Abstract
Context: Inadequate penetration of the blood–brain barrier (BBB) by systemically administered chemotherapies including carboplatin is implicated in their failure to improve prognosis for patients with glioblastoma. Convection-enhanced delivery (CED) of carboplatin has the potential to improve outcomes by facilitating bypass of the BBB.Objective: We report the first use of an implantable CED system incorporating a novel transcutaneous bone-anchored port (TBAP) for intermittent CED of carboplatin in a patient with recurrent glioblastoma.Materials and methods: The CED catheter system was implanted using a robot-assisted surgical method. Catheter targeting accuracy was verified by performing intra-operative O-arm imaging. The TBAP was implanted using a skin-flap dermatome technique modeled on bone-anchored hearing aid surgery. Repeated infusions were performed by attaching a needle administration set to the TBAP. Drug distribution was monitored with serial real-time T2-weighted magnetic resonance imaging (MRI).Results: All catheters were implanted to within 1.5 mm of their planned target. Intermittent infusions of carboplatin were performed on three consecutive days and repeated after one month without the need for further surgical intervention. Infused volumes of 27.9 ml per day were well tolerated, with the exception of a single seizure episode. Follow-up MRI at eight weeks demonstrated a significant reduction in the volume of tumor enhancement from 42.6 ml to 24.6 ml, and was associated with stability of the patient’s clinical condition.Conclusion: Reduction in the volume of tumor enhancement indicates that intermittent CED of carboplatin has the potential to improve outcomes in glioblastoma. The novel technology described in this report make intermittent CED infusion regimes an achievable treatment strategy.
Introduction
Despite advances in surgical methods and adjuvant therapies, the prognosis for patients with glioblastoma remains poor. Median survival with maximal treatment is reported as 14.6 months (Stupp et al., Citation2005).
The failure of systemically administered chemotherapies, other than temozolomide, to improve prognosis may result from inadequate penetration of the blood–brain barrier (BBB), and sub-therapeutic drug concentrations within the tumor mass and infiltrated peri-tumoral tissue. A number of pre-clinical studies have shown carboplatin to have significant cytotoxic effects against glioma (Wolff et al., Citation1999; Leuraud et al., Citation2004). However, clinical application of carboplatin to the treatment of glioma has proven disappointing, despite high-dose intravenous and intra-arterial administration (Follezou et al., Citation1989; Castello et al., Citation1990; Stewart et al., Citation1992; Larner et al., Citation1995; Cloughesy et al., Citation1997; Qureshi et al., Citation2001; Silvani et al., Citation2002).
Comparing the relationship between peak plasma and tumor concentrations of carboplatin in patients undergoing glioblastoma resection, Whittle et al. (Citation1999) demonstrated the peak plasma concentration following intravenous administration to be 0.044 mg/ml whilst the concentration in tumor tissue peaked at 0.013 mg/ml, below the IC50 of carboplatin. Consequently, convection-enhanced delivery (CED) has been proposed as a method of bypassing the BBB and facilitating the delivery of therapeutic carboplatin concentrations to the tumor (Yang et al., Citation2011; White et al., Citation2012a,Citationb).
CED describes direct delivery of drugs to the brain through intraparenchymal microcatheters (Bobo et al., Citation1994). By establishing a pressure gradient at the tip of the infusion catheter, CED facilitates bypass of the BBB, accurate anatomical targeting and distribution of the therapeutic agent through clinically relevant brain volumes whilst reducing the risk of systemic side effects. We recently described CED of carboplatin for the treatment of brainstem glioma (Barua et al., Citation2013a).
We describe the first use of intermittent CED of carboplatin for the treatment of recurrent glioblastoma using an implantable catheter system and transcutaneous bone-anchored port (TBAP). Repeated infusions at one-month intervals resulted in initial tumor regression, suggesting that intermittent CED might offer some hope for improving outcomes in glioblastoma.
Methods
History
A 50-year-old female presented with generalized seizures in December 2010. Magnetic resonance imaging (MRI) revealed enhancing lesions in the left insula and parietal lobe. The patient was commenced on dexamethasone and levetiracetam. The patient proceeded to image-guided biopsy in view of the multifocal nature of the disease and eloquent location of the tumor. Biopsy confirmed the tumor to be glioblastoma. Genetic analysis revealed loss of chromosomes 1p and 19q heterozygosity and unmethylated O6-methylguanine-DNA methyltransferase (MGMT) status.
