Abstract
Single-agent dacetuzumab has demonstrated antitumor activity in relapsed/refractory diffuse large B-cell lymphoma (DLBCL). Preclinical data demonstrated improved dacetuzumab antitumor activity in combination with rituximab, ± chemotherapy. We designed a phase 2b, double-blind, placebo-controlled trial to compare rituximab, ifosfamide, carboplatin and etoposide (R-ICE) + dacetuzumab with R-ICE + placebo in patients with DLBCL who relapsed after rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) (ClinicalTrials.gov #NCT00529503). The primary endpoint was complete response (CR); additional endpoints included failure-free survival and overall survival (OS). Overall, 151 patients were randomized (75 dacetuzumab, 76 placebo). No notable differences between arms in demographics or subsequent treatment parameters were observed. Cytopenias, cough and infection were more frequent with dacetuzumab. Futility analysis failed to demonstrate higher CR rates with dacetuzumab (36% dacetuzumab, 42% placebo); consequently, enrollment was stopped. Unplanned post hoc analysis showed that patients who underwent subsequent autologous stem cell transplant experienced improvement in OS (hazard ratio = 0.195, p = 0.004), which may be explained by potential immunomodulatory effects of dacetuzumab on antigen-presenting cells.
Introduction
For patients with diffuse large B-cell lymphoma (DLBCL), rituximab (R) plus CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) is the standard front-line chemotherapy. However, for the significant number of patients who subsequently relapse, aggressive salvage therapy and autologous stem cell transplant (aSCT) can only cure a relatively small minority. A recent randomized trial by Gisselbrecht and colleagues compared two common salvage regimens, R-ICE (R, ifosfamide, carboplatin, etoposide) vs. R-DHAP (R, methylprednisolone, cytarabine, platinum), and demonstrated similar overall and complete response rates of 63% and 38%, respectively. There was no significant difference in 3-year event-free survival after R-ICE (26%) vs. after R-DHAP (35%) [Citation1,Citation2]. Adverse prognostic factors to survival in this trial were international prognostic index (IPI) > 1, prior R exposure and relapse in less than 12 months. Given these results, and other similar reports, there is significant opportunity to improve outcomes for patients with relapsed DLBCL.
CD40 is a type-1 transmembrane protein of the tumor necrosis factor receptor superfamily. CD40 is expressed on B cells and their progenitors, epithelial and endothelial cells and many antigen-presenting cells (dendritic cells, activated B lymphocytes and activated monocytes) [Citation3,Citation4]. In addition, CD40 is expressed on most malignancies of B-cell origin, including non-Hodgkin lymphoma, multiple myeloma and chronic lymphocytic leukemia [Citation5–7]. The high prevalence of CD40 expression on B-cell malignancies makes it an attractive potential tumor target for antibody-based cancer therapy [Citation8].
Dacetuzumab (SGN-40; Seattle Genetics, Inc., Bothell, WA) is a humanized immunoglobulin G1 (IgG1) monoclonal antibody (mAb) against CD40, derived from the murine antibody, S2C6. Dacetuzumab has little effect on normal cells in vitro, but in the presence of cross-linking reagents and certain cytokines or growth factors (e.g. interleukin 4 [IL-4]), it enhances the proliferation of B cells from healthy donors [Citation9]. In contrast, on several transformed B-cell lymphoma lines, dacetuzumab sends a growth-inhibitory signal and induces apoptosis. Additionally, dacetuzumab has been shown to mediate antibody-dependent cellular cytotoxicity of B-cell lymphoma cell lines in vitro and increases survival in murine xenograft models of B-cell lymphomas [Citation9–11]. In another in vivo study, dacetuzumab, in combination with rituximab, acted synergistically, showing improved antitumor activity versus rituximab alone in a murine xenograft model of non-Hodgkin lymphoma (NHL) [Citation12].
