Abstract
Introduction: Liver cancer or hepatocellular carcinoma (HCC) is a major cause of death worldwide. Targeted delivery of drug to the carcinoma cell can be achieved by conjugation of ligand on the carrier system.
Methods and materials: In this study, oxaliplatin-loaded hepatoma-targeted liposome were designed and prepared using galactosylated distearoylphosphatidylethanolamine. The liposomes were prepared by cast film method and coupled with lactobionic acid (LA-LP) using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide as a coupling agent. The coupling was confirmed by infrared spectroscopy. They were further characterized for various parameters such as vesicle shape and surface morphology, size, entrapment efficiency and in vitro release pattern.
Results and discussion: The vesicle size of the uncoupled liposome (256 nm) was found to be less than LA-LP (310 nm). The uptake of LA-LP and uncoupled liposomes by BEL7402 HCC cell lines was visualized using fluorescence microscopy that revealed the dependence of liposomal recognition and higher uptake of the LA-LP. Organ distribution studies provided evidence that coupling of lactobionic acid on liposomal surface significantly enhanced the tumor uptake of drug, which is reflected by recovery of higher percentage of drug from tumor as compared to uncoupled liposomes or free drug.
Conclusion: These studies suggest them as effective vectors for HCC targeting.
Introduction
Cancer is a disease in which control on growth is lost in one or more cells, leading to a solid mass of cells known as a tumor. The initial tumor growth, known as the primary tumor, often becomes life threatening by obstructing vessels or organs. However, death is commonly caused by spread of the primary tumor to one or more other sites in the body (by a process called metastasis). Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide (Blum, Citation2005). It is one of the sixth commonest type of cancer all along the globe. For the treatment of liver cancer, several anticancer drugs are used; this therapy is termed as chemotherapy. Conventional chemotherapy lacks selectivity for cancerous cells and causes damage to rapidly proliferating normal cells. Now-a-days, targeted drug delivery systems are used for reducing the side effects of conventional drug delivery system. The phenomenon of ligand targeted therapeutics is useful in delivering antineoplastic drugs effectively at target organ using carriers like liposomes, nanoparticles, etc. (Allen, Citation2002).
Liposomes are “lipid spheres” in which aqueous phase is surrounded by lipid layer as a bilayer configuration. Liposomes could be specifically targeted on cancerous cells using a specific ligand (Mishra et al., Citation2009). Identification of specific interactions between ligands and tumor cells is a prerequisite for ligand-mediated targeting. Ligands intended for use in tumor targeting of liposomal drugs should have well-defined propensity, affinity and specificity toward selective receptors or antigens present on the surface (Maruyama et al., Citation1990).
Doxorubicin, cisplatin (de Lope et al., Citation2012) and cabozantinib (Xiang et al., Citation2014) are few of the drugs proven to be effective for the treatment of liver cancer. Oxaliplatin is a novel anticancer agent, which works by inhibiting DNA synthesis in cancerous cells. This is due to virtue of formation of inter- and intra-strand cross links in DNA, which leads to inhibition of DNA replication and transcription, resulting in cell death (Culy et al., Citation2000; Graham et al., Citation2004; Harper et al., Citation2010). Oxaliplatin is successfully used in patients with unresectable, metastatic or recurrent HCC (Yen et al., Citation2008; Alberts et al., Citation2012). Furthermore, oxaliplatin has been safely used in patient with metastatic breast cancer and combined renal and hepatic failure (Honecker et al., Citation2006).
In order to achieve high degree of selectivity, liposomes coupled with a ligand that can bind to the asialoglycoprotein receptor (ASGPR) overexpressed in HCC were designed, which can provide specific degree of drug delivery (Wang et al., Citation2006). In this study, oxaliplatin-loaded liposomes, composed of biodegradable and biocompatible components namely hydrogenated soya phosphatidylcholine (HSPC) and disteroylphosphatidylethanolamine (DSPE) were prepared, which were conjugated with lactobionic acid (LA) for preferential drug delivery to the liver tumor cells.
