Abstract
Context: The mucoadhesive gel formulations are helpful to prolong the residence time at the nasal absorption site and thereby facilitate the uptake of drug. Sumatriptan succinate has oral bioavailability of 15% and undergoes hepatic metabolism, hence it is suitable for nasal administration.
Objective: The objective of the investigation was to develop a mucoadhesive in situ gel to improve the bioavailability of the sumatriptan succinate.
Materials and methods: Deacetylated gellan gum was used as gelling agent. In situ gel was formulated by ion activation mechanism in simulated nasal fluid. A 32 factorial design was found suitable to optimize batch. In vivo study was carried out in Spraugue-Dawley rats, and drug was estimated in plasma by UPLC-MS.
Result: The optimized batch showed drug release of 98.57% within 5 h followed by Peppas model of drug release. Ex vivo studies on sheep nasal mucosa showed 93.33% within 5 h. In histopathological study, optimized batch was found to be safe and stable in accelerated stability study for three months. Optimized formulation, F7 has shown absolute bioavailability, which was found to be 164.70%. Drug targeting index for brain tissues was found to be 1.866.
Discussion: Concentration of the gelling polymer was compromised for satisfactory gel strength and an acceptable viscosity. The release depended on viscosity of formulation. Drug targeting index indicates sumatriptan can reach to brain via olfactory pathway.
Conclusion: In situ gel proved to be suitable for administration of sumatriptan succinate through nasal route. The ease of administration coupled with less frequent administration enhances patient compliance.
Introduction
Nasal drug delivery is one of the challenging endeavors facing the pharmaceutical scientist today. Nasal administration is significantly effective in case of oral administration of drug gives an undesirable side effect. From the pharmacokinetic standpoint, intranasal administration avoids first-pass metabolism and retards incomplete absorption in the gastrointestinal tract which leads to improve the bioavailability (Jadhav, Citation2007; Dey & Mahanti, Citation2011; Kushwaha et al., Citation2011).
Mechanism of permeation across the nasal mucosa is passive transportation, carrier mediated, transcytosis and transport through intercellular tight junctions. However, nasal delivery system has limitations which have restricted its use to delivery of few drug molecules and rapid clearance from nasal cavity. A greater emphasis on the properties of drug molecules, formulation matrices, the nasal mucosa itself and the drug delivery systems that affect drug absorption through the nasal route is invaluable (Ugwoke & Agu, Citation2005; Singh et al., Citation2012).
A significant challenge to the formulator is to overcome the protective barriers of the nasal cavity without causing permanent tissue damage. The major problems that persist with nasal solutions are cleared off rapidly from nasal cavity. The half-life of clearance for both liquid and powder formulations that are not mucoadhesive is in the order of 15–20 min. Therefore, another possible strategy is to decrease the mucocilliary clearance by the use of mucoadhesive gel formulations to prolong the residence time at the nasal absorption site and thereby facilitate the uptake of the drug. Approaches to enhance the nasal bioavailability aim at prolonging the contact time with the nasal surface by using viscosity-enhancing or in situ gelling polymers. An in situ gel is drug delivery system that exhibits sol-to-gel phase transition due to change in specific physicochemical parameters such as ionic, temperature or pH.
In situ gelling systems can be classified as ion-activated systems (e.g. gellan gum and sodium alginate), temperature-dependent systems (e.g. Pluronics, Tetronics and polymethacrylates) and pH-triggered systems (e.g. Carbopol and cellulose acetate phthalate). The principal advantage of in situ gels is that they can be easily administered with accurate and reproducible dose compared to that of ordinary gels, have an advantage over ordinary gels that they can be easily instilled in liquid form and are capable of prolonging the residence time of the formulation on the surface of the nasal cavity due to gelling (Gibaldi & Perrier, Citation1982; Noha et al., Citation2007; Nirmal et al., Citation2010).
Sumatriptan succinate is a 5-HT1D and 5-HT1B (5-hydroxy tryptamine) receptor agonist used in the treatment of migraine and cluster headache. Sumatriptan is generally given by oral or parental routes. However, a substantial proportion of patients suffer severe nausea or vomiting during their migraine attack, which may make oral treatment unsatisfactory.
Sumatriptan succinate has oral bioavailability of 15%; however, the problem associated with nasal delivery of sumatriptan solution is lower retention time in nasal cavity (15 minutes) resulting in lower bioavailability as well as lower transfer of sumatriptan directly to the brain through olfactory pathway. Moreover, the transport of sumatriptan across the blood–brain barrier is very poor. After 15 minutes, sumatriptan solution is swallowed and it enters the gastrointestinal tract where remaining dose is absorbed, which may get increased by intranasal delivery (Ryan et al., Citation1997; Majithiya et al., Citation2006). An intranasal-microemulsion drug delivery system to accomplish rapid delivery of sumatriptan to the brain in acute attacks of migraine has been reported (Vyas et al., Citation2005). The PF127 gel formulation of sumatriptan with in situ gelling and mucoadhesive properties with increased permeation rate is promising for prolonging nasal residence time and thereby nasal absorption (Majithiya et al., Citation2006).
However, mucoadhesive nasal in situ gel drug delivery was very beneficial in case of BCS class III drugs like sumatriptan succinate in presence of fulvic acid due to its permeation-enhancing effect (Badgujar et al., Citation2010).