In clinical trials, dacetuzumab has shown single-agent activity in relapsed/refractory DLBCL in phase 1 and 2 trials. In a phase 1 trial, patients received 2 mg/kg weekly for 4 weeks in the first cohort. In subsequent cohorts, intra-patient dose-escalation was implemented with increasing doses up to a maximum of 8 mg/kg. Four of 16 (25%) evaluable patients with DLBCL responded, including two durable responses (a complete response [CR] ongoing at ≥ 69 weeks and a partial response [PR] that evolved into a CR after study completion at ≥ 18 weeks); responses were seen at all dose levels, and no dose–response relationship was identified. The maximum tolerated dose was not reached at 8 mg/kg/week [Citation13]. In a phase 2 single-agent open label trial of dacetuzumab for DLBCL, patients received six intravenous infusions of dacetuzumab over 5 weeks (cycle 1) with intra-patient dose loading (1 mg/kg on day 1; 2 mg/kg on day 4; 4 mg/kg on day 8) and 8 mg/kg/week thereafter. Objective responses included two CRs (5%) and two PRs (5%), yielding an objective response rate (ORR) of 9%. Furthermore, 13 patients (28%) had stable disease (SD) as best response. Reductions in tumor size were seen in approximately one-third of patients [Citation14]. The treatment was well tolerated in this heavily treated group of patients. Based on this early evidence of activity, a randomized phase 2b clinical trial, comparing R-ICE + placebo vs. R-ICE + dacetuzumab in patients with first relapse DLBCL was planned.
The goals of this study were to evaluate the safety of adding dacetuzumab to an intensive chemoimmunotherapy regimen and to assess clinical outcomes of this regimen for patients who had failed frontline therapy for DLBCL, consisting of R plus multi-agent chemotherapy. CR after three cycles of R-ICE was the primary endpoint, based on data demonstrating that those patients who achieve positron emission tomography (PET)-negative status prior to aSCT have improved outcome [Citation15,Citation16]. Additional endpoints included ORR, stem cell mobilization and engraftment, failure-free survival (FFS) and overall survival (OS).
Methods
Patients
Eligibility criteria included: age ≥ 18 years; DLBCL (de novo, transformed or follicular lymphoma grade 3b [FL3b]) with measurable disease; prior therapy with R-CHOP or equivalent and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; and adequate blood counts and liver function tests. Exclusion criteria included: central nervous system (CNS) lymphoma; therapy for relapsed or progressive disease except for local radiation, steroids or single-agent R; prior hematopoietic stem cell transplant; or other serious medical illness.
Study design
In this phase 2b, randomized, placebo-controlled, double-blind trial, patients with relapsed DLBCL were randomized 1:1 to receive three cycles of R-ICE + dacetuzumab or R-ICE + placebo (Supplementary Figure 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2015.1007504). The study was conducted in compliance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practices and the applicable United States Food and Drug Administration (FDA) regulations. Institutional Review Boards approved the study for each site, and all patients provided written informed consent prior to any study procedures. The study was registered at ClinicalTrials.gov (NCT00529503).
A total of 224 patients were to be randomized. Study arms were stratified by time since completion of first-line therapy (≤ 12 months or > 12 since completion of first-line therapy) and disease histology (de novo DLBCL and FL3b, or transformed DLBCL). Ten days after completing or discontinuing study treatment, patients were seen for an end of treatment (EOT) visit and were restaged.
During the follow-up phase, patients were contacted by telephone 30 days after the EOT visit for safety follow-up and every 3 months until study closure to assess secondary and tertiary endpoints (stem cell transplant, failure-free progress and survival). Radiographic assessments to confirm ongoing response were to occur 1 and 2 years following randomization.
Treatment
Day 1 of each cycle was defined as the first day of etoposide (). During cycle 1, R was infused on day − 2 and investigational drug (dacetuzumab or placebo) was to be administered on days − 1, 3, 8 and 15.
Table I. Dosing and administration of R-ICE and dacetuzumab/placebo.