Materials and methods
Chemicals
Oxaliplatin was obtained as a gift sample from Dr. Reddys Laboratories (Hyderabad, India). HSPC and DSPE were obtained as a gift sample from Lipoid (Ludwigshafen, Germany). Lactobionic acids, cholesterol, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and Sephadex G-50 were procured from Sigma Chemicals (St Louis, MO). Hank’s balanced-salt solution, HEPES buffer medium and dialysis membrane were procured from Himedia (Mumbai, India). Chloroform and methanol were purchased from Spectrochem Pvt. Ltd. (Mumbai, India).
Preparation and characterization of ligand coupled liposomes
Preparation of liposomes
Liposomes were prepared by cast film method reported by Bangham et al. (Citation1965). HSPC, cholesterol, DSPE (7:3:1.5 molar ratio) were dissolved in minimum quantity of chloroform and methanol (2:1) in a round-bottom flask. A thin film of lipids was casted on the inner surface of the round-bottom flask by evaporating the solvent under reduced pressure using rotator flash evaporated (Buchi type, York Sci. Co., Mumbai, India). The flask was rotated continuously until the film was dried. Final traces of solvents were removed under vacuum when stored overnight. Drug was dissolved in phosphate-buffered saline (PBS) (pH 7.4), which was used for hydration of dried lipid film, followed by continuous vortexing of the flask for about an hour to get MLV. Liposomal suspension was allowed to stand for further 3–4 h in the dark at room temperature to allow complete swelling of the vesicles. Liposomal suspension was sonicated for different time period at 4 ± 1 °C using bath sonicator (Lark Innovative Technology India Ltd., Chennai, India) to form smaller vesicles. The liposomal suspension was then centrifuged at 2000 rpm for 3 min using Sephadex G-50 minicolumn to remove the free drug and stored in dark at low temperature (Sorensen et al., Citation1977).
Preparation of ligand-coupled liposomes
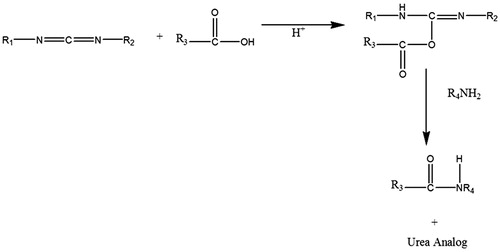
Lactobionic acid was attached on the surface of liposomes using the reported method (Bagari et al., Citation2011; Ma et al., Citation2011). The method involves covalent coupling of DSPE present on the surface of preformed drug-loaded liposomes to lactobionic acid via amide linkage using EDC as a coupling agent (). Briefly, in 2 mL of liposomal suspension, lactobionic acid (5 mg) dissolved in PBS (pH 7.4) was added. Then, 10 mg of EDC per mL was added to the reaction mixture. The mixture was incubated at room temperature (25 ± 2 °C) for 3 h. Excessive unbound lactobionic acid was removed by passing the dispersion through Sephadex G-50 column. The attachment of ligand to coupled liposomes through amide-bond linkage was confirmed by infrared (IR) spectroscopy.
HPLC method
The HPLC system (Class ATVP, Shimadzu, Kyoto, Japan) consisted of 2LC 10AT VP pumps, a variable wavelength programmable UV-VIS detector SPD-10AVP, a system controller SCL-10AVP and an RP C-18 column (150 × 4.6 mm ID, particle size 5 µm, E Merck, Darmstadt, Germany). It was equipped with the software class VP series, version 5.0. A manual injection valve was equipped with a 20-µL sample loop injector. All HPLC assays were performed isocratically at ambient temperature. The HPLC analysis of L-OHP was performed on Shimadzu LC-10A. The conditions of the experiment and the setup of the system were as follows: RP-C18 column; mobile phase: methanol and water (80/20, v/v). The flow rate was 0.6 mL/min.