Gellan gum is an anionic deacetylated, exocellular polysaccharide secreted by Pseudomonas elodea with a tetrasaccharide repeating unit of 1β-l-rhamnose, 1β d-glucuronic acid and 2β d-glucose. The mechanism of gelation involves the formation of double-helical junction zones followed by aggregation of the double-helical segments to form a 3-D network by complexation with cations and hydrogen bonding with water. Because human nasal mucosa is covered with approximately 0.1 ml mucus, which consists of sodium, potassium and calcium ions, a solution-gel phase transition can be expected (Bajaj et al., Citation2007; Pires et al., Citation2009; Dhuria et al., Citation2010; Morris et al., Citation2012).
Ion-activated nasal gels of sumatriptan succinate were prepared by using the polymer deacetylated gellan gum. This investigation deals with development and evaluation of ion-activated gel of sumatriptan succinate. The prepared dosage regimens provided ease in application and capable drug release with reduced frequency of administration.
Materials and methods
Sumatriptan succinate was obtained as a gift sample from Cipla Ltd., Mumbai, India. Gellan gum was obtained as gift sample from CP Kelco, Division of JM Huber (India) Pvt. Ltd., Mumbai, India. Polyethylene glycol 400 (PEG 400) was purchased from Loba Chemie Pvt. Ltd., Mumbai, India.
Preformulation studies
Determination of λmax of sumatriptan succinate
The stock solution of 100 µg/ml was prepared by dissolving sumatriptan equivalent to 10 mg sumatriptan succinate in 100 ml of distilled water. Then, spectrum of this solution was obtained by scanning between 400 and 200 nm using UV visible spectrophotometer (Shimadzu UV-1800, Japan). The absorption maximum wavelength was determined.
Fourier transforms infrared spectral studies
The compatibility study was carried out by using FTIR (Jasco M 4100, Mumbai, India) (Pavia et al., Citation2007). Before FTIR study, the physical mixtures of 1:1 w/w were prepared for sumatriptan succinate and gellan gum, drug and physical mixture (gellan gum + PEG 400 + mannitol) and also for gellan gum and individual dry ingredients of simulated nasal fluid that is potassium chloride (KCl), sodium chloride (NaCl) and calcium chloride (CaCl2). These mixtures were kept for one month at room temperature that is 25 °C ± 2 °C and 60% ± 5% relative humidity for complete interaction between the drug and polymer. The drug and drug-polymer samples were dried in hot air oven at 60°C for 30 min for removal of moisture. These samples were scanned from 4000 to 400 cm–1. Spectra obtained were compared with spectra of sumatriptan sample for changes in the peaks if any.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) was used to evaluate the thermal behavior of pure drug using a DSC-60 (Shimadzu Corporation, Japan) (Jess & Rhodes, Citation1990; Skoog et al., Citation2007). Samples 10 mg were weighed and sealed in standard aluminum pans and then scanned over a temperature range from 50 °C to 300 °C at a heating rate of 10 °C/min.
Preliminary studies for optimum concentration of deacetylated gellan gum for in situ gelation
Preliminary studies were carried out using different concentrations of gellan gum as shown in . The simulated nasal fluid was prepared by dissolving sodium chloride (2.1925 g), calcium chloride (0.145 g) and potassium chloride (0.745 g) into 250 ml of double distilled water (Mahajan et al., Citation2009).
Table 1. Gelation studies with deacetylated gellan gum.
The gelling concentration of deacetylated gellan gum was optimized on the basis of minimum possible concentration which would produce gelation with minimum viscosity. Gelation studies were carried out using simulated nasal fluid (pH 6.4 ± 0.1) at 34 ± 1 °C. Preliminary studies revealed optimum results with 0.2% w/v gellan gum in double distilled water. In trial batches optimized gelling concentration of gellan gum (0.2% w/v) was used to study the effect of PEG 400 on viscosity in the concentration range of 1–10% w/v.
Preparation of in situ gelling systems
Deacetylated gellan gum of different concentrations of (0.2% w/v, 0.3% w/v and 0.4% w/v) was dispersed in double distilled water, heated to 90°C while stirring, until all solids were dissolved and then cooled to room temperature (25 °C ± 2 °C). The sumatriptan succinate was added in distilled water containing PEG 400. Further, drug solution was mixed into the polymer solution and then mannitol and methyl paraben were added subsequently with continuous agitation. Mannitol was added as isotonic agent and methyl paraben as preservative. Finally, formulation was stored in properly capped glass container at room temperature and evaluated for different parameters.
Formulation optimization
In trial batches it was found that PEG 400 and gellan gum level mainly affects the viscosity, mucoadhesive strength and drug release. Preliminary studies were carried out using different concentrations of gellan gum ranging from 0.1% w/v to 0.4% w/v. To optimize the concentration, gelation studies were carried out using simulated nasal fluid (pH 6.4) at 34 ± 1 °C. Preliminary studies revealed optimum results with 0.2% w/v gellan gum. A 32 full factorial design (Design Expert Stat-Ease 8.0.4.1, USA), shown in , was applied to the formulation that showed the satisfactory viscosity, mucoadhesive strength and in vitro drug release, to see the effect of varying concentration of variables, gellan gum % w/v (X1) and PEG 400% w/v (X2) on various dependent variables, that is, viscosity, mucoadhesive strength, % cumulative drug release (Shastri et al., Citation2010).
Table 2. Factor and levels in the optimization of in situ gel.
Six trial batches were prepared for 2, 3, 4, 5, 6 and 10%w/v of PEG 400 with gellan gum 0.2%w/v constant for each. Results obtained by using 10% PEG 400 were not satisfactory in trial batches. The three levels of variable PEG 400 (X2) were selected as 4% w/v, 5% w/v and 6% w/v as low, medium and high, respectively.
