Abstract
The purpose of this study was to investigate the microdialysis pharmacokinetic of scopolamine in plasma, olfactory bulb and vestibule after intranasal administration. The pharmacokinetic study of subcutaneous and oral administration was also performed in rats. From the in vivo results, scopolamine intranasal administration can avoid hepatic first-pass effect. Tmax plasma samples after intranasal administration were significantly faster than oral administration and subcutaneous injection. The relative bioavailability of intranasal administrations was 51.8–70% when compared with subcutaneous injection. Moreover, one can see that in comparison with scopolamine subcutaneous administration, scopolamine intranasal gel and solutions can increased drug target index (DTI) with olfactory bulb 1.69 and 2.05, vestibule 1.80 and 2.15, respectively. The results indicated that scopolamine can be absorbed directly through the olfactory mucosa into the olfactory bulb, and then transported to various brain tissue after intranasal administration, with the characteristics of brain drug delivery.
Introduction
Motion sickness describes the discomfort felt by individuals caused by repetitive angular and linear acceleration and deceleration. The symptoms associated with motion sickness include nausea, vomiting, pallor, cold sweats, hypersalivation, hyperventilation and headaches and often occur during travel in vehicles or when in motion (Thornton & Bonato, Citation2013). Previous hypotheses about the development of motion sickness held that symptoms were due to either reduced cerebral blood flow or to motion of the viscera prompting stimulation of afferent nerves in abdominal organs (Oman, Citation1990). The most widely held explanation for the cause of motion sickness is described by the sensory conflict hypothesis (Reason, Citation1970; Reason & Brand, Citation1975; Yardley, Citation1992). Briefly, the hypothesis postulates that each person has an internal representation of bodily movement. This internal picture is continuously updated by information from sensory receptors such as the eyes, the vestibular system, and mechanoceptors in joints and muscles. Motion sickness develops when repeated and sustained mismatches occur between the information received from the sensory receptors and the expected internal model.
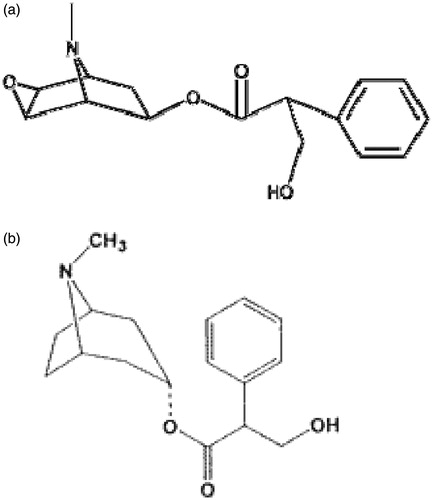
Scopolamine (SCOP, CAS:51-34-3, ), a naturally occurring anti-muscarinic agent, has been used for the prevention and treatment of nausea and vomiting associated with motion sickness for almost 200 years. About 60 years ago, the nasal absorption of SCOP in solution was reported by Hyde et al. (Citation1953). They found that SCOP could produce faster responses and greater therapeutic activity than an equivalent oral dose. In 2007, our group optimized the SCOP nasal dosage forms with gellan gum (Cao et al., Citation2007). It could prevent some actual problems, such as fast mucociliary beating; a short duration of therapeutic effect and a frequent dosing regimen.
Recently, intranasal drug delivery is verified as a reliable method to bypass the blood–brain barrier (BBB) and deliver therapeutic drug to the brain and has shown therapeutic effects in both animals and humans (Mittal et al., Citation2014). Other researchers of our group have found that, either methotrexate or apomorphine after intranasal administration, the brain targeting index were significantly higher than other routes of administration (e.g. intravenous, subcutaneous) (Wang et al., Citation2003; Lu et al., Citation2008), indicating a nasal drug delivery characteristics of the brain, some portion of the drug can be absorbed directly by the nasal cavity to the top of the central nervous system (CNS).