Particle shape and size
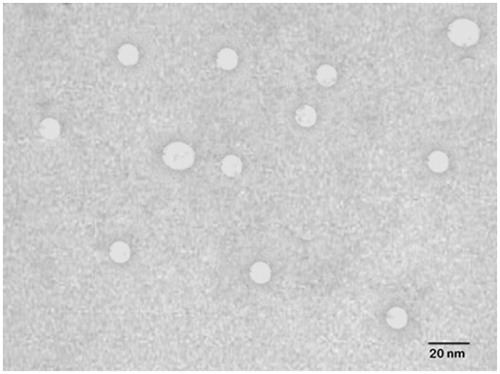
The uncoupled and lactobionic acid coupled liposomes (LA-LP) were characterized morphologically using transmission electron microscopy (TEM). A drop of the sample was placed on a carbon-coated copper grid to leave a thin film on the grid. Before drying, the film was negatively stained with 1% phosphotungstic acid. A drop of the staining solutions was added to the film, and excess quantity of solution was drained using a filter paper. The grid was air-dried thoroughly, and samples were viewed under a transmission electron microscope (Philips CM 10, Eindhoven, the Netherlands). Vesicle size of various formulations was determined by dynamic light-scattering method using a Zetasizer (Model 3000 HS, Malvern Instruments, Malvern, UK), and the measurements were conducted in triplicate.
Percentage entrapment efficiency
Entrapment efficiency of liposomes was determined using the Sephadex G-50 minicolumn centrifugation method (Sorensen et al., Citation1977). The unentrapped drug in liposomal formulation was separated using the Sephadex G-50 minicolumn centrifugation method. The separated liposomes were than disrupted using ethanol (2 mL ethanol for 10 µL of liposomes) and analyzed for drug content.
In vitro drug release
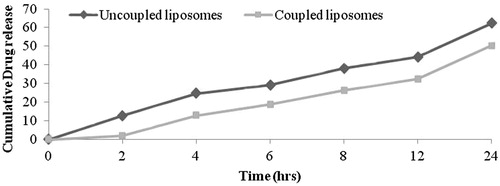
Drug release from coupled and uncoupled liposomes was determined using dialysis technique after separation of unentrapped drug using Sephadex G-50 minicolumn centrifugation technique. The in vitro drug release from the formulations was studied using dialysis membrane (molecular weight, cutoff point 2450 Da) (Himedia) in PBS buffer pH 7.4. Liposomal dispersion (20 mg/mL) free from any unentrapped drug was taken in dialysis membrane (Himedia) and suspended in a beaker containing 50 mL of PBS (pH 7.4). The content of the beaker was shaken using a magnetic stirrer at 37 ± 2 °C. Samples were withdrawn periodically and replaced with same volume of fresh PBS (pH 7.4); the amount of drug was quantified ().
Cellular-uptake studies
Cellular-uptake studies were performed using BEL7402 HCC cell line (procured from National Centre for Cell Science, Pune, India). The cell lines were cultured as monolayers in RPMI-1640 media supplemented with 1% l-glutamine, 0.1% penicillin, 0.1% streptomycin and 10% fetal bovine serum and propagated in a humidified atmosphere containing 5% CO2 at 37 °C. These cell lines were subcultured twice a week by simply resuspending the cells using 0.2% trypsin, 0.025% ethylenediaminetetraacetic acid and then replacing half of the cell suspension with a fresh medium. Adherent cells were grown to 80% confluence in tissue culture-grade flask and were subcultured by discarding the used medium, leaving the cells adhered to the bottom of the flask.
Liposomes loaded with calcein were prepared to investigate their uptake on BEL7402 HCC cell. Uncoupled calcein-loaded liposomes and lactobionic acid-coupled calcein-loaded liposomes were prepared. The calcein-loaded liposomes were prepared by the same method that was used to prepare drug-loaded liposomes. Calcein was used in concentration 0.5 mg/mL in water instead of drug. The calcein-loaded liposomes were purified from unloaded calcein with centrifugation at 3000 rpm for 10 min. Calcein-loaded coupled or uncoupled liposomes suspensions were diluted in 1 mL RPMI-1640 solution and added to monolayers of BEL7402 liver cancer cell lines (5 × 104) grown in 96-wells culture plate and incubated for 0.5, 1, 2 and 4 h at 37 °C.