During cycles 2 and 3, R was administered on day 1, and investigational drug was to be administered on days 1, 8 and 15. Delay in initiation of treatment during cycle 2 or 3 (up to 21 days) was allowed if clinically significant unresolved toxicity or cytopenia was present on scheduled day 1 that would preclude administration of chemotherapy. Dose modifications of R and investigational drug were not permitted. Investigational drug was discontinued if R-ICE chemotherapy was discontinued.
Investigators were permitted to use hematopoietic growth factors, blood transfusions and other supportive measures as per institutional standard practice. Furthermore, stem cell mobilization and aSCT were allowed following the final chemotherapy treatment.
Study assessments
DLBCL response assessments were performed using computed tomography (CT) and PET scans of the neck, chest, abdomen and pelvis at baseline, 10 days after completing or discontinuing study treatment, and at 1 and 2 years following randomization. A best clinical response of CR, PR, SD or progressive disease (PD) as per the Revised International Working Group Response Criteria for Malignant Lymphoma 2007 was to be determined by the investigator [Citation17]. An independent and blinded imaging core laboratory evaluated radiographic images, which were documented in a separate charter and reported independently. Safety assessments included adverse events (AEs), changes in laboratory values and vital signs, electrocardiogram (ECG) and physical examination findings. The National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 was used for grading AEs. An independent data monitoring committee (IDMC) reviewed blinded data at several pre-specified timepoints to oversee study conduct and patient safety. Other study assessments were to include dacetuzumab and R pharmacokinetic (PK) analysis, measurement of anti-drug antibodies, central pathology review of archived tumor specimens, DLBCL molecular subtype determination, CD40 expression in lymphoma cells, evaluation of the gene signature associated with response to CD40 therapy in DLBCL [Citation18] and changes in lymphocyte subsets.
Statistical methods
Approximately 224 patients were to be randomized to ensure that 200 would be evaluable for efficacy. The sample size of 200 would yield a trial with approximately 87% power at a one-sided type 1 error of 15% if the assumed treatment effect existed.
The primary efficacy endpoint was CR at EOT using the independent review committee (IRC) integrated CT + PET + oncology response assessment. Ninety-five percent confidence intervals (CIs) for the CR response rates, and 70% and 95% CIs for the rate difference between the treatment arms, were provided using normal approximations for the primary efficacy analysis set. Patients with missing clinical response were considered as not achieving CR.
FFS was the tertiary efficacy endpoint defined as time from randomization until treatment failure. Tertiary efficacy endpoints included clinical response (CR, PR, SD, PD and unknown) at EOT, OS and occurrence of hematopoietic SCT. The medians and first and third quartiles of FFS and OS were provided if the quartiles could be estimated by the Kaplan–Meier method. Log-rank test was used to compare the two treatment groups. Three analysis sets were used for the efficacy analysis. The primary efficacy analysis set was defined as patients who were randomized, received any dose of dacetuzumab or placebo, had measurable disease as determined by IRC and had disease diagnosis as confirmed by an independent pathologist. The modified intent-to-treat (mITT) set was defined as patients who were randomized and received any dose of study treatment (any agent). The futility analysis set included the first 112 patients in the mITT set. FFS and OS were analyzed based on the primary efficacy analysis set and the mITT set (Supplementary Material to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2015.1007504).
The planned futility analysis included the first 50% of patients who completed treatment, and CR rate based on the investigator assessment was used to assess futility.
Treatment-emergent AEs were summarized using Medical Dictionary for Regulatory Activities (MedDRA) version 13.0 preferred terms and included severity and relationship to study treatment (dacetuzumab/placebo or R-ICE). Laboratory values, change from baseline values and change from baseline in toxicity grade were summarized by descriptive statistics for each treatment group. Data were analyzed by representatives of Seattle Genetics, Inc., and all authors had access to the primary clinical trial data.