The patient was enrolled in the AVAglio trial and commenced radiotherapy, temozolomide and Avastin (Chinot et al., Citation2011). Disease control was maintained until August 2012, when surveillance imaging revealed progression of both the parietal and remote insular lesions. Despite procarbazine, lomustine and vincristine salvage chemotherapy, the lesions progressed whilst the patient maintained a high level of independent function (WHO performance status 1; ). Following review by the neuro-oncology multidisciplinary team and approval from our Institutional Review Board, a decision was made to proceed with resection of the parietal tumor mass followed by CED of carboplatin. A macroscopic resection of the parietal lesion was achieved without any adverse neurological sequelae ().
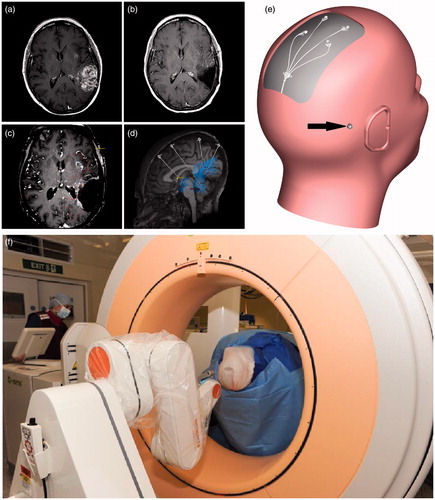
Figure 1. Progression of both the left parietal and left insular tumor masses occurred following treatment with PCV (a). A macroscopic resection of the parietal lesion was undertaken prior to catheter implantation (b). In the four-week interval between resective surgery and catheter implantation, significant tumor progression occurred with new enhancement around the resection cavity as well as progression of the insular lesion (c). Using an in-house modification to neuro | inspire™ stereotactic planning software, the volume of tumor enhancement was delineated (shown in outline) and four catheter trajectories were planned (d) as well as the site for implantation of the TBAP (e; black arrow). Targeting accuracy was determined by performing intra-operative O-arm imaging (f).
Pre-operative planning
MRI was undertaken to facilitate pre-operative planning (field strength 3 T, Philips Achieva TX, Philips, Amsterdam, The Netherlands) four weeks after resective surgery. This imaging confirmed a significant recurrence of tumor enhancement around the resection cavity with progression of the left insular lesion (). Using an in-house modification to neuro | inspire™ stereotactic software (Renishaw PLC, Wotton-under-Edge, UK), the enhancing tumor volume was delineated by drawing profiles on axial MRI images. The trajectories for implantation of four guide tube-directed CED catheters were planned targeting the areas of tumor enhancement as well as the infiltrated peri-tumoral penumbra (.). The CED catheters were an in-house design manufactured by Renishaw (Bienemann et al., Citation2012; Barua et al., Citation2013b).
Surgical procedure
The patient was anesthetized and placed in a Leksell frame. Intravenous flucloxacillin and gentamicin were administered at induction, but no further post-operative antibiotics were given. Pre-operative CT angiography was performed and co-registered with the post-contrast T1-weighted planning scan to facilitate output of stereotactic co-ordinates to a neuro | mate® robot (Renishaw) (Barua et al., Citation2013a). The site for implantation of a TBAP was also planned ().
Catheter and port implantation followed the pre-clinical procedure previously optimized in a large animal (porcine) model (Barua et al., Citation2013b). A linear paramedian scalp incision was made and the periosteum retracted. The robot was driven to the first position on the skull and a multi-featured burr hole made into which the guide tube hub would push fit. The dura was pierced, and a 1-mm guide rod was inserted to a pre-determined point in the peri-tumoral white matter, short of the planned catheter target. The guide tube was then implanted on a 0.6-mm outer diameter (OD) guide rod to maintain trajectory and the 0.6-mm guide rod extended to target. This process was repeated for all four guide tubes. Stylettes were inserted into the guide tubes to allow verification of targeting accuracy by performing post-contrast O-arm imaging (Medtronic, Minneapolis, MN; ). O-arm images were co-registered with the planning scan, and targeting accuracy ascertained by measuring the distance between the stylette tip and planned target in the trajectory plane. All stylettes were within 1.5 mm of their targets.