In , different formulation compositions with respective value of dependent variable are given and in situ gel was prepared. Evaluation of final formulations was done with respect to clarity, pH, content uniformity, viscosity, spreadability, gel strength, mucoadhesive force, diffusion through nasal mucosa, ex vivo permeation study, stability study, histopathological study and in vivo study.
Table 3. Composition of various ion-activated mucoadhesive nasal in situ gel formulations.
Clarity
Abbe's refractometer (RS 12-1, Rajdhani, India) has been used to measure the refractive index of liquids (More & Hajare, Citation2008; Gupta & Sharma, Citation2009). The clarity of sols, that is, formulations before gelling, was determined in term of refractive index using Abbes refractometer. The refractometer was calibrated with water as reference standard. The refractometer scale was adjusted in such a way that the cross wire of the telescope was exactly on the boundary between the bright and dark regions. The procedure was repeated for all formulations F1 to F9 and the results were compared with refractive index of water (1.33).
pH
Digital pH meter (Equip-Tronics EQ-610, India) was calibrated by using pH buffer of 4 and 7. Twenty milliliter of each formulation was taken in beaker and glass electrode was sufficiently dipped into the samples of formulations. Then, pH of the solution was determined in triplicate (Nisha et al., Citation2012).
Drug content
Drug contents of formulations were determined in triplicate by using double beam UV visible spectrophotometer (Shimadzu UV-1800) (Gaikwad, Citation2010). One milliliter of formulation was taken in capacity of 10 ml volumetric flask, diluted with double distilled water and volume adjusted to 10 ml. One milliliter quantity from this solution was again diluted with 10 ml of double distilled water. Finally, the absorbance of prepared solution was measured at 226 nm by using UV visible spectrophotometer.
Viscosity
The viscosity measurements were carried out by using Brookfield viscometer RVDV-II+ pro model. The viscosity measurements were performed using small volume adapter (Mahajan et al., Citation2009). The temperature sensing probe was lowered in gel and temperature of gel was recorded. Viscosity at 32–34 °C temperatures was noted.
Mucoadhesive strength
It is the force required to detach the formulation from nasal mucosal tissue. The mucoadhesive force, the detachment stress of the formulation was determined using a modification of the mucoadhesive force measuring device. The modified balance technique using two-glass vials and sheep nasal mucosa was used. A nasal mucosa with thickness of 0.6 mm and surface area 2.835 cm2 was cut from the olfactory region of sheep nasal cavity and instantly secured with the mucosal side out onto each glass vial using a thread. The vials were stored at 32–34 °C for 10 min. One vial was attached to one side of balance and 0.5 ml of gel sample was placed between the two mucosal membranes attached to the bottom of the vials. The minimum weight of water required to break the mucosal adhesion was measured (Gaikwad, Citation2010; Jaya Raj Kumar et al., Citation2010).
Where m is weight required for detachment in g, g is acceleration due to gravity (980 cm/s2) and A is surface area of mucosa exposed (cm2)
In vitro drug release
In vitro drug diffusion study of various formulations was performed using Franz diffusion cell (Gowda et al., Citation2011). Dialysis membrane having molecular weight cut-off range of 12 000–14 000 kDa was used as diffusion membrane. Dialysis membrane was allowed to soak in phosphate buffer pH 6.4 for 24 h before experiment. Diffusion cell was filled with 21 ml phosphate buffer pH 6.4 and dialysis membrane was mounted on cell. The gel containing drug equivalent to 10 mg was placed onto donor chamber. The temperature was maintained at 32–34 °C by circulating water bath. Samples of 1 ml were withdrawn at different time intervals replaced with same volume of fresh solution, filtered and amount of drug was determined by UV visible spectrophotometer at 226 nm.
Dissolution kinetics
Various models like zero-order, first-order, Higuchi models, Korsemeyer and Peppas were tested for explaining the kinetics of drug release.
Gel strength determination
A sample of (50 g) was placed in a 100-ml graduated measuring cylinder and gelled in a thermostatically controlled water bath at 32–34 °C by addition of simulated nasal fluid. The weight of 35 g was then placed onto the disk whose diameter was 2.3 cm, clearance from side wall of cylinder 0.4 cm, thickness 0.5 cm and this disc was put onto the gel as shown in . The gel strength was measured as the time (seconds) required to moving the piston 5 cm down through the gel. Gel strength is related to molecular weight, degree of cross-linking, etc. In cases that took more than five minutes to drop the apparatus into the gel, additional weights were placed on top of the apparatus and gel strength was described by the minimal weights that pushed the apparatus 5 cm down through the gel (Uttarwar, Citation2012).
Spreadability
Spreadability is the area traveled per unit time (cm2/min) by the gel formulation. Whatmanns filter paper (0.45 μm) was used for determination of spreadability of solution formulations F1 to F9. A 1-ml graduated pipette with rubber bulb was clamped vertically to the stand in such a way that the tip of pipette was at 2 cm above the horizontal surface of round shape filter paper. A 0.1-ml sol formulation was dropped at center of filter paper. At fixed time interval, 20 s, the surface area covered by the formulation was measured (Shinde & Mali, Citation2008).
Ex vivo permeation studies
Fresh nasal mucosa from olfactory region was carefully removed from the nasal cavity of sheep obtained from the local slaughterhouse. Nasal mucosa was inserted into phosphate buffer pH 6.4. Tissue samples were placed on diffusion cells immediately. Phosphate buffer solution pH 6.4 at 34 °C was added to the acceptor chamber. Formulation equivalent to 10 mg of sumatriptan succinate was placed in the donor chamber. At predetermined time points, 1 ml samples were withdrawn from the acceptor compartment, replacing the sampled volume with phosphate buffer pH 6.4 after each sampling, for a period of 5 h. The samples withdrawn were filtered and used for analysis. Blank samples (without sumatriptan succinate) were run simultaneously throughout the experiment to check for any interference. The amount of permeated drug was determined using a UV-visible spectrophotometer at 226 nm (Linearity range = 1 to 8 μg/ml, R2 = 0.998) (Badgujar et al., Citation2010; Basu & Bandyopadhyay, Citation2010).