Results
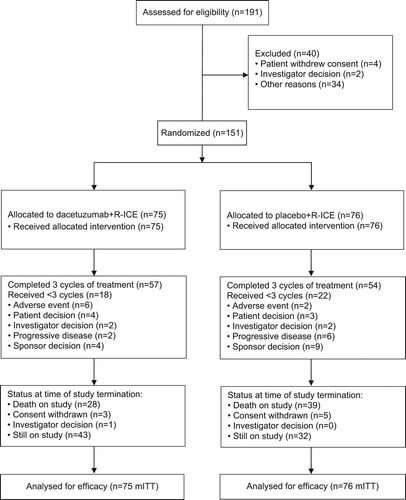
A total of 151 patients were enrolled and randomized (75 dacetuzumab, 76 placebo) between December 2007 and September 2009. This international study enrolled patients at 52 sites: 19 sites in the United States, 19 in Western Europe (Belgium, France, Germany, Italy and Spain), 10 in Eastern Europe (Czech Republic, Hungary and Poland) and four in Australia (Supplementary Material to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2015.1007504). Patients enrolled were equally randomized between the two treatment arms by time since completion of first-line therapy (≤ 12 months: 67% dacetuzumab, 66% placebo; and > 12 months: 33% dacetuzumab, 34% placebo) and by disease histology (FL3b or de novo DLBCL: 92% each dacetuzumab and placebo; and transformed DLBCL: 8% each dacetuzumab and placebo).
Among the 151 randomized patients, a total of 111 patients (74%) completed the treatment phase (57 patients [76%] dacetuzumab, 54 patients [71%] placebo), and all randomized patients received the correct study treatment. Forty patients (26%) discontinued study treatment: eight patients (5%) discontinued due to disease progression (3% dacetuzumab, 8% placebo), eight (5%) due to AE (8% dacetuzumab, 3% placebo), seven (5%) due to patient decision (5% dacetuzumab, 4% placebo) and four (3%) due to investigator decision (3% each dacetuzumab and placebo); 13 patients (9%) discontinued treatment due to a sponsor decision to terminate study treatment based on the futility analysis. Patients were followed after study treatment discontinuation (or completion) for a median of 27.3 months. At the time of study termination, 54 patients (36%) had died due to disease progression, 13 (9%) had died due to other causes, eight (5%) withdrew consent and one (1%) discontinued due to an investigator decision. Seventy-five patients (50%) were in follow-up when the study was terminated by the sponsor ().
Overall, treatment arms were well balanced with respect to age, gender, race, ECOG status, disease diagnosis, time since initial diagnosis and prior therapy, with the exception of best response to first-line therapy. Slightly more patients in the placebo arm had a best response of CR to first-line therapy (n = 45, 59%) as compared to the dacetuzumab arm (n = 38, 51%) and more patients randomized to the dacetuzumab arm had a best response of PR to first-line therapy (n = 31, 41%) than patients in the placebo arm (n = 25, 33%). One patient was enrolled who had progressed during first-line therapy; this was recorded as a major protocol violation. All patients had received first-line therapy of R-CHOP or equivalent with a median of 6 treatment cycles (range, 4–9), and the median time since completion of first-line therapy was 7 months. Few patients (8%) had received maintenance R since completing first-line therapy. Based on local pathology review, disease diagnoses for the 151 enrolled patients were de novo DLBCL for 131 patients (87%), transformed DLBCL for 13 patients (9%) and FL3b for seven patients (5%). However, 50 patients were not included in the primary efficacy analysis set due to either insufficient measurable disease by the independent radiographic review or inability to confirm the disease diagnosis based on central pathology review. The disease diagnosis for the remaining 101 patients in this analysis set was de novo DLBCL for 93 patients (92%), transformed DLBCL for five patients (5%) and FL3b for three patients (3%). The median time since initial diagnosis for this set of patients was 9.6 months (range, 2–124). There were a total of 19 protocol violations where eligibility criteria were not met; no other major protocol violations were reported. Patient characteristics are summarized in .
Table II. Patient characteristics (mITT population).