Carbothane microcatheters (0.6 mm OD) were cut to length, attached to the port assembly and primed with artificial cerebrospinal fluid (aCSF; Torbay Pharmaceutical Manufacturing Unit, Torbay, UK; ). This catheter material and OD were selected following extensive pre-clinical studies, which confirmed long-term patency after implantation in the brain, even without continuous infusion (Bienemann et al., Citation2012). The site for implantation of the TBAP prepared and draped. The TBAP implantation procedure was modeled on the skin-flap dermatome technique commonly used in bone-anchored hearing aid surgery (; Stalfors & Tjellstrom, Citation2008; Dun et al., Citation2011). A skin-flap dermatome (Osscora, Cochlear Europe, Surrey, UK) was used to elevate a 0.6 mm by 25 mm skin-flap pedicled inferiorly. The underlying subcutaneous tissue was excised taking care to maintain periosteal integrity. The skin flap was temporarily replaced, and a hole made in the skin flap and periosteum for placement of the port. The skin flap was then re-opened and a robot-assisted burr hole drilled. A linear incision was made in the periosteum, through which a trench was drilled in the skull to accommodate the port tubing.
Figure 2. A CED catheter system incorporating an external guide tube (black arrow) and 0.6-mm OD carbothane catheter (white arrow) was implanted (a). The catheters were attached to a transcutaneous bone-anchored port assembly (b). The transcutaneous port was implanted using a skin-flap dermatome technique behind the right ear (c and d). Infusions were performed by attaching a needle administration set incorporating in-line gas and bacterial filters to the port using MRI-compatible pumps (e and f).
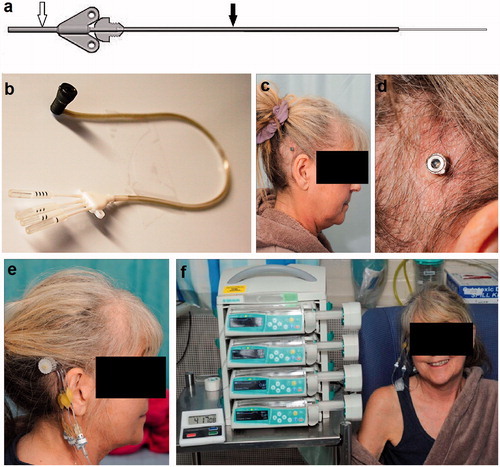
The catheters were attached to the port assembly and implanted, and their winged hubs were secured with titanium screws. The TBAP was tunneled to the skin graft site and implanted into the burr hole and trench. The surgical sites were closed and the patient woken from anesthesia.
Infusions of carboplatin
Infusions were commenced on the third post-operative day by attaching a custom-made needle administration set with in-line gas and bacterial filters to the port (). The needle administration set was attached to 3 m extension lines to allow infusions to be performed inside the scan room by placing syringe drivers into an MRI-compatible unit (B Braun, Melsungen, Germany). Infusions of carboplatin diluted in aCSF to a concentration of 0.18 mg/ml were commenced using the following infusion regime:
0.5 µl/min for 10 min, 1 µl/min for 5 min, 2.5 µl/min for 5 min, 5 µl/min for 5 min, 7.5 µl/min for 5 min, 10 µl/min until completion.
Serial T2-weighted MRI scans were performed in order to allow areas of hyperintense signal change to be used as a proxy measure for drug distribution (Sampson et al., Citation2007; Iyer et al., Citation2011; Barua et al., Citation2013a).
Results
Infused volumes
The infusions were planned to be repeated on three consecutive days in order to maintain exposure of the tumor to therapeutic drug concentrations for greater than 72 h (based on pre-clinical analysis of drug clearance (White et al., Citation2012a)). A total volume of 27.9 ml (6.98 ml per catheter) was delivered over a 12-h period, followed by a 12-h rest period. The first two infusions were well tolerated, without any adverse neurological effects or change in conscious level. However, the third infusion was terminated prematurely due to a generalized seizure 2 h after commencement. The seizure pattern and transient postictal right-sided hemiparesis was consistent with the patients’ pre-existing seizure disorder. Neurological function returned to baseline over a period of 24 h, and the patient was discharged on the 10th post-operative day.
