Abstract
The goal is to develop an in situ gel system comprising anionic liposomes (AL) containing bleomycin A6 (BLM A6) dispersed within the thermosensitive in situ gel for sustained release. The results indicated that the gelation temperature decreased due to AL within gel. Similarly, viscosity and mechanical parameters, such as gel strength for gel, could be enhanced by inducing lipid material with negative charge (phosphatidylglycerol) at 37 °C, which provided against corrosion at physiological condition. The in vitro release experiments performed with a dialysis method revealed that in situ gel with AL exhibited the longer drug-release period compared to that with or without nonionic liposomes. An in vivo fluorescence imaging study suggested that the gel with AL loading FITC-BLM A6 stayed in administration site at least for five days. A thermosensitive in situ gel with anionic liposome was a promising carrier for hydrophilic BLM A6, to be used in parenteral delivery system for anti-tumor treatment.
Introduction
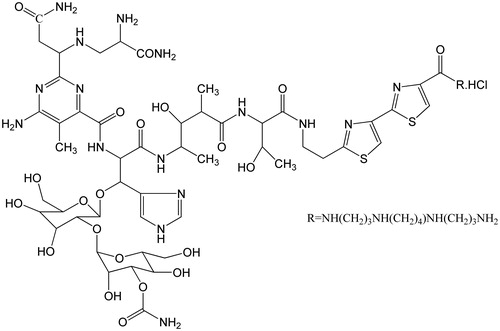
Bleomycin A6 (BLM A6), a derivative from bleomycin, is a glycopeptides antibiotic originally produced by the bacterium Streptomyces verticillus, inducing DNA strand breakage (Moeller et al., Citation2008) (). It is commonly used in the treatment of esophageal cancer, hepatocellular carcinoma, colon cancer hepatic metastasis and colorectal cancer (Jiang & Zhen, Citation1987; Deng et al., Citation2001; Liu et al., Citation2001; Li et al., Citation2002; Tang & Yang, Citation2002). However, higher doses and frequent dosing are required due to a narrow therapeutic window, a short half-life and fast clearance speed in vivo for BLM A6 (Ma et al., Citation2005). Thus, there is a need for the development of some suitable delivery system like long-acting injections prolonging the release of drugs for an extended duration of time (Sadekar & Ghandehari, Citation2012).
During the last two decades, injectable in situ gels have attracted considerable attention as polymeric drug carriers (Haglund et al., Citation1996), and then great interest has arisen on the applications of in situ gels in injectable drug delivery systems (Park et al., Citation2013; Fan et al., Citation2014; Lopez-Noriega et al., Citation2014; Ni et al., Citation2014; Peng et al., Citation2014). These systems are in situ gel delivery systems, exposed to body temperature (∼37 °C), are capable of getting converted to a very high viscous gel, though remaining fluid at room temperature (He et al., Citation2008). The gel network that remains insoluble in water and retains shape for a long period can become an appropriate carrier for biomacromolecules (Baroli, Citation2010). For localized therapy, injection of in situ gel will cause the formation of a depot at the site of administration, which continuously and slowly releases the drug to the target tissue. Besides, the gel can deliver a drug throughout the tumor, thereby decreasing systemic toxicity, which is also an advantage over actively or other passive targeted therapies (Yang et al., Citation2009). Commercially available Pluronic F127 (F127) has been introduced in the late 1950s and since then it has been proposed for diverse pharmaceutical applications (e.g. IV, inhalation, oral solution, suspension, ophthalmic or topical formulations) (Rowe et al., Citation2005). Presented by FDA guide as an inactive ingredient, it is one of the most important thermoreversible gels and has been applied in local drug delivery such as intraperitoneal, intramuscular and subcutaneous injections (Gong et al., Citation2009; Lippens et al., Citation2010), and is listed in the US and European Pharmacopoeia. Liposomes, widely used in parenteral systems, provide many advantages for delivery, in that they can increase drug loading and yield slower, more prolonged drug release compared with pure gel besides drug targeting. On the other hand, a system containing pluronic together with liposome could be of great interest for they fully integrate two characteristics such as the thermogelling properties of F127 and the sustained release ability of the liposome.