After several decades of development and improvement, microdialysis sampling technique has gradually become mature, and its essence is a new technology developed and extended from the perfusion sampling techniques in the early neurochemistry laboratory (Melgaard et al., Citation2013; Navailles et al., Citation2013; Shannon et al., Citation2013). As a research tool in pharmacokinetics, it has the following significant advantages: (1) Time-resolved: the continuous tracer of the law that the concentration of a variety of compounds in vivo changes with time; (2) spatial resolution: positioning may be required in accordance with the test site visits required sampling, even at the same time in different parts of the drug that changes over time; (3) samples were free of macromolecules of proteins and enzymes, without pre-treatment, easy to determinate; (4) can get a complete drug curve from the same animal, reduce individual differences.
The purpose of this study: First, using microdialysis method for the determination of SCOP concentration in the olfactory bulb to verify whether SCOP pass through the nose into the brain after intranasal administration. Second, because the nerve endings in the vestibule area is the main receptor of human balance system as well as the main site of action SCOP, use microdialysis method to measure the SCOP concentration in the vestibule area to compare the pharmacokinetic difference between in situ gel and common solutions.
Materials and methods
Materials
SCOP was gifted by the Department of Pharmaceutics, School of Pharmacy, Fudan University (Shanghai, China). Gellan gum was purchased from ZhongWei Biochemical Ltd (Shanghai, China). The SCOP injection (0.3 g/L) was obtained from Shanghai Harvest (Shanghai, China). MD 2212 micro-dialysis probe and MD 2255 bushing (BAS USA); micro-infusion pump (CMA/100 CMA Sweden); Stereotactic (IC Chuan sha hua mu agricultural machinery, Shanghai).
Artificial cerebrospinal fluid (ACF) included NaCl 145 mM; CaCl2 1.2 mM; MgCl2 1.0 mM; KCl 2.7 mM; Na2HPO4 1.6 mM; NaH2PO4 0.4 mM, prepared according to previous report (Robert et al., Citation1996). Purified water from a Milli-Q system (Millipore, Bedford, MA) was used throughout the experiment. All other reagents were of commercially analytical grade.
Sprague–Dawley rats (220 ± 20 g) were supplied by animal center of Fudan University. Prior to use, all rats were maintained under standard laboratory conditions on a 12 h light–dark cycle and were fed standard rat chow and sterilized tap water. All experimental procedures were carried out in accordance with the guidelines of Animal Use and Care by Medical Center of Fudan University.
Microdialysis location
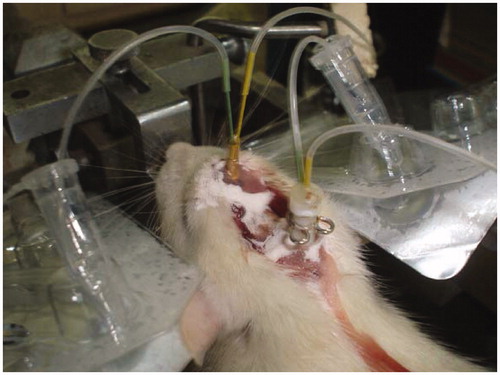
Briefly, SD rats were anesthetized with 10% chloral hydrate (4 mL/kg) by intraperitoneal injection and then the rats were moved to the stereotactic and fixed the rats' head. Use scalpel to cut the duramater on skull and wipe with hydrogen peroxide to expose the sagittal suture. Use bregma as the original point, in the forward 2 mm, 1 mm right to punch by dental drill; buried the microdialysis tube at the drilling point down to 2 mm, the first cannula tip was placed vertically above the olfactory bulb. Next use fonticuli minor as the original point, in the forward 1 mm, 2 mm right to punch by dental drill; buried the microdialysis tube at the drilling point down to 9 mm, the second cannula tip was placed vertically above the vestibule area. Then tooth powder and tooth dehydration were used to fix the two tubes (). After the test, the rats were perfused and rinse the remaining cerebrovascular blood, brain tissues immersed in 4% paraformaldehyde (w/v) for 3 d, and then sliced to confirm the position of the microdialysis probe. Slice observation indicates the location of microdialysis probes are in rat olfactory bulb and vestibular area without shifting.
In vitro recovery
Since the analyte's concentration in effluent (dialysate, Ces) is usually lower than the concentrate intercellular fluid around membrane probe (Cos), which means that the former one is part of the latter one. Due to the different experimental conditions, the ratios of the two were different. Therefore, it is necessary to pre-test simulations obtained by the ratio between in vitro, in vitro recovery rate. In vivo microdialysis test data divided by the recovery rate was the true concentration of intercellular fluid.