Futility analysis
A planned futility analysis was conducted after 112 patients (50% of planned patients) were enrolled and randomized (55 patients in the dacetuzumab + R-ICE arm [dacetuzumab], 57 patients in the placebo + R-ICE arm [placebo]). CR rate at EOT was the primary efficacy endpoint evaluated. The results of the analysis showed that 20 patients in the dacetuzumab arm (36% [95% CI, 24% to 49%]) and 24 patients in the placebo arm (42% [95% CI, 29% to 55%]) had a CR at EOT per investigator assessment (Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2015.1007504). Because the CR rate at EOT was not improved with dacetuzumab (− 6% [95% CI, − 24% to + 12%]), the IDMC recommended that enrollment be stopped and treatment with dacetuzumab be discontinued for all patients. All patients already enrolled at the time of the results for the futility analysis (n = 151) subsequently continued to receive R-ICE therapy.
Final analysis
Analysis of the primary efficacy endpoint based on the primary efficacy analysis set (n = 101) also showed no difference in the CR rate between arms, 33% (95% CI, 20% to 46%) in the dacetuzumab arm and 36% (95% CI, 23% to 49%) in the placebo arm using the investigator assessment; the CR rates using the IRC assessment were 35% (95% CI, 22% to 48%) in the dacetuzumab arm and 40% (95% CI, 26% to 54%) in the placebo arm (). Furthermore, the ORRs as assessed by the investigator at the EOT were similar between arms, 67% (95% CI, 54% to 80%) in the dacetuzumab arm and 64% (95% CI, 51% to 77%) in the placebo arm.
Table III. Clinical response at end of treatment: primary efficacy analysis set.
For the mITT set of patients (n = 151), there remained no difference in the CR rate between arms based on the investigator assessment. Twenty-seven patients in the dacetuzumab arm (36% [95% CI, 25% to 47%]) had a CR at EOT, and 28 patients (37% [95% CI, 26% to 48%]) had a CR at EOT in the placebo arm. Based on central review of radiographic assessments, the number of patients achieving a CR at EOT in the dacetuzumab arm was 22 (29% [95% CI, 19% to 40%]), and was 28 (37% [95% CI, 26% to 48%]) in the placebo arm. Among patients who were assessed for the 15-gene signature previously described as being associated with response to CD40 therapy in DLBCL [Citation18], no correlation between the dacetuzumab arm and CR rate was observed in this study (data not shown).
Among all patients enrolled in the study, 35 patients (47%) in the dacetuzumab arm and 40 patients (53%) in the placebo arm underwent aSCT after completion of study treatment. Collection of an adequate number of stem cells was similar in both arms (n = 41, 80% dacetuzumab, n = 43, 78% placebo) for the patients for whom stem cell mobilization (n = 51 dacetuzumab, n = 55 placebo) was attempted; reasons that patients did not receive transplant were balanced between arms.
An analysis of FFS using the investigator's response assessment for the mITT set (n = 151) revealed a hazard ratio (HR) of 0.797 and a p-value of 0.259 []. A subgroup analysis of FFS was conducted using the investigator's response assessment by post-treatment aSCT in the mITT analysis set. The p-value for patients who had not received post-treatment aSCT was 0.036 (HR = 0.591), while the p-value for patients who underwent post-treatment aSCT was 0.399 (HR = 0.750) [].
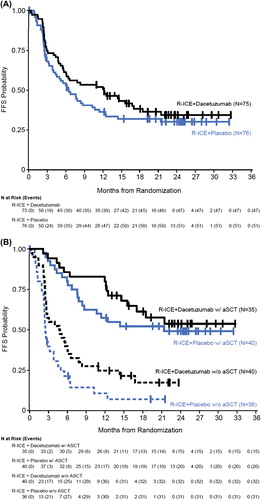
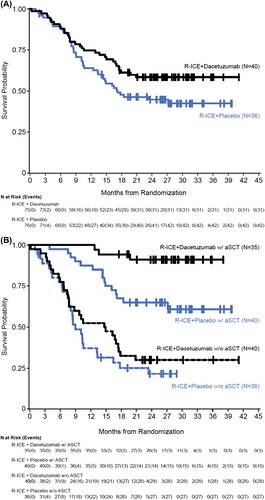
Using the mITT analysis set, the estimated median OS for patients receiving placebo was 17.2 months and the median OS for patients receiving dacetuzumab had not been reached at the time of the analysis (HR = 0.661, p = 0.078) []. A subgroup analysis was performed for OS by post-treatment aSCT, which revealed a survival advantage with dacetuzumab in patients who had subsequently received an aSCT (HR = 0.195, p = 0.004) []. However, these results were based on a small sample size for each of the subgroups. In addition, follow-up was halted at study termination and patients were censored at the date of last contact if the patient was alive at that time.