The aim of this study was to investigate the effectiveness of local injection of BLM A6-loaded liposomes within the thermosensitive in situ gel in order to extend the release and action time of drug. In this study, vesicle size, encapsulation efficiency, gelation temperature, rheological characteristic, texture analysis, in vitro release and in vivo retention were investigated. This work lays the foundation for developing a BLM A6 delivery system combining the in situ gel characteristic of pluronic with the merits offered from liposomes.
Materials and methods
Materials
BLM A6 was provided by Tianjin Hebei Pharm. (Tianjin, China). Phosphatidylcholine (PC S100), dipalmitoylphosphatidylglycerol (DPPG) was obtained from Lipoid (Ludwigshafen, Germany). Cholesterol (Chol) was purchased from Sigma-Aldrich Co. (St Louis, MO). F127 and Pluronic F68 were obtained from BASF Corp. (Shanghai, China). Other reagents were analytic graded.
Animals
Healthy male Kunming mice weighing 20 ± 1 g were supplied by the Animals Center of Peking University Health Science Center. All care and handling of animals were performed with the approval of Institutional Authority for Laboratory Animal Care of Peking University.
Liposome preparation by the reverse-phase evaporation method
BLM A6-loaded nonionic liposomes (BLM A6-NL) were prepared by the reverse-phase evaporation method described by Szoka & Papahadjopoulos (Citation1978). PC S100 (40 mmol) and Chol (20 mmol) dissolved in chloroform were placed in a round-bottom flask and dried in a rotary evaporator under reduced pressure at 30 °C to form a thin film on the inner flask surface. The film was hydrated with 4 ml of the aqueous phase containing BLM A6 (4 mg) in a ratio of 4:1 (v/v) between organic and aqueous phases. The mixture was sonicated at room temperature for 5 min and placed in a round-bottom flask. The organic solution was removed under reduced pressure at room temperature. Finally, the material formed a viscous gel that became an aqueous suspension by shaking in a vortex. After size-exclusion chromatographic separation on Sephadex G25 (Pharmacia Biotech, Uppsala, Sweden) in order to remove encapsulated drug, the liposomes were diluted with water until a final phospholipid concentration of 10 mmol/L was obtained. They were then extruded at room temperature into a Liposofast device (Avestin Inc., Ontario, Canada) through two polycarbonate membranes with a pore size of 0.2 µm (MacDonald et al., Citation1991).
BLM A6-loaded anionic liposomes (BLM A6-AL) were prepared following the same procedure but with the addition of DPPG (20 mmol).
Liposome characterization
The particle size, polydispersity index (PDI) and zeta potential of the vesicle was measured by the dynamic light scattering method using a Zetasizer Nano-Z (Malvern Instruments, Worcestershire, UK) by diluting the liposomal dispersion with ultrapure water. The zeta potential (an indirect measurement of surface charge) of the various formulations was measured in ultrapure water using the same instrument.
The entrapment efficiency (EE) of the liposomes was determined by centrifugation in an Eppendorf centrifuge (11 000 × g; 20 min) through Microcon YM-10 Centrifugal Filter Devices (Millipore Inc., Billerica, MA) with a cut-off value of 3 kDa for 1 h at 4 °C. BLM A6 concentration was determined by high-performance liquid chromatography (HPLC) as described below. The EE expressed in percentage (%) was calculated by dividing the drug to lipid ratio that was recovered after ultrafiltration in the final formulation by the initial amount of drug and lipid.
BLM A6 HPLC assay
An HPLC system (LC-10AT; Shimadzu Corporation, Tokyo, Japan) was used in the reverse-phase mode. Analysis was performed on a reverse-phase column (250 mm× 4.6 mm ID). The mobile phase was mixed with a methanol, acetonitrile and sodium hexanesulfonate solution (pH 4.3) at a volume ratio of 7:3:15 (v/v/v), vacuum-filtered through a 0.45-μm filter and degassed before use. The flow rate was kept constant at 1 ml/min at room temperature. An ultraviolet detector (SPD-10A; Shimadzu) was used at 254 nm.