Recovery test method is as follows: The microdialysis probe immersed in ACF containing SCOP's (Cos = 200 ng/mL), a flow rate of 2.5 μL/min infusion of ACF from the inlet pipe, balance 1 h after collection from the outlet pipe four parallel effluent samples, to determine the drug concentration (Ces), calculated in vitro recovery (R): R = Ces/Cos.
Pharmacokinetics evaluation
Twenty rats were used to investigate the microdialysis pharmacokinetics of SCOP formulations. Briefly, rats were divided into four groups at random and given a single dose of SCOP formulation by different administration route (). Before the test, the anesthetized rats were fixed on microdialysis shelves, injected ACF after pipe connection was completed and balanced for 2 min.
Table 1. Studies schedule in rats for microdialysis.
Blood (500 µL) and microdialysis (20 µL) samples were collected into heparinized tubes from the caudal vein at 5, 10, 15, 30, 60, 90, 120, 180 min after administration. Blood was immediately processed for plasma by centrifugation at 3000 × g for 10 min. Then, plasma and microdialysis samples were frozen and maintained at −70 °C until analysis.
LC-MS/MS analysis
The liquid chromatography-tandem mass spectrometry (LC-MS/MS) experiments were conducted with an API4000 tandem mass spectrometer (Applied Biosystems) equipped with a binary and a quaternary pump (Agilent series 1200). Analyses were performed on a 5-µm Diamonsil C18 column (150 mm × 2.1 mm) at a flow rate of 0.3 mL/min. The gradient of mobile phase was composed of solution A (10 mM ammonium formate, formic acid adjusted to pH = 4.0) and solution B (methanol). The column temperature was maintained at 35 °C. LC-MS/MS was carried out using nitrogen to assist nebulization. The Multiple Reaction Monitoring (MRM) transitions are 304.1→166.2 for SCOP; 290.2→124.2 for atropine (internal standard, I.S. CAS: 51-55-8). The mass spectrometer was run in positive ion mode and tuned for unit mass resolution in the mass range used in the experiments. The exact source conditions were: drying gas temperature 350 °C, drying gas flow 10 L/min, nebulizer pressure 40 p.s.i.g., quadrupole temperature 100 °C and capillary voltage 4000 eV. After the acquisition of the resultant single ion monitoring (SIM), chromatograms were integrated by the HP Chemstation software.
A total of 100 µL volume of the plasma sample was transferred to a 1.5 mL plastic test tube together with 5 µL of I.S. solution (5 µg/mL). After vortex shaking for 1 min, 1 mL of diethyl ether was added and the mixture was vortexed for 2 min. After centrifugation at 3000 × g for 10 min, the upper organic layer was quantitatively transferred to a 5 mL glass tube and evaporated to dryness using evaporator at 40 °C. The residue was reconstituted in 20 µL of the mobile phase, and then vortex-mixed. 5 µL aliquot of the solution was injected into the LC-MS/MS system for analysis. Microdialysis samples (5 µL) were directly injected for analysis.
Statistical analysis
Values were expressed as mean ± SD for each group. Statistical evaluation of the experimental data was performed using a one-way ANOVA, post-hoc Dunnett's test. A (p < 0.05) was considered statistically significant. The free drug concentration of olfactory bulb and vestibule were calculated by the formula (1); AUC0–T was calculated by using the trapezoidal rule. Drug Target index (DTI) was used to evaluate the characteristics of brain drug delivery after intranasal administration, which was calculated as follows (2):
(1)
(2)
Results and discussion
A thorough and complete method validation of SCOP in rats' plasma and dialysate were validated for selectivity, sensitivity, linearity, precision and accuracy, recovery and stability. The calibration curves for SCOP in plasma and dialysate were linear from 10 to 1200 ng/mL and 5 to 400 ng/mL with correlation coefficient r = 0.9999 and r = 0.9993, respectively. The data indicated that intra-assay and inter-assay RSDs were meet the requirement of biosample determination. Stock solution of SCOP and IS were stable at room temperature for 12 h and at 4 °C for 30 d with mean %change well within 10%. Both the analytes were found stable at room temperature up to 24 h and for at least three freeze and thaw cycles.