Safety
All patients received at least one dose of study drug (dacetuzumab or placebo), and many patients completed planned therapy (n = 111, 74%). The median compliance rate (actual dose over intended dose) for study treatment and for R-ICE dosing was 100% (range, 79–117%) for all drugs. The proportion of patients with dose eliminations of study treatment due to AE was higher in the dacetuzumab arm (16%) than in the placebo arm (7%). There were also more dose delays in the dacetuzumab arm.
Overall, 80% of patients in the dacetuzumab arm and 75% of patients in the placebo arm had at least one AE grade 3 or higher in severity. The incidence of cytopenias (≥ grade 3) was greater in the dacetuzumab arm (). Serious AEs (SAEs) also occurred more frequently in the dacetuzumab arm (44% dacetuzumab, 30% placebo). SAEs occurring in more than two patients were: febrile neutropenia (12% dacetuzumab, 7% placebo), neutropenia (7% dacetuzumab, 0% placebo), thrombocytopenia (7% dacetuzumab, 1% placebo), pancytopenia (4% dacetuzumab, 0% placebo), acute renal failure (4% dacetuzumab, 0% placebo) and pyrexia (3% dacetuzumab, 4% placebo). Again, cytopenias (SAEs) were more common in the dacetuzumab arm.
Table IV. Grade 3 or higher adverse events* and causes of death.
Death was reported for a total of 73 patients in the safety population; four patients (two in each arm) died within 30 days of last study treatment. Disease progression was reported as the primary cause of death in more patients in the placebo arm (n = 34) than in the dacetuzumab arm (n = 20) ().
Six patients (8%) had an AE leading to treatment discontinuation in the dacetuzumab arm, compared with two patients (3%) in the placebo arm. Sepsis was reported as an adverse event leading to treatment discontinuation in two patients (one patient per treatment arm). AEs leading to treatment discontinuation in the dacetuzumab arm were increased alanine aminotransferase, asphyxia, neutropenia, septic shock and ventricular extrasystoles (one patient each). One patient in the placebo arm discontinued treatment due to an AE of thrombocytopenia.
For hematologic laboratory abnormalities, the addition of dacetuzumab to R-ICE was associated with an increased incidence of thrombocytopenia, anemia and neutropenia, compared to placebo plus R-ICE. In addition, there was an increase in febrile neutropenia in the dacetuzumab arm. However, the increased rate of hematologic AEs did not result in a clinically meaningful change in disposition. Similar numbers of patients in each arm were able to complete the intended treatment regimen and proceed to aSCT.
There did not appear to be any difference in the laboratory abnormality incidence or severity of electrolytes, blood urea nitrogen (BUN), creatinine, bilirubin, alkaline phosphatase, alanine aminotransferase or aspartate aminotransferase between the study arms. Overall, the incidence of grade 3 or higher non-hematological laboratory abnormalities was low in this study.
Results of anti-drug antibody assays were available for 121 patients and no immunogenicity against dacetuzumab was detected; however, the possibility that the presence of dacetuzumab in the sample interfered with detection of the anti-drug antibody cannot be ruled out.