Preparation of in situ gels
The cold method, as formerly described, was applied (Choi et al., Citation1998; Cafaggi et al., Citation2008). F127 and F68 were slowly added in the calculated amount of cold water at room temperature to obtain an aqueous solution. Both solutions were stored in a refrigerator until use. After cooling in a refrigerator, an appropriate amount of these solutions was added to 1 mg/ml (w/v) BLM A6 solutions or liposomes containing the same amount of BLM A6, which were further diluted with water to obtain the desired weight fraction. All solutions were stored in a refrigerator (4 °C) until use.
Visual measurement of gelation temperature (Tsol–gel)
The Tsol–gel of the prepared formulations was determined as described previously (Kim et al., Citation1998; Yong et al., Citation2001). A 50-ml transparent vial containing a magnetic bar and 10 g of the gel was placed in a low-temperature thermostat water bath at < 10 °C with a thermosensor immersed in the gel and heated at a speed of 0.5 °C/min with constant stirring. The Tsol–gel – the point at which the magnetic bar stopped rotating because of gelation – was recorded by the thermistor. Measurements were performed in triplicate.
Rheological studies
The viscosity of the prepared formulations was determined using a Brookfield DV-II + viscometer (Brookfield, Middleborough, MA) on 10 ml of the sample. The shear stress of the sample solutions was measured at different shear rates at 25 °C and 37 °C. A typical run consisted of changing the shear rate from 0 to 200 s−1 at a controlled ramp speed within 2 min. The hierarchy of the shear rate was then reversed (200–0 s−1) for a similar 2-min period. The average value of two readings was used to calculate the shear stress.
Mechanical characterization: texture analysis
A software-controlled dynamometer (CT3 Texture Analyzer; Brookfield Engineering Laboratories, Inc.) was used for the mechanical characterization of the gel samples (Alves et al., Citation2000). The gels were kept in thermostat-controlled baths at 25 °C and 37 °C. After different intervals of time, the systems were tested using penetration/withdrawal experiments. From a penetration/withdrawal experiment, several parameters can be derived: system hardness and adhesive force, i.e. the maximum positive force and the maximum negative force registered while attaining the imposed deformation, respectively; the work of cohesion (gel strength), i.e. the positive area under the force–time curve from zero to the maximum imposed deformation; and the work of adhesion (adhesiveness), i.e. the negative area under the force–time curve.
The gel resistance to penetration and withdrawal of an ebonite cylindrical probe with a diameter of 10 mm (P10) was measured. The test and post-test speeds were 0.5 m/s, and the penetration depth was 20 mm, being imposed a fixed deformation of 20% with an acquisition rate of 200 points/s. All measurements were performed in triplicate.
In vitro drug release behavior
Drug release studies were performed in an incubation room (37 °C) for different gel formulations loading BLM A6 (Liu et al., Citation2007; Patois et al., Citation2009). The in situ gel samples (5 ml) were inserted into the semipermeable dialysis bag. The bag, sealed with a clamp to prevent leakage, was then placed in a dissolution tester. A release test was performed with 100 ml of PBS (pH 7.2) as a release medium and stirred at speed of 100 rpm. At specified time intervals, 5 ml of the medium was withdrawn and filtered. After sampling, an equivalent amount of PBS (pH 7.2) preheated at 37 °C was replenished to maintain a constant volume. The amount of BLM A6 in the release medium was measured by HPLC as described above. The halftimes for drug release (t1/2) were calculated from exponential best fits to the release profiles as the time at which the internal drug concentration was half the initial concentration (Johnston et al., Citation2007).
Studies of in vivo retention
To examine the gel retention properties, an in vivo multispectral imaging system (Kodak, Eastman Kodak Company, Rochester, NY) was used (Ur-Rehman et al., Citation2011). FITC is an amine reactive fluorescent dye. The isothocyanate group readily reacts with the primary amine groups of BLM A6. The covalent attachment of the FITC to BLM A6 was carried out as follows. FITC (0.5 mg) was reacted with BLM A6 (120 mg) in carbonate buffer solution (pH 8.0) for 24 h in the dark. After the reaction, unconjugated FITC was removed through column chromatography. The FITC-labeled BLM A6 (FITC-BLM A6) was lyophilized after freeze-drying.