Histopathological evaluation of nasal mucosa
Fresh nasal mucosa was carefully removed from the nasal cavity of sheep and was stored in 10% of formalin solution. Phosphate buffer pH 6.4 and isopropyl alcohol were used as negative and positive controls, respectively (Majithiya et al., Citation2006). Histological studies were carried out on sheep nasal mucosa that had been used for ex vivo permeation study which was treated with 1 ml optimized in situ gel for 5 h. After treatment, tissue were cut and stained with eosin. Sections were examined under a light microscopy (Motic DMW B1-223ASC) to detect any damage to the tissue during in vitro permeation.
Stability study
Stability study was carried out at elevated temperature 40 °C ± 2 °C and 75 ± 5% relative humidity, at room temperature (25 °C ± 2) and 65 ± 5% relative humidity, and at 4 °C ± 2 and 55 ± 5% relative humidity for 3 months (Gupta & Sharma, Citation2009). Samples were withdrawn every 1 month and analyzed for parameters like appearance, viscosity, drug content, pH, % drug released, etc. At the end of 3 months, the values of each observation were compared with the initial parameters.
In vivo study
Animal experiment and drug administration
Animal experiments were carried out in accordance with protocol submitted to IAEC as per CPCSEA guidelines. The protocol was approved by the Institutional Animal Ethical Committee of Modern College of Pharmacy, Nigdi, Pune- 44 with reference No. MCP/IAEC/71/2012 as per CPCSEA guidelines. Animals were obtained from National Toxicological Centre (NTC), Pune, India. The rats were fasted for 18 h prior to and during the pharmacokinetic study. The Sprague-Dawley male rats weighing approximately 230–270 g were used for the study and they were allowed free access to food and water (Vyas et al., Citation2005). The animals were divided into two groups A and B as shown in .
Table 4. In vivo study.
Group A received optimized in situ gel formulation and Group B aqueous solution. The blood samples and brain tissue were collected from each group to analyze the quantity of drug after administration through both routes.
Sample collection and analysis
After dose administration of formulation to each group, blood samples were withdrawn from retro orbital vein at time intervals of 15, 60, 120, 300 min and were collected in EDTA tubes to prevent clotting of blood. Blood samples were centrifuged at 5000 rpm for 10 min to separate plasma. Animals were kindly sacrificed for collection of brain tissue at time intervals of 15, 60, 120 and 300 min from each group. Brain tissue was separated carefully then rinsed with sufficient volume of normal saline solution and kept in filter paper to remove the excess of blood. Brain tissue was mixed with one volume normal saline solution, and homogenates in a tissue homogenizer and supernatant were collected for analysis. Plasma samples and brain tissue homogenates were stored at –22 °C until analysis. Drug concentrations in plasma samples and brain tissue homogenates were determined by using predeveloped UPLC/MS (Q-Exactive Orbitrap) technique (Kumbhar et al., Citation2013). Sumatriptan from the plasma was extracted using protein precipitation extraction technique. The supernatant is taken and transferred to vials and used for UPLC/MS analysis (Elshafeey et al., Citation2009; Piao et al., Citation2010).
Pharmacokinetic studies
Pharmacokinetic parameters were derived from plasma concentration versus time plot. Area under the curve (AUC), peak plasma concentration (Cmax) and time to attain peak concentration (Tmax) were obtained from these plots (Brahmankar & Jaiswal, Citation2002; Zaki et al., Citation2007). The drug targeting to brain after intranasal administration can be evaluated from drug targeting index DTI given by following equation:
Results
FTIR
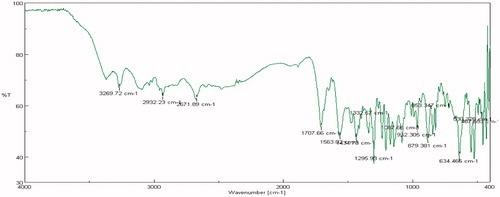
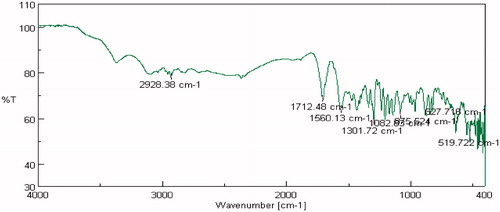
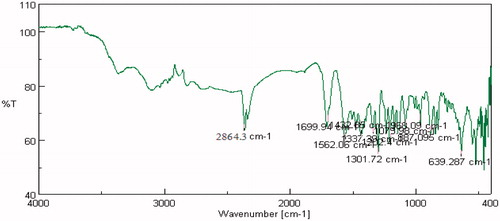
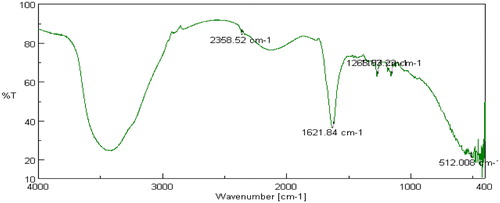
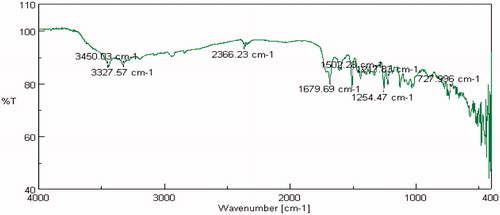
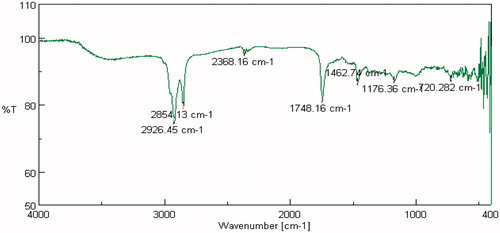
FTIR studies were carried out on pure drug as well as in combination with selected polymers. Drug spectrum shows prominent peaks at 3376.44 cm–1, 3104.2 cm–1, 1343.41 cm–1, 1205.73 cm–1 and 1139.88 cm–1 corresponding to the –NH stretching aromatic ring (C-H), sulfonamide, ter. amine and O–H stretching, respectively, as shown in . Drug:polymer mixture spectrum in shows absence of characteristic drug peaks at 3376.44 cm–1 corresponding to –NH stretching. In both cases, it was observed that the characteristic bands did not shift appreciably, suggesting the lack of any interaction between the drug and polymer. The infrared spectra of sumatriptan succinate and physical mixture of formulation are shown in . IR spectra of gellan gum with ingredients of simulated nasal fluid, that is, NaCl, KCl, show no significant changes in spectra peak comparative to pure gellan gum IR peaks shown in and . But for IR spectra with CaCl2, as shown in , the characteristic peak at 1748.16 cm–1, indicate the interaction of COO– with Ca++.