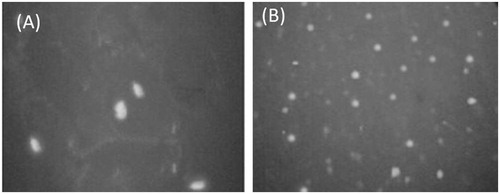
To determine the cellular uptake efficiency of liposomes, the cells were seeded on to 96-well microtitre plates at 5 × 104 cells/well and incubated for 24 h. The medium in the well was replaced with 100 µl of liposomal suspension and incubated for 24 h. At the end of the incubation period, the liposomal suspension was removed from the wells, and the cells monolayer was rinsed three times with cold PBS (pH 7.4) to remove un-internalized liposomes. The fluorescence of cells was measured with a FLUOstar OPTIMA microplate reader (BMG Lab Technologies, GmbH, Germany). The cells were mounted on slide by xylene-diluted Canada balsam and incubated it for 24 h and viewed by Olympus fluorescence microscopy (Tokyo, Japan) ().
Cytotoxic studies: MTT assay
The in vitro cytotoxicity of free oxaliplatin, drug-loaded liposomes and blank liposomes against BEL7402 cells were compared using the MTT assay as reported by Mosmann (Citation1983) with little modifications. Cell lines (5 × 104 cells each of BEL7402) in exponential growth phase were washed, trypsinized and resuspended in complete culture medium. Each well contained approximately 5 × 104 cells along with 100 µl culture medium on a microtitre plate and incubated for 24 h during which a partial monolayer was formed. The outermost wells were not used and were filled with sterile distilled water to maintain humidity. The plate were incubated at 37 °C, 5% Co2 and 90% humidity for 24 h, centrifuged and were washed with PBS (pH 7.4) three times. Following the final wash, the cells were resuspended in 0.2 mL of McCoy’s medium, then returned to the incubator, and the number of cells remaining after aspiration was quantified by treatment with MTT.
After 48 h following drug addition, the plates were again centrifuged; media was removed and 50 µl of MTT was added. The assay determines the cells viability based on mitochondrial conversion of a water-soluble pale yellow dye (MTT) by dehydrogenase enzyme (present in metabolically active cells) and indirect approximates the cell viability (Mosmann, Citation1983). During this incubation period, temperature was maintained at 37 °C and 90% humidity for 4 h. The absorbance was read on ELISA plate reader at wavelength of 650 nm. The percentage cell viability was calculated using the formula:
where Abs sample is the absorbance of cells tested with various formulations and Abs control is the absorbance of control cells (incubated with cell culture media only).
Animals
Mice weighing 20–22 g were selected for the study. The studies were carried out after taking approval of Institutional Animal Ethics Committee, Shri Ram Institute of Technology (Pharmacy), Jabalpur, India.
In vivo organ distribution studies
For in vivo biodistribution studies, six-week-old Balb/c mice weighing 20–22 g were selected and divided into four groups with three mice in each group. Liver tumor was induced in mice by injecting MDA-MB-231 liver cancer cells (1.0 × 107 cells) subcutaneously into the right flank of the Balb/c mice, and then tumor was allowed to grow until the mean tumor volume was 100 ± 10 mm3. The animals were fasted overnight before administration of dose. The animals of first group were administered plain drug solution (10 mg/kg body weight) intravenously, whereas animals of second and third groups received drug-loaded uncoupled and coupled liposomes intravenously; dose equivalent to 10 mg oxaliplatin/kg body weight. The animals of fourth group received identical volume of 0.09% NaCl solution were kept as control. At 0.5, 1, 2, 4 and 6 h, mice were killed and blood samples were collected by cardiac puncture. The organs liver, kidney, spleen, heart, lung and tumor were excised, isolated and washed with Ringer’s solution.
Various isolated organs (liver, kidney, spleen, heart, lung and liver tumor) were first dried with tissue paper and were weighed and minced into small pieces. One gram of each organ was homogenized with 2.0 mL of PBS (pH 7.4). In the organs weighing <1 g, whole organ was used. To tissue homogenate, 2 mL of acetonitrile was added and kept for 30 min. The resultant suspension was centrifuged for 20 min at 5000 rpm and was filtered through 0.45-µm-membrane filter. Tissue homogenate was analyzed for drug content as in serum by HPLC method.