In vitro recovery (R) of this microdialysis method was 10.06 ± 3.4%. The time course of the plasma concentrations of SCOP of four administration routes were summarized in . The pharmacokinetic parameters calculated from the plasma drug concentration versus time profiles were listed in . Cmax, maximum plasma concentration; Tmax, time to maximum plasma concentration; AUC0–∞, area under the concentration–time curve extrapolated to infinity; F, relative bioavailability. The dialysate concentration of SCOP in rats olfactory bulb and vestibular area following subcutaneous, oral administration, intranasal solution and intranasal in situ gel were summarized in and . The pharmacokinetic parameter of SCOP in rats olfactory bulb and vestibular area following subcutaneous, oral administration, intranasal solution and intranasal in situ gel in and . was the DTI value of SCOP in olfactory bulb after intranasal and oral administration.
Figure 3. Plasma concentration of SCOP in rats following intranasal in situ gel, subcutaneous, intranasal solution and oral administration. Data represent the mean ± SD (n = 5). *p < 0.05, intranasal in situ gel or intranasal solution or oral administration versus subcutaneous.
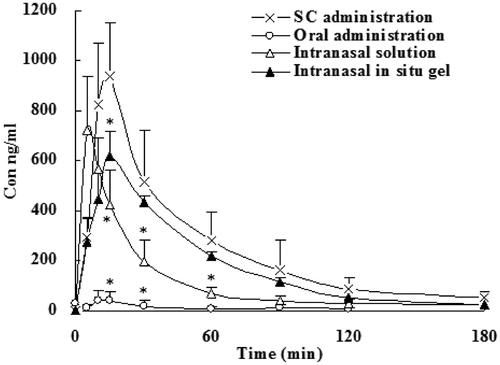
Figure 4. Dialysate concentration of SCOP in rats' olfactory bulb following subcutaneous, oral administration, intranasal solution and intranasal in situ gel. Data represent the mean ± SD (n = 5). *p < 0.05, intranasal in situ gel or intranasal solution or oral administration versus subcutaneous.
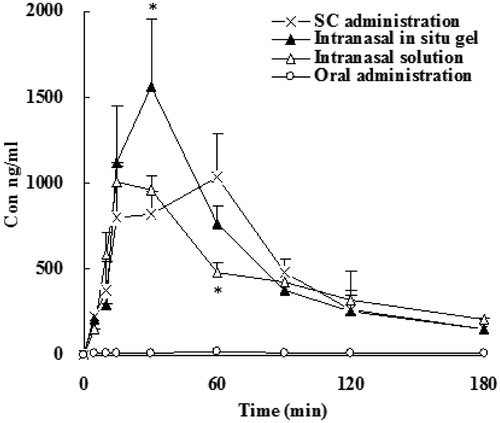
Figure 5. Dialysate concentration of SCOP in rats' vestibular area following subcutaneous, oral administration, intranasal solution and intranasal in situ gel. Data represent the mean ± SD (n = 5). *p < 0.05, intranasal in situ gel or intranasal solution or oral administration versus subcutaneous.
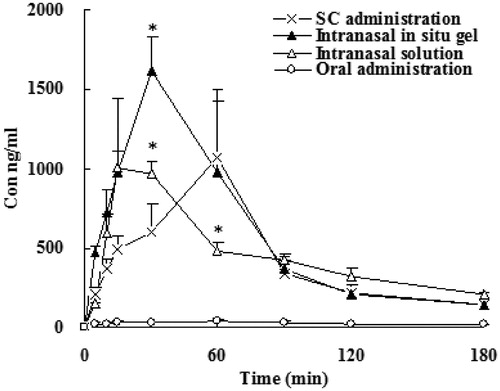
Table 2. Pharmacokinetic parameter of SCOP in rats following subcutaneous, oral administration, intranasal solution and intranasal in situ gel.
Table 3. Pharmacokinetic parameter of SCOP in rats' olfactory bulb following subcutaneous, oral administration, intranasal solution and intranasal in situ gel.
Table 4. Pharmacokinetic parameter of SCOP in rats' vestibular area following subcutaneous, oral administration, intranasal solution and intranasal in situ gel.