DSC
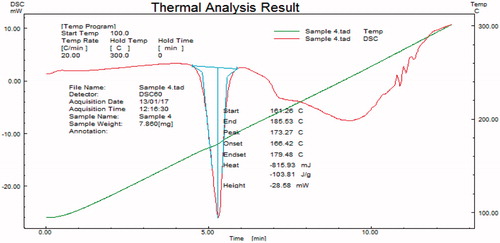
Samples subjected to DSC were observed for any change in characteristics of thermogram. From , it was confirmed that drug was crystalline in nature with a melting point 173.27 °C.
pH of formulation
The pH of all formulation was found to be in the range of 4.66 to 5.53 which is in the nasal (4.5–6.4) pH range (Nisha et al., Citation2012).
Drug content
shows that the percent drug content for all formulations of the drug contents were found in the range of 96–101%. The tests were carried out in triplicate.
Table 5. Result of optimized batches (for sol).
Refractive index
Refractive index was close to refractive index of water, that is 1.33. Hence, all formulations shown in were clear in appearance.
Viscosity
shows the viscosity values obtained for all formulation using Brookfield viscometer. Formulation F7 containing gellan gum 0.2% w/v exhibited optimum viscosity as well as mucoadhesive strength. The results also showed that the increase in PEG400 decreases the viscosity.
Table 6. Result of optimized batches (for gel).
Mucoadhesive strength
showed mucoadhesive strength for formulations F1 to F9 with increased concentration of PEG400 from 4% to 6% w/v. The stronger the mucoadhesive force is, the more it can prevent the gelled solution coming out of the nasal cavity as well as drain into the nasopharynx.
Spreadability
It is very important for in situ gel to have suitable spreadability to administer easily and to spread easily on nasal mucosa without leakage after administration. shows the data of spreadability measurement. Formulation F7 shows maximum spreadability due to more surface area covered by in situ gel after placing on filter paper.
Gel strength
The gel strength was found to be affected by concentrations of gelling and bioadhesive polymers. It is very important that the nasal gel formulation must have suitable gel strength. shows the data of gel strength measurement. This study revealed that the gel strength of nasal gel formulation is at 33–34 °C, increased as the concentration of gellan gum increased.
In vitro drug release study
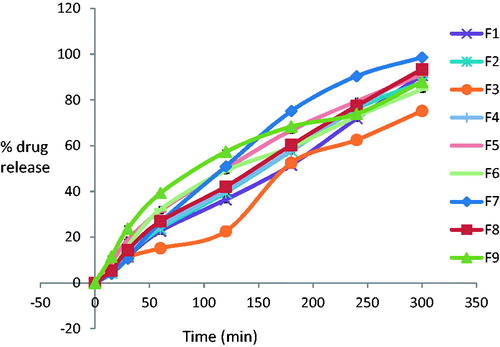
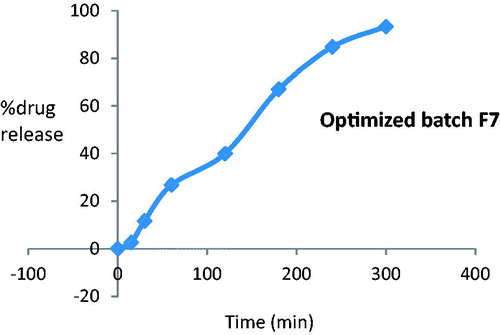
The in vitro release was carried out for all formulation using phosphate buffer pH 6.4 as medium. The in vitro drug release study revealed that the release rate depended on gellan gum concentration. The higher the gellan gum concentration, the lower the rate of drug release. The data of these studies are presented in . The formulation F7 containing 0.2% w/v gellan gum and 6% w/v PEG 400 showed 98.57% drug release within 5 h which was satisfactory. In general, it was found that drug release for all formulations, F1 to F9, was more than 90% after 5 h. This is shown in .
Drug release mechanism
The correlation coefficient (r2) values for various release models, that is, zero-order, first-order, Higuchi and Korsemeyer and Peppas, were found. Whereas release exponent, n, was less than 0.5, for F-1 to F-8 as shown in , indicating that release mechanism followed Fickian release and suggesting erosion diffusion mechanism for the tested sumatriptan succinate mucoadhesive system.