Results
Physicochemical characteristics of liposomes
Liposomes were prepared using cast film method reported by Bangham et al. (Citation1965). EDC was used to modify the surface of the liposomes by coupling COOH group of lactobionic acid molecule with NH2 group present on liposomal surface (). LA-LP was prepared, and coupling was confirmed by the formation of amide bond through IR spectrum of the prepared coupled liposomes. The IR spectra of coupled liposomes exhibited peaks at 1568 cm−1, which is characteristic of amide bond. Peaks at 3363 cm−1 and 1466 cm−1 confirmed the presence of N–H and C–N group, respectively. Uncoupled and coupled liposomes bearing drug were characterized for shape, surface morphology, vesicle size, drug entrapment efficiency and in vitro drug release. The shape of the vesicles was observed under TEM and was found to be unilamellar and spherical in shape. The shape and surface morphology of the uncoupled and lactobionic acid-coupled liposomes were evaluated by TEM (). The vesicle size of the coupled liposome (310 ± 7.24 nm) was larger than uncoupled liposome (256 ± 6.31 nm) (). The entrapment efficiency of drug in coupled liposome (42 ± 4.75) is less than the uncoupled liposome (45 ± 3.89) ().
Table 1. Particle size, percentage entrapment efficiency and Zeta potential of uncoupled and coupled liposomes.
The in vitro drug-release profile of LA-LP and uncoupled liposomal formulations were studied using dialysis membrane. The uncoupled liposomal formulation showed 62.2 ± 1.5% drug release, whereas LA-LP formulation showed 50.3 ± 1.2% drug release after 24 h. A constant slow drug release was noted for 24 h. The release from liposome dispersion occurred by a diffusion controlled process ().
Cell-uptake studies
The uptake of the LA-LP and uncoupled liposomes by BEL7402 HCC cell line was assessed using FLUOstar OPTIMA microplate reader and visualized under Fluorescence microscopy. Higher fluorescence intensity (9856) was observed with the coupled liposomes which, in the case of uncoupled was only 3266, respectively. Similar observation was recorded in respective Fluorescence photomicrograph, which revealed the dependence of liposomal recognition and higher uptake of LA-LP than that observed in uncoupled liposomes ().
Cytotoxic studies: MTT assay
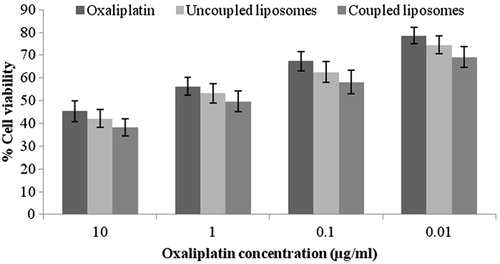
Percentage cell viability of drug-loaded uncoupled and coupled liposomes against free oxaliplatin were compared for its cytotoxic effect against BEL7402 liver cancer cells grown in vitro. It was observed that the cytotoxic effect of drug against melanoma cells was significantly higher when formulated as liposomes as compared to its free form. The cytotoxic effect of ligand coupled liposomes was significantly higher than uncoupled liposomes and free drug suggesting that pronounced cytotoxic action is exhibited by liposomes when coupled with a ligand ().
Organ distribution studies
Biodistribution of oxaliplatin in organs was evaluated in Balb/c mice for 6 h at definite time interval after administration of dose. Free drug and different formulations were given to mice of different groups in a dose of 10 mg oxaliplatin/kg body weight. As shown in , free drug cleared at a faster rate from the blood circulation. The estimation of amount of drug present in liver, kidney, spleen, heart, lung and liver tumor at different time intervals after intravenous administration of free oxaliplatin revealed that maximum accumulation of the drug in these organs was achieved within 1 h (). The accumulation in different organs was 8.02 ± 0.08% in liver, 4.82 ± 0.05% in kidney, 8.52 ± 0.9% in spleen and 1.87 ± 0.12% in tumor after 6 h from uncoupled liposomal formulation and was 10.02 ± 0.08% in liver, 1.72 ± 0.05% in kidney, 2.83 ± 0.9% in spleen and 16.58 ± 0.24% in tumor after 6 h from coupled liposomal formulation.
Table 2. Organ distribution study of plain drug and various formulations after IV administration.