To evaluate the gel retention in vivo, subcutaneous injections of the gels (0.5 ml each) containing a comparable amount of FITC-BLM A6 loaded in anionic liposome within gel (FITC-BLM A6-AL) were injected on the dorsal side of the necks of mice. These mice were scanned at predetermined intervals in an IVIS® In Vivo Imaging System (Xenogen, Alameda, CA) over a time period up to five days. At selected times, side-view images of the mice were collected at a wavelength of 540 nm (excitation wavelength, 500 nm). To confirm the general traceability of the fluorescent label, 100 µL PBS (pH 7.4) containing FITC-BLM A6 (0.05%, w/v) was injected subcutaneously.
Results
Liposomal characterization
The liposomal vesicles presented with a narrow particle size distribution (a very low PDI of <0.23 was obtained for all formulations) (). The average vesicle size was 215.1 ± 4.2 nm for nonionic liposomes and 233.3 ± 2.7 nm for AL. The zeta potential of the AL was significantly lower than that of the nonionic liposomes, indicating the negative potential of the liposomes with DPPG. The encapsulation efficiency of the BLM A6-NL was 86.29 ± 3.04%, whereas that of the BLM A6-AL was 96.12 ± 2.88%. The significant difference in EE between the two formulations was attributed to the electrostatic interaction of the AL giving the BLM A6 a positive charge that hampered the leakage of BLM A6 from the liposomes during the preparation process.
Table 1. Characterization of various liposomal formulations (n = 3).
Phase transition temperature
The Tsol-gel of the different formulations is listed in . The mixed solution containing F127 (19% w/v), F68 (0.8% w/v) and BLM A6 (0.1% w/v) was 35.4 ± 0.5 °C. Based on the same concentration for F127 and for F68, temperatures of the gel with BLM A6-NL and with BLM A6-AL decreased to 30.4 ± 0.6 °C and 31.7 ± 0.5 °C, respectively. Decreased concentrations of F127 (18% w/v) and F68 (0.8% w/v) resulted in a Tsol–gel of the BLM A6-NL and BLM A6-AL at 36.2 ± 0.4 °C and 35.8 ± 0.6 °C, respectively (Cabana et al., Citation1997; Peng et al., Citation2013). The adjusted BLM A6-NL and BLM A6-AL formulations were evaluated as follows.
Table 2. Gelation temperature for different formulations with the same concentration of pluronic F127 (19% w/v) and Pluronic F68 (0.8% w/v) (n = 3).
Rheological studies
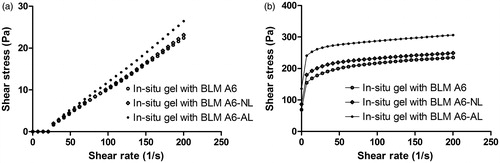
At 25 °C, the preparations were in a solution state and displayed a Newtonian behavior that demonstrated a linear relationship between shear rate and shear stress (). As the temperature was increased to 37 °C, the preparations entered a gel state, and the rheological behavior became non-Newtonian (). Fitted by Herschel–Bulkley equation, the rheograms described a plastic behavior of the gel at 37 °C. The influence of the presence of liposomes on the viscosity of the in situ gels composed of F127 (18% w/v) together with F68 (0.8% w/v) was then investigated. The results obtained with the gel containing BLM A6-NL showed little difference in shear stress (viscosity) compared to the in situ gels free of liposomes based on the same shear rate. With AL introduced, a clear increase in the shear stress (viscosity) of the in situ gel was observed, indicating that the addition of negative DPPG induced the gain of thermosensible properties for the in situ gel systems.