Table 7. Model fitting of experimental formulation.
Ex vivo permeation studies
Ex vivo permeation was observed for formulation F7 by using sheep olfactory nasal mucosa which comprises of thickness of about 0.6 mm. It was observed that permeation of optimized formulation F7 has shown release of 93.33% at the end of 300 min as shown in .
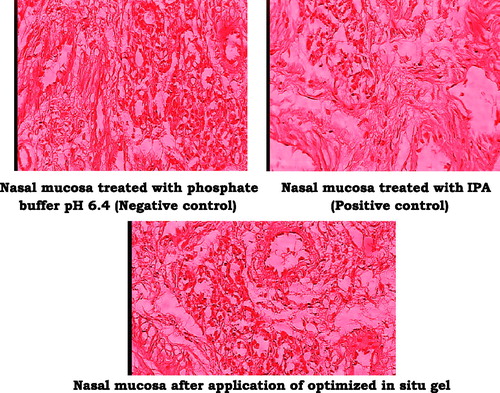
Histopathological evaluation of nasal mucosa
The microscopic observation indicates that the optimized formulation has no any significant effect on the microscopic structure of mucosa as shown in . Neither cell necrosis nor removal of the epithelium from the nasal mucosa was observed after application of formulation and buffer pH 6.4.
Stability study
Stability study was carried out in humidity control chamber (Oswal scientific) where the temperature was set at 40 ± 2 °C/75% ± 5% relative humidity, at room temperature (25 °C ± 2 °C) and at 4 °C ± 1 °C for 3 months as shown in .
Table 8. Stability study.
Effect of temperature/time on drug content
At the room temperature (25 °C ± 2 °C), at 4 °C ± 1 °C and at 40 ± 2 °C/75% ± 5% relative humidity, drug content remains constant during and at the end of 3rd month.
Effect of temperature/time on drug release
In vitro drug release study showed gradual decrease in drug release with respect to initial drug release. The decrease in drug release at all stated conditions of storage was found significant at the end of 3rd month. This was attributed to significant increase in viscosities of preparation at the same conditions. However, no significant change observed in drug release during and at the end of 2nd month.
Effect of temperature/time on viscosity
Viscosity showed significant increase at the end of 3rd month due to evaporation of vehicle of the preparation and reduction in the total volume of sol. This was observed at all stated conditions of storage.
In vivo study
Concentration of sumatriptan succinate in plasma
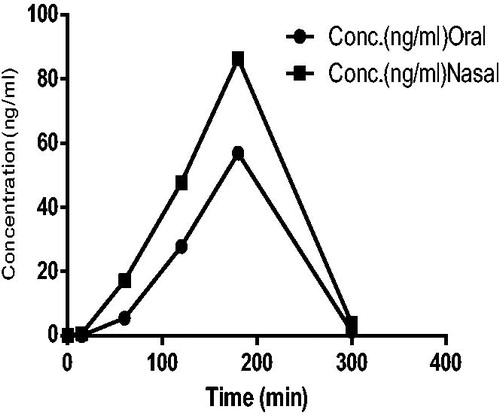
Pharmacokinetic parameters were calculated from the observed plasma concentration time profiles. The values of Cmax, Tmax, AUC 0–5 (ng.h/ml) and ratio of (AUC brain tissue/AUC plasma) % are shown in . Sumatriptan succinate attained a high concentration of 86.34 ng/ml and decreases after 3H for nasal in situ gel and for oral aqueous solution it was 56.90 ng/ml. Concentration of drug in plasma Cmax was higher by nasal in situ gel comparative to oral aqueous solution as shown in . The Tmax remains constant for nasal in situ gel and oral aqueous solution, respectively. The AUC 0–5 was 11 778 and 7151 ng.h/ml for nasal in situ gel and oral aqueous solution, respectively.
Table 9. Pharmacokinetic parameters of sumatriptan succinate in situ gel for oral and nasal route.
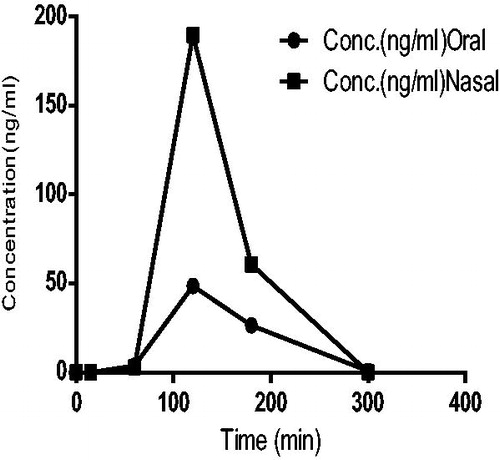
Concentration of sumatriptan succinate in brain tissues
The Cmax, AUC 0–5 (ng.h/ml) of the groups received nasal in situ gel of sumatriptan succinate were found to be higher than the groups receiving orally as shown in . Ratio of (AUC brain tissue/AUC plasma) % for nasal and oral groups of in situ gel of sumatriptan succinate and aqueous solution of drug were 144.43% and 77.40%, respectively. From this it is being concluded that concentration of drug in brain tissue administered through nasal route could be increased compared with oral route. In addition, the AUC values for brain tissues of nasal administration were higher than oral route administration as shown in .
Drug targeting index of sumatriptan succinate in brain tissues after intranasal administration in rats was found to be 1.866.