After four weeks, infusions were repeated on three consecutive days at a higher infused carboplatin concentration of 0.24 mg/ml, delivering a total volume of 27.9 ml per day. The infusions were performed by attaching a needle administration set to the TBAP, without the need for further surgery. In order to prevent further seizures, the patient was given a loading dose of phenytoin prior to the infusions and maintained on high dose dexamethasone (16 mg/d) for a period of one week. No further seizures occurred.
Volume of distribution analysis
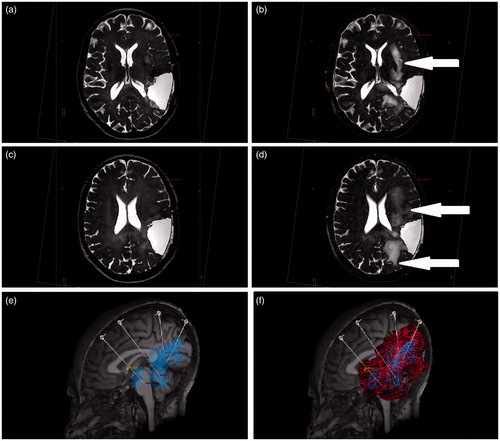
Serial T2-weighted MRI was performed to allow changes in hyperintensity to be used as a proxy measure of drug distribution (Sampson et al., Citation2007; Iyer et al., Citation2011). Pre- and post-infusion MRI scans were overlaid using neuro | inspire™ stereotactic software and profiles drawn around areas of new T2 signal change in order to approximate the volume of distribution (). The contrast level of the baseline and post-infusion images was standardized using an automatic contrasting function of the software. After infusion of 27.9 ml, the volume of new T2 hyperintensity was 97.6 ml, resulting in an approximate volume of infusion (Vi) to distribution (Vd) ratio of 3. This Vi:Vd ratio is consistent with our pre-clinical studies of white matter CED (White et al., Citation2012a). The changes in hyperintensity on post-infusion imaging encompassed the enhancing regions around the resection cavity as well as the remote insular region ().
Figure 3. The volume of T2 hyperintensity on real-time MRI scans was used as a proxy measure of drug distribution. Pre-infusion (a and c) and post-infusion (b and d) T2-weighted images were overlaid using neuro | inspire™ stereotactic software and profiles drawn around areas of new T2 hyperintensities (arrows, b and d). The total volume of T2 signal change on completion of the infusions was measured as 97.6 ml. Analysis of contrast enhancement on T1-weighted MRI was used to determine response to CED. Prior to catheter implantation, a total volume of 42.6 ml of tumor enhancement was measured (e; blue). By superimposing the volume of T2 signal change (red) onto the volume of tumor enhancement on T1-weighted imaging (blue), it was possible to ensure drug distribution was achieved throughout the targeted tumor volume as well as the infiltrated peri-tumoral penumbra (f). (Colour online). Please refer to online colour images.
Radiological follow-up
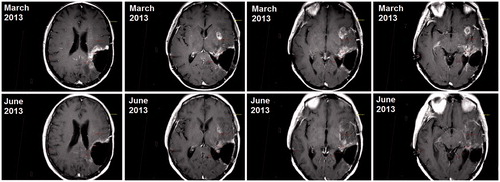
Analysis of the volumes of contrast enhancement on T1-weighted MRI was used to determine tumor response to CED. The total volume of enhancing tissue around the tumor resection cavity and of the remote insular lesion was 42.6 ml on baseline imaging. Follow-up MRI was performed four and eight weeks after completion of the infusions. Imaging at eight weeks demonstrated an almost 50% reduction in the volume of contrast enhancement, which was measured as 24.6 ml (). During this period, the patient remained clinically stable whilst maintained on a 2 mg/d maintenance dose of dexamethasone.
Figure 4. Follow-imaging at eight weeks post infusion demonstrated an almost 50% reduction in the volume of tumor enhancement. At baseline (top row), the volume of contrast enhanced on T1-weighted imaging was 42.6 ml compared with 24.6 ml at follow-up (bottom row).