Mechanical characterization: texture analysis
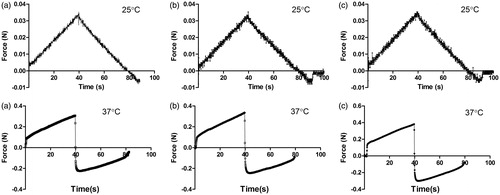
represents the profiles obtained from the penetration/withdrawal experiments investigated on in situ gel samples at 25 °C and 37 °C. Several parameters are shown in . First, all of the in situ gel samples showed no adhesiveness at 25 °C, indicating a solution state. Second, there was no significant difference among the in situ gel samples (p > 0.05) for any of the mechanical parameters, indicating that the liposomes within the gels had no effect on the in situ gels at room temperature. In contrast, at 37 °C, all of the mechanical parameters increased remarkably, indicating gel formation. Moreover, at increased hardness, the gel strength and adhesive force of the BLM A6-NL gel was observed, showing that the in situ gel imported by liposomes induced enhanced gel strength to a certain degree. Similar enhancement of all parameters was shown by BLM A6-AL compared to BLM A6-NL.
Table 3. Mechanical parameters for in situ gel systems at 25 °C and 37 °C.
Based on the rheology assay and texture analysis results, we report that in situ gel with negative lipids caused higher shear stress and gel strength compared with nonionic liposomes in the in situ gels, so that with BLM, A6-AL was used for the in vitro release and retention experiments.
In vitro drug release behavior
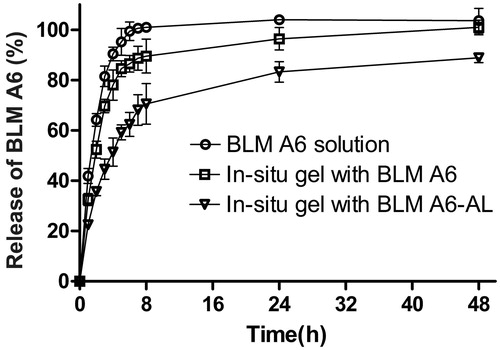
The effect of liposomes on the in vitro release of BLM A6 from the gels could be identified using the dialysis method. As shown in , the in situ gels exhibited typical biphasic release pattern with faster and constant continuous release at initial stages and slower release at later stages. It could be observed that the in situ gel with BLM A6 displayed a linear release characteristic of approximately 78% before 4 h compared with the BLM A6 solution control displayed a rapid release of nearly 90%, and then logarithmically increased release of 22% up to 48 h. In contrast, the in situ gel with BLM A6-AL showed a significantly retarded in vitro release of approximately 51% at 4 h with total release of approximately 83% at 48 h. T1/2 of gel with BLM A6 and BLM A6-AL was 1.97 and 3.82 h, respectively, indicating that the release rate of the in situ gel with BLM A6-AL was slowest among the above samples, and AL played an important role in impeding the release of BLM A6 from the gels.
In vivo retention
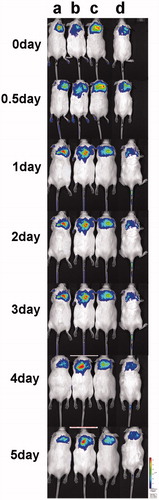
To gain information about the sustained characteristics in vivo, gels with FITC-labeled BLM A6-AL (FITC-BLM A6-AL) were injected subcutaneously into mice, and the arising fluorescence was imaged in vivo. It was assumed that the decrease of fluorescence intensity at the administration site over time largely reflected the release of FITC-BLM A6 from the gels. Using a FITC-BLM A6 solution without polymers as a control, it could be confirmed that free FITC-BLM A6 did not stay localized in the subcutaneous tissue at the administration site but was rapidly distributed such that no fluorescence signal could be detected after 24 h (Supplemental file). Therefore, the signals obtained within the long-term experiments with FITC-BLM A6-AL gels indicated successfully detainment of FITC-BLM A6 in the gel network. shows the fluorescence images of four mice (A–D) scanned at defined time points. Fluorescence emission of the FITC-BLM A6-AL in the gels lasted longer (five days), revealing a slower loss of fluorescence intensity. We also confirmed that the fluorescence signals of FITC-BLM A6-AL in the gel gradually disappeared seven days after administration.