Discussion
Recent advance in novel drug delivery has created a stride and has been regarded as an important tool for delivery of pharmaceuticals. Lipid-based drug delivery systems have specially been recognized for drug delivery at desired site (Muthu & Singh, Citation2009). Liposomes are “self-assembled nanoparticles” that are widely used to deliver hydrophobic and hydrophilic drugs. Being colloidal phospholipidic vesicles, liposomes are widely investigated as a potential carrier for drug delivery. They are also recognized for biocompatibility, biodegradability, non-toxicity and controlled release behavior. Along with this, unilamellar liposomal vesicles are capable to enter inside the cells resulting in better drug uptake (Torchilin, Citation2005; Elaine & Gary, Citation2008; Mishra et al., Citation2010). Moreover, liposomes are cleared by reticuloendothelial system on parenteral administration and are rapidly uptaken by nonparenchymal Kupffer cells in the liver (Hashida et al., Citation1995). Ligand-mediated drug targeting is amongst a promising approach, which is known for site-specific delivery of drugs. Many endeavors have been attempted to deliver drugs exclusively to hepatocytes. ASGPRs are recurrently utilized as an effective means for hepatic targeting. It is due to high expression on the surface of hepatocytes. Modification with D-galactose or N-acetylgalactosamine residues is successfully utilized for targeting drugs to hepatocytes (Rensen et al., Citation2001). Thus, this strategy could be fruitful in delivery of cytotoxic agents selectively to hepatocytes.
In this work, lactobionic acid-coupled liposomes (LA-LP) were prepared, and coupling of same was confirmed by the formation of amide bond through IR spectrum of the coupled liposomes. EDC reacts with carboxylic acid group and activates the carboxyl group, allowing it to be coupled to amino group (R4NH2) present in the reaction mixture. EDC is released as a soluble urea derivative after displacement by the nucleophile, R4NH2. The lactobionic acid-coupled liposomes exhibited anchoring over the liposomal surface, which clearly distinguished the coupled liposome from uncoupled liposome.
The vesicle size of the coupled liposome was found to be larger than that of the uncoupled liposome, which could be due to anchoring of lactobionic acid on the surface of liposomes. The entrapment efficiency of drug in coupled liposome is less than that of the uncoupled liposome, which can be attributed due to the loss of drug during the coupling reaction.
The in vitro drug-release profiles of LA-LP and uncoupled liposomal formulations clearly exhibited that the release behavior of drug from the liposomal dispersion showed a diffusion controlled release, where liposome act as a reservoir system for continuous release of encapsulated drug. The decrease in drug release from LA-LP could be due to double-barrier effect for drug diffusion across the phospholipid membrane.
In cellular uptake studies, the cell associated fluorescence was measured. The mean fluorescence intensity of coupled liposomes was found to be greater as compared to uncoupled liposomes, which can be accounted due to greater cellular association of coupled liposomes with the receptors over-expressed on HCC. The drug-loaded coupled liposomal formulations exhibited higher cytotoxicity as compared to drug-loaded uncoupled liposomal formulations after incubating with cancer cell lines. This phenomenon is in agreement with the results of cellular uptake studies.
The clearance rate and the tissue distribution of intravenously injected liposomes, on the one hand, depend on the vesicle size distribution. Free drug was removed at a faster rate from the blood circulation as compared to drug encapsulated in uncoupled and coupled liposomes (Figure 6). Maximum amount of drug accumulated in kidney, liver, lung, spleen and heart on administration of free drug than various ligand-coupled and non-ligand-coupled formulations. By passive targeting, the concentration of drug from plain liposome was higher than that observed from free drug in liver and spleen at corresponding time, which can be attributed due to greater RES uptake of drug-loaded liposomal formulation to liver tissues. Accumulation of drug from coupled liposomes in the liver tumor was significantly higher than that of plain drug-loaded liposomes, which could be due to organ specific targeting efficacy toward the receptor over expressed on the HCC cells.
Conclusion
LA-LP with high entrapment efficiency was formulated for targeting of liver tumors. Liver tumors overexpress ASGPR, which were utilized for effective localization of LA-LP through receptor-mediated endocytosis. From the results obtained, it can be concluded that lactobionic acid is a promising ligand that can be exploited for drug targeting to the liver tumors.