Discussion
During the last decade, the addition of R to frontline treatment for DLBCL has improved clinical outcomes [Citation19–22]. However, for those who fail to achieve a complete remission or relapse after R-CHOP, new treatment options are needed [Citation2,Citation23]. Multiple studies have demonstrated that achieving a PET-negative CR at the conclusion of salvage therapy predicts lower relapse rates after aSCT [Citation15,Citation16]. Accordingly, in this randomized, placebo-controlled phase 2b trial, the primary objective was an improved CR rate after three cycles of R-ICE therapy when dacetuzumab is added to the regimen.
Dacetuzumab is an IgG1 mAb targeting CD40, a member of the tumor necrosis factor receptor superfamily, which is expressed on B-cell malignancies as well as antigen-presenting cells (APCs) and endothelial cells. This mAb has shown modest activity in DLBCL when administered as a single agent. Since dacetuzumab has limited overlapping toxicities with R-ICE, the addition of dacetuzumab to R-ICE was considered to be a reasonable approach to improve clinical outcomes.
This study represents the largest population of patients with DLBCL in second-line who had all received prior R-CHOP. The patient population was well balanced in both arms () with respect to age, sex, histology, time since diagnosis and performance status. There was a modestly higher percentage of patients who achieved a CR after front-line therapy in the placebo group.
In this international study, the overall CR rate was approximately 35–45% after three cycles of R-ICE, establishing an important benchmark for future studies of second-line DLBCL in which all patients received front-line chemoimmunotherapy including R. However, the trial failed to demonstrate an improvement in CR rate or ORR for those patients who received dacetuzumab. As a result, the IDMC recommended that the trial be terminated due to futility after enrollment of 151 of 224 planned subjects.
From a safety perspective, the addition of dacetuzumab did not affect the administration of R-ICE or the ability to complete treatment when compared to the placebo arm. Furthermore, a similar percentage of patients attempted and completed adequate stem cell mobilization and collection in both arms. However, the dacetuzumab arm reflected increased incidence and severity of hematopoietic cytopenias and febrile neutropenia when compared to the placebo arm.
During planned follow-up of the 151 patients enrolled in this trial, an unexpected trend of improved FFS and OS emerged. With a median follow-up of ∼27 months, there were 42 deaths in the placebo arm (55%) vs. 31 deaths in the dacetuzumab arm (41%). The source of this difference was a reduction in deaths due to disease progression: 31 (41%) vs. 20 (27%) for patients who received dacetuzumab. Post hoc subset analyses generated several intriguing results. The survival advantage for patients receiving dacetuzumab was not apparent until several months after completion of treatment, was greatest for those patients who underwent aSCT, and appeared to correlate with those patients who achieved PR or SD after R-ICE but was equivalent for those who achieved CR. Furthermore, there was no pattern of improved survival related to the DLBCL subtype analysis (i.e. germinal center B-cell-like [GCB] vs. non-GCB), nor was there a difference among those patients with the highest tumor CD40 expression by immunohistochemistry.
Because CD40 is expressed on both malignant lymphoma cells as well as APCs, the mechanism of action for dacetuzumab could be a combination of direct effects on malignant cells and immunomodulatory effects on APCs, especially dendritic cells. In this phase 2b trial, the data suggest that dacetuzumab may have a greater effect as an immunomodulator, resulting in a trend toward improved OS despite no improvement in CR rate at the end of treatment. Additional studies would be required to test this hypothesis as well as developing a regimen that is optimized for immunotherapy.
The results of this study are intriguing and may require further studies with different design to determine whether there is any value to the addition of this CD40 partial agonist mAb in this disease.
Supplementary material available online
Supplementary Material, Additional methods and results and list of contributors
ilal_a_1007504_sm1292.zip
Download Zip (12.2 MB)ilal_a_1007504_sm1291.pdf
Download PDF (99.9 KB)Acknowledgments
The authors thank the patients who participated in this study and their families, and would like to thank the members of the IDMC, including Joseph M. Connors, Alan K. Burnett, Jerald P. Radich, Claudio Anasetti and Daniel L. Gillen. The authors also thank Natalie Masterton of Seattle Genetics, Inc. for her project management support and Tiffany Griffin of Seattle Genetics, Inc. for her writing support.