Further imaging was performed after an interval of eight weeks. Unfortunately, this confirmed substantial tumor progression, which correlated with a decline the patient’s neurological function with progression of right-sided hemiparesis. The areas of tumor progression were outside of the volumes of T2 signal change observed during the infusions and included invasion of the left cerebral peduncle. Further infusions of carboplatin were considered to be futile, and the patient died eight months after implantation of the CED catheter system and 33 months after initial diagnosis.
Discussion
CED has the potential to improve outcomes for patients with glioblastoma by allowing therapeutic drug concentrations to be more effectively delivered to the tumor. However, a number of technical challenges have emerged in recent trials, which have hindered progression of this novel treatment strategy. The major barriers to effective clinical translation include reflux of infusate, which results in sub-therapeutic drug concentration within the target structure and off-target side-effects and poor drug distribution within the target volume (Sampson et al., Citation2010; Mueller et al., Citation2011). Furthermore, the majority of clinical trials to date have been restricted to either single infusions or continuous slow infusions over a number of days using implantable or ambulatory pumps (Bogdahn et al., Citation2011; Bruce et al., Citation2011; Mueller et al., Citation2011).
In this case, we were able to safely and accurately deliver multiple microcatheters with 0.6-mm OD using a robot-assisted method. The use of a novel TBAP facilitated repeated infusions on three consecutive days, followed by a second course of infusions one month later, without the need for additional surgical intervention. High volume, high flow rate infusions were well tolerated, with the exception of a single seizure episode (in a patient with pre-existing seizures). Serial real-time T2-weighted MRI gave us confidence that drug distribution had been achieved throughout the targeted tumor volume, as well as the infiltrated peri-tumoral penumbra.
Reduction in the volume of tumor enhancement on follow-up imaging, in combination with stability of the patient’s clinical condition suggest that intermittent CED of carboplatin has potential to improve outcomes in patients with glioblastoma. With hindsight, administering further infusions at the time of tumor regression may have had a beneficial effect on prognosis, and may be preferable to re-infusing at the time of tumor regrowth.
This case demonstrates the feasibility of accurately and safely delivering very small diameter catheters to target recurrent glioblastoma and performing intermittent infusions of carboplatin. However, for a conventional chemotherapy to have a significant impact on the disease, it may be necessary for repeated infusions to be performed, perhaps for the lifetime of the patient. The novel technology described in this report make intermittent CED an achievable goal. We are hopeful that a treatment strategy of intermittent infusions could favorably impact the prognosis of patients with glioblastoma, and it is our intention to use the experience gained in this case to develop a robust protocol for clinical trial of CED of carboplatin for recurrent glioblastoma.
Acknowledgements
We would like to thank Maureen Wiltshire for her administrative and organisational support. We would also like to thank Dave Johnson, Owen Lewis, Charlie Irving, Gavin Murray, Catriona Fennelly, Stuart Campbell and Paul Skinner for their assistance in catheter and TBAP development. Finally, we would like to acknowledge the courage of this patient and her family.
Declarations of interest
N. U. B. and A. S. B. are consultant advisors to Renishaw Plc. and S. S. G. is Renishaw’s clinical director.
Other authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding for the 3T MRI scanner was provided by The Gatsby Foundation. The manufacture of catheters was funded from The Functional Neurosurgery Research Fund and Friends of Bristol Oncology Centre.