Acknowledgements
We thank Dr. Reddys for providing Oxaliplatin as a gift sample. The authors are also thankful to Lipoid, Germany, for providing HSPC and DSPE as a gift sample. Authors extend their acknowledgement to Rewa Shiksha Samiti, Jabalpur, India, for providing necessary support during their studies.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Alberts SR, Sargent DJ, Nair S, et al. (2012). Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 307:1383–93
- Allen TM. (2002). Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer 2:750–63
- Bagari R, Bansal D, Gulbake A, et al. (2011). Chondroitin sulfate functionalized liposomes for solid tumor targeting. J Drug Target 19:251–7
- Bangham AD, Standish MM, Watkins JC. (1965). Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol 13:238–52
- Blum HE. (2005). Hepatocellular carcinoma: therapy and prevention. World J Gastroenterol 11:7391–440
- Culy CR, Clemett D, Wiseman LR. (2000). Oxaliplatin. A review of its pharmacological properties and clinical efficacy in metastatic colorectal cancer and its potential in other malignancies. Drugs 60:895–924
- de Lope CR, Tremosini S, Forner A, et al. Management of HCC (2012). J Hepatol 56:S75–87
- Elaine MM, Gary GL. (2008). Drug nanoparticles: formulating poorly water-soluble compounds. Toxicol Pathol 36:43–8
- Graham J, Mushin M, Kirkpatrick P. (2004). Oxaliplatin. Nat Rev Drug Discov 3:11–2
- Harper BW, Krause-Heuer AM, Grant MP, et al. (2010). Advances in platinum chemotherapeutics. Chemistry 25:7064–77
- Hashida M, Nishikawa M, Takakura Y. (1995). Hepatic targeting of drugs and proteins by chemical modification. J Control Release 36:99–107
- Honecker FU, Brümmendorf TH, Klein O, Bokemeyer C. (2006). Safe use of oxaliplatin in a patient with metastatic breast cancer and combined renal and hepatic failure. Onkologie 29:273–5
- Ma K, Shen H, Shen S, et al. (2011). Development of a successive targeting liposome with multi-ligand for efficient targeting gene delivery. J Gene Med 13:290–301
- Maruyama K, Holmberg E, Kennel SJ, et al. (1990). Characterization of in vivo immunoliposome targeting to pulmonary endothelium. J Pharm Sci 79:978–84
- Mishra B, Patel BB, Tiwari S. (2010). Colloidal nanocarriers: a review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine 6:9–24
- Mishra PK, Gulbake A, Jain A, et al. (2009). Targeted delivery of an anti-cancer agent via steroid coupled liposomes. Drug Delivery 16:437–47
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
- Muthu MS, Singh S. (2009). Targeted nanomedicines: effective treatment modalities for cancer, AIDS and brain disorders. Nanomedicine (Lond) 4:105–18
- Rensen PC, Sliedregt LA, Ferns M, et al. (2001). Determination of the upper size limit for uptake and processing of ligands by the asialoglycoprotein receptor on hepatocytes in vitro and in vivo. J Biol Chem 276:37577–84
- Sorensen EN, Weisman G, Vidaver GA. (1977). A sephadex column procedure for measuring uptake and loss of low molecular weight solutes from small, lipid-rich vesicles. Anal Biochem 82:376–84
- Torchilin VP. (2005). Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4:145–60
- Wang SN, Deng YH, Xu H, et al. (2006). Synthesis of a novel galactosylated lipid and its application to the hepatocyte-selective targeting of liposomal doxorubicin. Eur J Pharm Biopharm 62:32–8
- Xiang Q, Zhang D, Wang J, et al. (2014). Cabozantinib reverses multidrug resistance of human hepatoma HepG2/adr cells by modulating the function of P-glycoprotein. Liver Int doi: 10.1111/liv.12524. [Epub ahead of print]
- Yen Y, Lim DW, Chung V, et al. (2008). Phase II study of oxaliplatin in patients with unresectable, metastatic, or recurrent hepatocellular cancer: a California Cancer Consortium Trial. Am J Clin Oncol 31:317–22