Discussion
The goal of this work was to evaluate a novel BLM A6 delivery system based on incorporation of BLM A6-loaded liposomes within an in situ gel composed of pluronic, allowing for in vivo retention and leading to a long-lasting delivery of BLM A6 from this formulation.
Tsol–gel, i.e. the temperature at which the liquid phase transferred to a gel, was obviously a key parameter for in situ gel-forming systems. Based on the suitable Tsol–gel range (25–35 °C), pluronic F127, reported to be the less toxic of the commercially available pluronic (Talasaz et al., Citation2008), was an appropriate gelling agent. In our experiments, two liposomal formulations showed much lower Tsol–gel values compared with the blank in situ gel based on the same concentration of F127 and F68, indicating that liposome vesicles improved gelation. Moreover, there was no significant difference in Tsol–gel between the two liposome formulations (p > 0.05), indicating no effect of DPPG in a liposomal formulation on Tsol–gel. After appropriate decreases of the F127 concentration, the adjusted formulations showed similar Tsol–gel values, indicating comparability. Of course, another advantage of the formulations with lower polymer concentrations is the relatively lower incidence of adverse reactions.
Texture analysis, a penetrometry technique that has historically been used in the mechanical characterization of food materials (Barrett et al., Citation1994), has been employed increasingly for pharmaceutical gel analysis (Pons & Fiszman, Citation1996; Coviello et al., Citation2003, Citation2005). Liposomes containing BLM A6 within gels had little effect on fluid type at either 25 °C or 37 °C but showed some degree of increased viscosity (), indicating that the liposome was a vital factor that determined the rheological modification observed after liposome addition in gels (Boulmedarat et al., Citation2003; Mourtas et al., Citation2008). Similarly, as shown in , the addition of liposomes to the in situ gel solution clearly enhanced the mechanical parameters at 37 °C, inferring a lower probability of in vivo corrosion for gels with vesicles.
The release profile of the BLM A6 formulations exhibited a diphasic release pattern (). The first phase corresponded to the initial fast release in the first 2 h (∼30%; the “burst release”), which was due to the amount of BLM A6 that was present on or near the gel surface. This kind of release was advantageous as it took rapidly pharmacodynamic effect. The second phase demonstrated a constant continuous release of BLM A6 that was controlled by diffusion from the gel and liposomes. The in vitro release pattern of in situ gel with BLM A6-AL revealed that the presence of vesicles conferred sustained release of the entrapped molecule since the drug has first to pass across the phospholipid layer and then be released from the polymer. That is to say, as a result of the rigidity of the lipid bilayers and increased gel strength, the in situ gel with liposomes exhibited a significantly sustained release profile. More importantly, the electric static interaction brought about by DPPG decreased the escape opportunities of the loaded molecules with a positive outward charge. This fast–slow release pattern was potentially beneficial as a clinical regime that usually needed to quickly reach a therapeutic level and maintained the appropriate levels for prolonged amounts of time.
The effect of the presence of a vesicle within the gel on the release of the encapsulated drug was investigated by studying the release of encapsulated FITC-labeled BLM A6 from the gel. The experimental results showed () that after injection in the mice, the gel remained at the injection site, allowing for a slow release effect over a prolonged period of time (five days). The release properties of these delivery systems were affected by the gel network structure, polymer type and concentration, and, more importantly, the mechanical properties of the gels. The higher gel strength, hardness, adhesiveness and adhesive force of the BLM A6-AL gels could both dramatically slow down the gel corrosion and delay the diffusion of BLM A6 outward from the gels in vivo. Further studies of the long-term effect of the gel system on antitumor pharmacodynamics will be undertaken.
Conclusion
The results presented in this work indicated that the in situ gel with the anionic liposome delivery system could be used for the sustained delivery of small-molecular-weight hydrophilic compounds. The solution gels rapidly at 37 °C as demonstrated by the rheological analysis, and the release profile of the incorporated compound can be controlled by liposome introduction. High gel strength and interactions between liposomes and the in situ gel allowed for the sustained delivery of BLM A6. Such a formulation would be best suited for local sustained release of pharmaceutical delivery systems (e.g. sustained release of anticancer drugs injected intratumorally).