Table 5. DTI value of SCOP in olfactory bulb after intranasal and oral administration.
As shown in and , Cmax and bioavailability of SCOP after subcutaneous administration were the highest among the all groups. In the orally administration group, AUC was very low, and significant individual differences, erratic absorption, although the dose increased by 2.5 times. After dose calibration with respect to subcutaneous injection, the bioavailability was only 8.9%. It illustrated that orally administration of SCOP had serious first-pass effect (Nachum et al., Citation2001; Renner et al., Citation2005). The nasal gel administered after that totally absorbed more rapidly, with the relative bioavailability of 70.2%, further described SCOP nasal administration can avoid hepatic first-pass effect. Tmax plasma samples after intranasal administration were significantly faster than oral administration and subcutaneous injection, the drug can be rapidly absorbed by the body. Tmax was between 6 and 18 min and tips nasal administration does have the advantage of rapid onset. Meanwhile, the relative bioavailability of intrasal administrations was 51.8 to 70% when compared with subcutaneous injection. Furthermore, the bioavailability of gel group were higher compared to that of intranasal solutions, which may be due to the formation of a gel can reduce the loss of the drug, prolonged contact time of the drug in the nasal mucosa, thereby increasing the absorption of the drug.
It can be seen from and and and , first, although the AUC of intranasal administration group was lower than that of subcutaneous administration group, the concentration in the olfactory bulb and the vestibule was relatively high, indicating the presence of direct access transport from the olfactory bulb to the brain. Second, from the data of Tmax, we can see that the drug administered through the nose into the brain was faster, whether the solution or gel group reached peak within 30 min.
From , one can see that in comparison with SCOP subcutaneous administration, SCOP intranasal gel and solutions can increase DTI with olfactory bulb 1.69 and 2.05, vestibule 1.80 and 2.15, respectively. The results indicated that SCOP can be absorbed directly through the olfactory mucosa into the olfactory bulb, and then transported to various brain tissue after intranasal administration, with the characteristics of brain drug delivery.
SCOP is an alkaloid drug which is derived from Solanaceous plants, chiefly from henbane (Hyoscyamus niger). Its pharmacological properties arise through interference with the transmission of vestibular input to the CNS. This acts to inhibit the vomiting impulse normally activated by motion sickness. SCOP can be delivered through a variety of means, including intravenous injection, ingestion of tablets or liquid formulations, or topical application with adhesive transdermal patches or intranasal administration. The duration of treatment effectiveness varies according to the means of administration. For example, transdermal patches may be effective for up to 3 d, while tablets may need to be taken every 6 h for continued efficacy. Adult doses are typically 0.3 to 0.6 mg daily, while smaller doses of approximately 0.006 mg/kg are administered to children. Adverse effects experienced are typical of parasympathetic system depression and include drowsiness, dilated pupils, rapid heartbeat, and dry skin, mouth and respiratory passages. Overdose of scopolamine may cause symptoms of delirium, delusions, memory disturbances, paralysis and stupor. Withdrawal symptoms have also been noted after discontinuation following prolonged use and include dizziness, nausea, headache and vomiting (Spinks & Wasiak, Citation2011).
The BBB represents a stringent barrier for delivery of drugs in vivo. An attempt to overcome this barrier is represented by the direct transport of drugs from the nose to the brain along the olfactory and trigeminal nerve pathways. These nerve pathways initiate in the nasal cavity at olfactory neuroepithelium and terminate in the brain. An enormous range of neurotherapeutics, both macromolecules and low molecular weight drugs, can be delivered to the CNS via this route (Jiang et al., Citation1995). Since there are many capillaries and the lymphatic vessels of the nasal mucosa, drugs can rapidly absorbed directly into the systemic circulation. Therefore, intranasal administration can avoid first-pass effect of the liver and increase the bioavailability of the drug. Recent studies have found that, by nasal administration of drugs to increase brain delivery of beneficial in the treatment of brain diseases (Illum, Citation2000), which is receiving more and more attention from researchers.