This work was supported by research funding from Seattle Genetics, Inc. and Genentech, Inc. for all authors and sites listed in the Supplementary Material. to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2015.1007504
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- Gisselbrecht C, Glass B, Laurent G, et al. Maintenance with rituximab after autologous stem cell transplantation in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL): CORAL final analysis. J Clin Oncol 2011;29(15 Suppl.): Abstract 8004.
- Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010;28:4184–4190.
- Stamenkovic I, Clark EA, Seed B. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J 1989;8:1403–1410.
- van Kooten C, Banchereau J. Functions of CD40 on B cells, dendritic cells and other cells. Curr Opin Immunol 1997;9:330–337.
- Gruss HJ, Herrmann F, Gattei V, et al. CD40/CD40 ligand interactions in normal, reactive and malignant lympho-hematopoietic tissues. Leuk Lymphoma 1997;24:393–422.
- Gruss HJ, Dower SK. Tumor necrosis factor ligand superfamily: involvement in the pathology of malignant lymphomas. Blood 1995; 85:3378–3404.
- Uckun FM, Gajl-Peczalska K, Myers DE, et al. Temporal association of CD40 antigen expression with discrete stages of human B-cell ontogeny and the efficacy of anti-CD40 immunotoxins against clonogenic B-lineage acute lymphoblastic leukemia as well as B-lineage non-Hodgkin's lymphoma cells. Blood 1990;76:2449–2456.
- Tong AW, Stone MJ. Prospects for CD40-directed experimental therapy of human cancer. Cancer Gene Ther 2003;10:1–13.
- Law C-L, Gordon KA, Collier J, et al. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40. Cancer Res 2005;65:8331–8338.
- Gowda AC, Zhao XB, Cheney C, et al. Humanized anti CD-40 antibody SGN-40 effectively induces cytotoxicity against chronic lymphocytic leukemia (CLL) cells through antibody mediated cytotoxicity and demonstrates modest biologic evidence of CD40 activation. Blood 2005;106(Suppl. 1): Abstract 2966.
- Drachman JG, Law C-L, Furman RR, et al. A humanized anti-CD40 monoclonal antibody (SGN-40) demonstrates antitumor activity in non-Hodgkin’s lymphoma: Initiation of a phase I clinical trial. J Clin Oncol 2005;23(16 Suppl.): Abstract 6572.
- Lewis TS, McCormick RS, Emmerton K, et al. Distinct apoptotic signaling characteristics of the anti-CD40 monoclonal antibody dacetuzumab and rituximab produce enhanced antitumor activity in non-Hodgkin lymphoma. Clin Cancer Res 2011;17:4672–4681.
- Advani R, Forero-Torres A, Furman RR, et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin's lymphoma. J Clin Oncol 2009;27:4371–4377.
- De Vos S, Forero A, Ansell S, et al. A phase II study of dacetuzumab (SGN-40) in patients with relapsed diffuse large B-cell lymphoma (DLBCL) and correlative analyses of patient-specific factors. J Hematol Oncol 2014;7:44.
- Schot BW, Zijlstra JM, Sluiter WJ, et al. Early FDG-PET assessment in combination with clinical risk scores determines prognosis in recurring lymphoma. Blood 2007;109:486–491.
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Heamtology Am Soc Hematol Educ Program 2011:498–505.
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–586.
- Burington B, Yue P, Shi X, et al. CD40 pathway activation status predicts response to CD40 therapy in diffuse large B cell lymphoma. Sci Transl Med 2011;3:74ra22.
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235–242.
- Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 2005;23:4117–4126.
- Molina A. A decade of rituximab: improving survival outcomes in non-Hodgkin's lymphoma. Annu Rev Med 2008;59:237–250.
- Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006;7:379–391.
- Gisselbrecht C. Use of rituximab in diffuse large B-cell lymphoma in the salvage setting. Br J Haematol 2008;143:607–621.