Supplemental Material.pdf
Download PDF (144.2 KB)Acknowledgements
We gratefully acknowledge support from the National Major Science and Technology Project of China (Pharmaceutical Research for Microbial Medicine and Biopharmaceutics, 2012ZX09301002-001).
Declaration of interest
The authors report no declarations of interest.
References
- Alves MM, Antonov YA, Goncalves MP. (2000). Phase equilibria and mechanical properties of gel-like water-gelatin-locust bean gum systems. Int J Biol Macromol 27:41–7
- Baroli B. (2010). Injectable hydrogels: from basics to nanotechnological features and potential advances. Berlin, Germany: Springer
- Barrett AH, Rosenberg S, Ross EW. (1994). Fracture intensity distributions during compression of puffed corn meal extrudates: method for quantifying fracturability. J Food Sci 59:617–20
- Boulmedarat L, Grossiord JL, Fattal E, Bochot A. (2003). Influence of methyl-beta-cyclodextrin and liposomes on rheological properties of Carbopol 974P NF gels. Int J Pharm 254:59–64
- Cabana A, Ait-Kadi A, Juhasz J. (1997). Study of the gelation process of polyethylene oxidea – polypropylene oxideb – polyethylene oxidea copolymer (poloxamer 407) aqueous solutions. J Colloid Interface Sci 190:307–12
- Cafaggi S, Russo E, Caviglioli G, et al. (2008). Poloxamer 407 as a solubilising agent for tolfenamic acid and as a base for a gel formulation. Eur J Pharm Sci 35:19–29
- Choi H-G, Jung J-H, Ryu J-M, et al. (1998). Development of in situ-gelling and mucoadhesive acetaminophen liquid suppository. Int J Pharm 165:33–44
- Coviello T, Alhaique F, Parisi C, et al. (2005). A new polysaccharidic gel matrix for drug delivery: preparation and mechanical properties. J Control Release 102:643–56
- Coviello T, Coluzzi G, Palleschi A, et al. (2003). Structural and rheological characterization of scleroglucan/borax hydrogel for drug delivery. Int J Biol Macromol 32:83–92
- Deng YC, Zhen YS, Zheng S, Xue YC. (2001). Activity of boanmycin against colorectal cancer. World J Gastroenterol 7:93–7
- Fan R, Deng X, Zhou L, et al. (2014). Injectable thermosensitive hydrogel composite with surface-functionalized calcium phosphate as raw materials. Int J Nanomedicine 9:615–26
- Gong CY, Shi S, Dong PW, et al. (2009). In vitro drug release behavior from a novel thermosensitive composite hydrogel based on Pluronic f127 and poly(ethylene glycol)-poly(epsilon-caprolactone)-poly(ethylene glycol) copolymer. BMC Biotechnol 9:8
- Haglund BO, Joshi R, Himmelstein KJ. (1996). An in situ gelling system for parenteral delivery. J Control Release 41:229–35
- He C, Kim SW, Lee DS. (2008). In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J Control Release 127:189–207
- Jiang M, Zhen YS. (1987). Antitumor activity of bleomycin A6 against human liver cancer in cell culture and in nude mice. Acta pharmaceutica Sinica 22:881–5
- Johnston MJ, Semple SC, Klimuk SK, et al. (2007). Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin. Biochim Biophys Acta 1768:1121–7
- Kim C-K, Lee S-W, Choi H-G, et al. (1998). Trials of in situ-gelling and mucoadhesive acetaminophen liquid suppository in human subjects. Int J Pharm 174:201–7
- Li ZD, Li Y, Zhen YS. (2002). Chemotactic peptide fMLP enhances antitumor activity of boanmycin. Chin J Canc 21:828–32
- Lippens E, Vertenten G, Girones J, et al. (2010). Evaluation of bone regeneration with an injectable, in situ polymerizable Pluronic F127 hydrogel derivative combined with autologous mesenchymal stem cells in a goat tibia defect model. Tissue Eng 16:617–27