References
- Barua NU, Lowis SP, Woolley M, et al. (2013a). Robot-guided convection-enhanced delivery of carboplatin for advanced brainstem glioma. Acta Neurochir (Wien) 155:1459–65
- Barua NU, Woolley M, Bienemann AS, et al. (2013b). Intermittent convection-enhanced delivery to the brain through a novel transcutaneous bone-anchored port. J Neurosci Methods 214:223–32
- Bienemann A, White E, Woolley M, et al. (2012). The development of an implantable catheter system for chronic or intermittent convection-enhanced delivery. J Neurosci Methods 203:284–91
- Bobo RH, Laske DW, Akbasak A, et al. (1994). Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA 91:2076–80
- Bogdahn U, Hau P, Stockhammer G, et al. (2011). Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol 13:132–42
- Bruce JN, Fine RL, Canoll P, et al. (2011). Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery 69:1272–9
- Castello MA, Clerico A, Deb G, et al. (1990). High-dose carboplatin in combination with etoposide (JET regimen) for childhood brain tumors. Am J Pediatr Hematol Oncol 12:297–300
- Chinot OL, De La Motte Rouge T, Moore N, et al. (2011). AVAglio: phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther 28:334–40
- Cloughesy TF, Gobin YP, Black KL, et al. (1997). Intra-arterial carboplatin chemotherapy for brain tumors: a dose escalation study based on cerebral blood flow. J Neurooncol 35:121–31
- Dun CA, Faber HT, De Wolf MJ, et al. (2011). An overview of different systems: the bone-anchored hearing aid. Adv Otorhinolaryngol 71:22–31
- Follezou JY, Fauchon F, Chiras J. (1989). Intraarterial infusion of carboplatin in the treatment of malignant gliomas: a phase II study. Neoplasma 36:349–52
- Iyer RR, Butman JA, Walbridge S, et al. (2011). Tracking accuracy of T2- and diffusion-weighted magnetic resonance imaging for infusate distribution by convection-enhanced delivery. J Neurosurg 115:474–80
- Larner JM, Phillips CD, Dion JE, et al. (1995). A phase 1-2 trial of superselective carboplatin, low-dose infusional 5-fluorouracil and concurrent radiation for high-grade gliomas. Am J Clin Oncol 18:1–7
- Leuraud P, Taillandier L, Medioni J, et al. (2004). Distinct responses of xenografted gliomas to different alkylating agents are related to histology and genetic alterations. Cancer Res 64:4648–53
- Mueller S, Polley MY, Lee B, et al. (2011). Effect of imaging and catheter characteristics on clinical outcome for patients in the PRECISE study. J Neurooncol 101:267–77
- Qureshi AI, Suri MF, Khan J, et al. (2001). Superselective intra-arterial carboplatin for treatment of intracranial neoplasms: experience in 100 procedures. J Neurooncol 51:151–8
- Sampson JH, Archer G, Pedain C, et al. (2010). Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg 113:301–9
- Sampson JH, Raghavan R, Provenzale JM, et al. (2007). Induction of hyperintense signal on T2-weighted MR images correlates with infusion distribution from intracerebral convection-enhanced delivery of a tumor-targeted cytotoxin. AJR Am J Roentgenol 188:703–9
- Silvani A, Eoli M, Salmaggi A, et al. (2002). Intra-arterial ACNU and carboplatin versus intravenous chemotherapy with cisplatin and BCNU in newly diagnosed patients with glioblastoma. Neurol Sci 23:219–24
- Stalfors J, Tjellstrom A. (2008). Skin reactions after BAHA surgery: a comparison between the U-graft technique and the BAHA dermatome. Otol Neurotol 29:1109–14
- Stewart DJ, Belanger JM, Grahovac Z, et al. (1992). Phase I study of intracarotid administration of carboplatin. Neurosurgery 30:512–6; discussion 516–7
- Stupp R, Mason WP, Van den Bent MJ, et al. (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–96
- White E, Bienemann A, Pugh J, et al. (2012a). An evaluation of the safety and feasibility of convection-enhanced delivery of carboplatin into the white matter as a potential treatment for high-grade glioma. J Neurooncol 108:77–88
- White E, Bienemann A, Taylor H, et al. (2012b). A phase I trial of carboplatin administered by convection-enhanced delivery to patients with recurrent/progressive glioblastoma multiforme. Contemp Clin Trials 33:320–31
- Whittle IR, Malcolm G, Jodrell DI, Reid M. (1999). Platinum distribution in malignant glioma following intraoperative intravenous infusion of carboplatin. Br J Neurosurg 13:132–7
- Wolff JE, Trilling T, Molenkamp G, et al. (1999). Chemosensitivity of glioma cells in vitro: a meta analysis. J Cancer Res Clin Oncol 125:481–6
- Yang W, Huo T, Barth RF, et al. (2011). Convection enhanced delivery of carboplatin in combination with radiotherapy for the treatment of brain tumors. J Neurooncol 101:379–90