Discussion
The prolonged residence of drug formulation in the nasal cavity is of utmost importance for intranasal drug delivery. The proposed aim was to develop successfully mucoadhesive ion-activated in situ gel of sumatriptan succinate with the help of ion-activated polymer deacetylated gellan gum in presence of cations of simulated nasal fluid at physiological temperature of nasal cavity, that is 32–34 °C. From compatibility studies, it was concluded that there was no significant interaction found between drug and excipients. The results revealed that all the formulations provided acceptable pH range. This will help to avoid local irritation to nasal mucosa and other related changes produced by formulation. Content uniformity was found to be uniform in all the formulation and the values of refractive index were compared with water. Good clarity was found in formulations. The sols were mixed with simulated nasal fluid, immediately viscous gel was formed. In the selection of the concentration of the gelling polymer, a compromise is sought between satisfactory gel strength for use as a delivery vehicle and an acceptable viscosity for ease of spraying. The viscosities directly depend on polymeric content of formulation. The viscosity of gels increased with increasing concentrations of gellan gum (0.2%w/v, 0.3%w/v and 0.4%w/v).
The mucoadhesive force is an important physicochemical parameter for prolonging nasal retention time; assessment of mucoadhesive strength in terms of detachment stress showed that gellan gum preparation possessed adhesive properties. Mahajan et al. (Citation2009) reported that if the mucoadhesive force is too excessive, the gel can damage the nasal mucosal membrane.
Assessment of spreadability in terms of area covered by in situ nasal gels per unit time revealed that spreadability was related to the viscosity of in situ nasal gels. Increase in concentration of mucoadhesive polymer decreases the distance traveled by in situ nasal gels, because mucoadhesive polymer increases the viscosity of in situ nasal gels. Optimal in situ gel must have suitable gel strength so as to be administered easily and can be retained at nasal mucosa without leakage after administration. The release of the drug from the formulation depends upon viscosity of the formulation. The results clearly showed that the mucoadhesive nasal in situ gel have the ability to retain sumatriptan succinate in its matrix network. Gellan gum undergoes gelation in the presence of cations via a chemical bonding between the divalent cations and two COO− groups of glucuronic acid molecules in gellan chains. Even at lower concentrations of gellan gum, the drug release was sustained. Drug release seemed to slow down with an increase in gellan concentration. As the formulation becomes gel at the site of application, drug release takes place from high concentration to low concentration, that is, it can follow Peppas model for drug release. From R2 value, it was concluded that the drug release profile from most of the batches followed Peppas model. Formulation F7 exhibited good drug release profile with favorable rheological properties. The release of sumatriptan succinate from the gel formulation was found to be up to 5 h and that may be due to the inverse relationship between viscosity and drug release. The gel formulation seems to be safe with respect to nasal administration.
The sol form of preparation was found stable at 40 ± 2 °C/75% ± 5% relative humidity, room temperature (25 °C ± 2 °C) and at 4 °C ± 1 °C with respect to drug content and drug release up to two months, though there was little increase in viscosity of the sol. From pharmacokinetic studies, it was concluded that nasal route provides a passage for drugs acting on brain tissues. In addition, it is suitable for sumatriptan succinate which has limited capacity to cross blood–brain barrier. The components of optimized in situ gel were helpful for permeation of drug molecules across the nasal mucosa through olfactory pathways.
Conclusion
Ion-activated in situ gel of sumatriptan succinate can be prepared successfully by using gellan gum. A 32 full factorial design was found suitable to optimize formulation. The optimized batch showed drug release of 98.57% within 5 h. The inclusion of polyethylene glycol polymer counteracted the effect of mucoadhesive polymer whereby it decreased the gel consistency as well as increases in vitro drug diffusion. Ex vivo studies on sheep olfactory nasal mucosa showed 93.33% within 5 h. Histopathological studies of optimized batch was found to be safe and it was stable for three months in the accelerated stability studies.
Sumatriptan was estimated in rat plasma by using UPLC/MS technique. The concentration (Cmax) of sumatriptan in plasma and brain tissues was achieved higher by nasal in situ gel comparative to oral aqueous solution. Animal studies have shown that AUC was higher in plasma and brain for in situ gel administered by nasal route than AUC obtained by oral administration. The AUC of sumatriptan in brain tissues was achieved 1.44 times higher by nasal in situ gel comparative to AUC in plasma. Drug targeting index for brain tissues was found to be 1.866, this indicates sumatriptan can reach to brain via olfactory pathway.
Thus, the prepared in situ gel proved to be suitable for administration of sumatriptan succinate through nasal route. Hence, this can be viewed as a viable alternative to conventional nasal drops by virtue of its ability to enhance nasal residence time and thereby intranasal bioavailability. The ease of administration coupled with less frequent administration, thus enhancing patient compliance.
Declaration of interest
Authors report no declaration of interest.
Acknowledgements
The authors are grateful to the National Chemical Laboratory, Pune, India, for providing instrumentation and necessary facilities to carry out bioanalytical work and Cipla Ltd. Mumbai, India, for providing gift sample of pure sumatriptan succinate.