- Liu XJ, Li Y, Zhen YS. (2001). Inhibitory effect of boanmycin on the growth of colon carcinoma 26 and hepatic metastasis in mice. Acta Pharmaceutica Sinica 36:14–8
- Liu Y, Lu WL, Wang JC, et al. (2007). Controlled delivery of recombinant hirudin based on thermo-sensitive Pluronic F127 hydrogel for subcutaneous administration: in vitro and in vivo characterization. J Control Release 117:387–95
- Lopez-Noriega A, Hastings CL, Ozbakir B, et al. (2014). Hyperthermia-induced drug delivery from thermosensitive liposomes encapsulated in an injectable hydrogel for local chemotherapy. Adv Healthc Mater [Epub ahead of print]
- Ma J, Feng F, Zhou L, et al. (2005). Pharmacokinetics study and clinical evaluation on boanmycin. China Pharm 17:1314–6
- MacDonald RC, MacDonald RI, Menco BP, et al. (1991). Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta 1061:297–303
- Moeller A, Ask K, Warburton D, et al. (2008). The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol 40:362–82
- Mourtas S, Haikou M, Theodoropoulou M, et al. (2008). The effect of added liposomes on the rheological properties of a hydrogel: a systematic study. J Colloid Interface Sci 317:611–9
- Ni P, Ding Q, Fan M, et al. (2014). Injectable thermosensitive PEG-PCL-PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects. Biomaterials 35:236–48
- Park MR, Seo BB, Song SC. (2013). Dual ionic interaction system based on polyelectrolyte complex and ionic, injectable, and thermosensitive hydrogel for sustained release of human growth hormone. Biomaterials 34:1327–36
- Patois E, Osorio-da Cruz S, Tille J-C, Walpoth B. (2009). Novel thermosensitive chitosan hydrogels: in vivo evaluation. J Biomed Mater Res A 91:324–30
- Peng M, Xu S, Zhang Y, et al. (2014). Thermosensitive injectable hydrogel enhances the antitumor effect of embelin in mouse hepatocellular carcinoma. J Pharm Sci 103:965–73
- Peng Q, Sun X, Gong T, et al. (2013). Injectable and biodegradable thermosensitive hydrogels loaded with PHBHHx nanoparticles for the sustained and controlled release of insulin. Acta Biomater 9:5063–9
- Pons M, Fiszman SM. (1996). Instrumental texture profile analysis with particular reference to gelled systems. J Texture Stud 27:597–624
- Rowe R, Sheskey P, Owen S. (2005). Pharmaceutical handbook of pharmaceutical excipients, 5th ed. Washington, DC: Pharmaceutical, London UK and American Pharmaceutical Association
- Sadekar S, Ghandehari H. (2012). Transepithelial transport and toxicity of PAMAM dendrimers: implications for oral drug delivery. Adv Drug Deliv Rev 64:571–88
- Szoka F Jr, Papahadjopoulos D. (1978). Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A 75:4194–8
- Talasaz AHH, Ghahremankhani AA, Moghadam SH, et al. (2008). In situ gel forming systems of poloxamer 407 and hydroxypropyl cellulose or hydroxypropyl methyl cellulose mixtures for controlled delivery of vancomycin. J Appl Polymer Sci 109:2369–74
- Tang H, Yang XP. (2002). Effect of boanmycin on apoptosis and cell cycle of human esophageal cancer(Eca-109) cells. Chin J Cancer 21:855–9
- Ur-Rehman T, Tavelin S, Gröbner G. (2011). Chitosan in situ gelation for improved drug loading and retention in poloxamer 407 gels. Int J Pharm 409:19–29
- Yang Y, Wang J, Zhang X, et al. (2009). A novel mixed micelle gel with thermo-sensitive property for the local delivery of docetaxel. J Control Release 135:175–82
- Yong CS, Choi JS, Quan Q-Z, et al. (2001). Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int J Pharm 226:195–205