There are many examples where microdialysis has been used in animal studies to help elucidate the pharmacokinetics of certain drugs (Alavijeh & Palmer, Citation2010). Excellent data can be gathered from regular blood sampling and cerebral microdialysis, and conclusions drawn about the interplay between blood and brain. These studies are used to predict therapeutic doses and dosing intervals for human clinical trials. However, there may be significant differences between how the drug behaves in an animal model and in humans. For example, there may be different substrate specific transporters operating at the BBB. In addition, cerebral microdialysis is an invasive technique that cannot be applied to healthy human volunteers. So it is important to take account of these differences between species when results are interpreted and compared.
This study determined the concentration of SCOP in brain with improved microdialysis methods to confirm whether SCOP enter into the brain through the olfactory mucosa after intranasal administration. Its deeper significance lies in the method to establish a platform for other brain delivery of drug research studies, such as Alzheimer's psychosis.
Declaration of interest
This work was supported by the National Science Foundation of China(81072175;81372854;81102010)and the Shanghai Committee of Science and Technology, China (Grant No.13NM1401504).
References
- Alavijeh MS, Palmer AM. (2010). Measurement of the pharmacokinetics and pharmacodynamics of neuroactive compounds. Neurobiol Dis 37:38–47
- Cao SL, Zhang QZ, Jiang XG. (2007). Preparation of ion-activated in situ gel systems of scopolamine hydrobromide and evaluation of its antimotion sickness efficacy. Acta Pharmacol Sin 28:584–90
- Hyde RW, Tonndorf J, Chinn HE. (1953). Absorption from the nasal mucous membrane. Ann Otol Rhinol Laryngol 62:957–68
- Illum L. (2000). Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci 11:1–18
- Jiang XG, Cui JB, Fang XL, et al. (1995). Toxicity of drugs on nasal mucocilia and the method of its evaluation. Acta Pharm Sin 30:848–53
- Lu W, Jiang W, Chen J, et al. (2008). Modulation of brain delivery and copulation by intranasal apomorphine hydrochloride. Int J Pharm 349:196–205
- Melgaard L, Hersini KJ, Gazerani P, Petersen LJ. (2013). Retrodialysis: a review of experimental and clinical applications of reverse microdialysis in the skin. Skin Pharmacol Physiol 26:160–74
- Mittal D, Ali A, Md S, et al. (2014). Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv 21:75–86
- Nachum Z, Shahal B, Shupak A, et al. (2001). Scopolamine bioavailability in combined oral and transdermal delivery. J Pharmacol Exp Ther 296:121–3
- Navailles S, Lagière M, Contini A, De Deurwaerdère P. (2013). Multisite intracerebral microdialysis to study the mechanism of L-DOPA induced dopamine and serotonin release in the parkinsonian brain. ACS Chem Neurosci 4:680–92
- Oman CM. (1990). Motion sickness: a synthesis and evaluation of the sensory conflict theory. Can J Physiol Pharmacol 68:294–303
- Reason JT. (1970). Motion sickness: a special case of sensory rearrangement. Adv Sci 26:386–93
- Reason JT, Brand JJ. (1975). Motion sickness. : Academic Press
- Renner UD, Oertel R, Kirch W. (2005). Pharmacokinetics and pharmacodynamics in clinical use of Scopolamine. Ther Drug Monit 27:655–65
- Robert F, Bert L, Lambas-Senas L, et al. (1996). In vivo monitoring of extracellular noradrenaline and glutamate from rat brain cortex with 2-min microdialysis sampling using capillary electrophoresis with laser-induced fluorescence detection. J Neurosci Methods 70:153–62
- Shannon RJ, Carpenter KL, Guilfoyle MR, et al. (2013). Cerebral microdialysis in clinical studies of drugs: pharmacokinetic applications. J Pharmacokinet Pharmacodyn 40:343–58
- Spinks A, Wasiak J. (2011). Scopolamine (hyoscine) for preventing and treating motion sickness. Cochrane Database Syst Rev 6:CD002851
- Thornton WE, Bonato F. (2013). Space motion sickness and motion sickness: symptoms and etiology. Aviat Space Environ Med 84:716–21
- Wang F, Jiang XG, Lu W. (2003). Profiles of methotrexate in blood and CSF following intranasal and intravenous administration to rats. Int J Pharm 263:1–7
- Yardley L. (1992). Motion sickness and perception: a reappraisal of the sensory conflict approach. Br J Psychol 83:449–71