson B, Svärdsudd K, Welin L, Eriksson H, Wilhelmsen L, Björntorp P, et al. 1985. The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes 34:1055–1058.
- Perito ER, Rodriguez LA, Lustig RH. 2013. Dietary treatment of nonalcoholic steatohepatitis. Curr Opin Gastroenterol 29:170–176.
- Ravnskov U. 1995. Quotation bias in reviews of the diet-heart idea. J Clin Epidemiol 48:713–719.
- Rees K, Hartley L, Flowers N, Clarke A, Hooper L, Thorogood M, Stranges S. 2013. ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 8:CD009825.
- Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. 2012. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 308:1024–1033.
- Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, et al. 2015. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 372:1489–1499.
- Scientific Advisory Committee on Nutrition. (2014). Draft carbohydrates and health report. London: Public Health England.
- Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. 2010. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr 91:502–509.
- Tai V, Grey A, Bolland MJ. 2014. Results of observational studies: analysis of findings from the Nurses’ Health Study. PLoS One 9:e110403.
- Te Morenga L, Mallard S, Mann J. 2013. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 346:e7492.
- Unal B, Critchley JA, Capewell S. 2004. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation 109:1101–1107.
- US Department of Agriculture & US Department of Health and Human Services. (2010). Dietary guidelines for Americans. 7th ed. Washington (DC): US Government Printing Office.
- White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, et al. 2013. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 369:1327–1335.
- World Health Organisation (WHO). (2015a). Sugars intake for adults and children. Geneva: WHO.
- World Health Organisation (WHO). (2015b). Cardiovascular diseases. Fact sheet N°317. [Online]. Available at: www.who.int/mediacentre/factsheets/fs317/en/. Accessed on 22 May 2015.
- World Health Organisation (WHO) and Food and Agriculture Organization of the United Nations (FAO). (2003). Diet, nutrition and the prevention of chronic diseases. WHO Technical Report Series 916. Geneva: FAO/WHO.
- Yu D, Zhang X, Xiang YB, Yang G, Li H, Gao YT, Zheng W, et al. 2014. Adherence to dietary guidelines and mortality: a report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am J Clin Nutr 100:693–700.