References
- Badgujar SD, Sontakke MA, Narute DR. (2010). Formulation and evaluation of sumatriptan succinate nasal in-situ gel using fulvic acid as novel permeation enhancer. Int J Pharm Res Develop 2:1–8
- Bajaj IB, Survase SA, Saudagar PS. (2007). Gellan gum: fermentative production, downstream processing and applications. gellan gum – review. Food Technol Biotechnol 45:341–54
- Brahmankar DM, Jaiswal SB. (2002). Biopharmaceutics and pharmacokinetics. A Treatise. 1st Edition. Delhi: Vallabh Prakashan, 1–74
- Basu S, Bandyopadhyay A. (2010). Development and characterization of mucoadhesive in- situ nasal gel of midazolam prepared with Ficus carica mucilage. Eur J Pharma Sci 11:1223–31
- Dey S, Mahanti B. (2011). Nasal drug delivery: an approach of drug delivery through nasal route. Der Pharmacia Sinica 2:94–106
- Dhuria SV, Hanson LR, Frey WH. (2010). Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci 99:1654–73
- Elshafeey AH, Bendas ER, Osama HM. (2009). Intranasal microemulsion of sildenafil citrate: in-vitro evaluation and in-vivo pharmacokinetic study in rabbits. AAPS Pharm SciTech 10:361–7
- Gaikwad V. (2010). Formulation and evaluation of in-situ gel of metoprolol tartrate for nasal delivery. J Pharm Res 3:788–93
- Gowda DV, Tanuja D, Khan MS. (2011). Formulation and evaluation of in situ gel of diltiazem hydrochloride for nasal delivery. Der Pharmacia Letter 3:371–81
- Gupta H, Sharma A. (2009). Ion activated bioadhesive in-situ gel of clindamycin for vaginal application. Int J Drug Delivery 1:32–40
- Pio HM, Balakrishnan P, Cho HJ. (2010). Preparation and evaluation of fexofenadine microemulsion for intranasal delivery. Int J Pharm 395:309–16
- Jadhav KR. (2007). Nasal drug delivery system-factors affecting and applications. Current Drug Therapy 2:27–38
- Jaya Raj Kumar K, Jayachandran E, Srinivas GM. (2010). Formulation and evaluation of pH–induced povidone iodine in-situ gel for oralthrush. J Pharm Sci & Res 2:294–301
- Jess C, Rhodes CT. (1990). Drug stability principles and practices. 3rd edition. Delhi, India: CBS Publisher, 252–5
- Kumbhar AB, Galgatte UC, Warkad S, Santhakumari B. (2013). Development and validation of a sensitive bioanalytical method for the determination of sumatriptan in rat plasma by UPLC-MS. Int J Pharm Pharm Sci 5:78–82
- Kushwaha KS, Keshari R, Rai AK. (2011). Advances in nasal trans-mucosal drug delivery. J Appl Pharm Sci 1:21–8
- Gibaldi M, Perrier D. (1982). Pharmacokinetics. New York: Marcel Dekker
- Majithiya RJ, Ghosh PK, Umrethia ML. (2006). Thermoreversible mucoadhesive gel for nasal delivery of sumatriptan. AAPS Pharm Sci Tech 7:1–7
- Mahajan H, Shaikh H, Gattani S, Nerkar P. (2009). In situ gelling system based on thiolated gellan gum as a new carrier for nasaladministration of dimenhydrinate. Int J Pharm Sci Nanotechnol 2:544–9
- More HN, Hajare AA. (2008). Practical physical pharmacy. 1st edn. India: Career Publications, 236–8
- Morris ER, Nishinari K, Rinaudo M. (2012). Gelation of gellan – A review. Food Hydrocolloids 28:373–411
- Nisha GS, Maithi P, Charyulu RN. (2012). Formulation and development of nasal in-situ gels of triptans for anti migraine activity. Int J Pharm Biomedical sci 3:861–4
- Nirmal HB, Bakliwal SR, Pawar SP. (2010). In-situ gel: new trends in controlled and sustained drug delivery system. Int J Pharm 2:1398–408
- Noha MZ, Gehanne AA, Nahed DM. (2007). Enhance bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesive in-situ gel with modulated rheological and mucociliary transport properties. Eur J pharm sci 32:296–307
- Pavia DL, Gary ML, George SK. (2007). Spectroscopy. Indian edition. Delhi, India: Cengage Learning, 26–107
- Pires A, Fortuna A, Alves G, Falcao A. (2009). Intranasal drug delivery: how, why and what for? J Pharm Pharm Sci 12:288–311
- Ryan R, Elkind A, Baker CC. (1997). Sumatriptan nasal spray for the acute treatment of migraine: results of two clinical studies. Neurology 49:1225–30
- Skoog DA, Holler FJ, Crouch SR. (2007). Instrumental analysis. Indian edition. Delhi, India: Cengage Learning, 477–8
- Shastri DH, Prajapati ST, Patel LD. (2010). Thermoreversible mucoadhesive ophthalmic in-situ hydrogel: design and optimization using a combination of polymers. Acta Pharm 60:349–60
- Shinde JV, Mali KK. (2008). In-situ mucoadhesive nasal gels of metoclopramide hydrochloride: preformulation and formulation studies. J Pharm Res 1:88–96
- Singh AK, Singh A, Satheesh Madhav NV. (2012). Nasal cavity: a promising transmucosal platform for drug delivery and research approaches from nasal to brain targeting. J Drug Delivery Ther 2:22–33
- Ugwoke MI, Agu RU. (2005). Nasal mucoadhesive drug delivery: background, applications, trends and future perspectives. Adv Drug Dev Rev 57:1640–65
- Uttarwar S. (2012). Formulation and development of in-situ gelling system for nasal administration for an antiemetic drug ondansetron hydrochloride by using pluronics 127P and pluronics 68. Int J Res Pharm Biomed Sci 3:1103–18
- Vyas TK, Babbar AK, Sharma RK. (2005). Preliminary brain-targeting on intranasal mucoadhesive microemulsion of sumatriptan. AAPS Pharm Sci Tech 7:E1–9
- Zaki NM, Awad GA, Mortuda ND. (2007). Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesive in-situ gel with modulated rheological and mucociliary transport properties. Eur J Pharm Sci 32:296–300