Abstract
In recent years, polysaccharides, including starch and its derivatives, have been widely used in the pharmaceutical industry, including as diluents, fillers, binders, disintegrants and glidants. The use of native starch as excipient in extended-release tablets is limited due to its low compactibility and enzymatic degradability, leading to the formation of weakly structured tablets. To overcome these limitations and expand the application of starch as an excipient, researchers have modified starch by physical and chemical methods, as well as by enzymatic hydrolysis. Some starch derivatives, including retrograded starch, pregelatinized starch, carboxymethyl starch, starch acetate, cross-linked starch and grafted starch have recently been introduced as excipients in oral tablets to control drug release. In this review, applications of starch and its derivatives as extended release excipients are reviewed and future frontiers are described.
Introduction
In recent years, modified starches have been extensively used in pharmaceutical companies worldwide in different stages of drug development. Excipient dominates in solid dosage formulation by rendering mechanical strength, stability and tablet disintegration.
Native starches, as natural and secure excipients, have been used as classic tablet disintegrants, fillers and binders. However, they suffer from low compactibility and elastic compression behaviors. Meanwhile, orally administered drugs comprising native starches are subject to being eroded by α-amylase in the gastrointestinal tract and thus fail to extend the drug release (Prado et al., Citation2009). Therefore, natural starches should be modified by physical and chemical methods, as well as by enzymatic hydrolysis, in order to overcome these limitations and expand the application of starch as an excipient.
During the past two decades, numerous studies have prepared oral extended release tablets by starch and its derivatives. Many starch-based extended release excipients have successfully retarded drug releases. This review aims to summarize the recent development of starch-based excipients used in matrix tablets and to be aware of the extended release delivery systems relating to controlled release.
Retrograded starch
During the retrogradation of starch, the chains re-associate through intramolecular hydrogen bonding and intermolecular van der Waals forces. Meanwhile, starch paste is recrystallized, thus raising the enzymatic resistance (Tako et al., Citation1996), the amount and polymorph of which are relevant to the retrogradation conditions such as temperature and retrogradation rate. Commonly, the polymorph of retrograded starch contains more B-type crystals characterized by double helices (Marsh & Blanshard, Citation1988), and adding water can mobilize the starch chains. Starch is optimally retrograded and recrystallized at 4 °C and 50%–60% of water content. Furthermore, mild heating during refrigeration facilitates the growth of starch crystals (Yoon et al., Citation2009), and temperature cycling elevates the pasting temperature and viscosity of retrograded starches compared with isothermal storage does. Thus, retrogradation increases the content of slowly digested starch without altering that of resistant starch, which becomes more prominent by temperature cycling rather than isothermal storage (Zhou & Lim, Citation2012). Besides starch crystals, amorphous starch, which plays an essential role in the formation of gel network structure, also dominates in the drug release from extended release tablets. The amorphous and non-ordered starch chains develop into a non-ordered gel network after association, which highly resists to the erosion of gastrointestinal enzymes and benefits the controlled release of drugs from tablet matrix.
The effects of retrogradation on the drug release from retrograded starch-based tablets were further investigated by Yoon’s research group (Yoon et al., Citation2009). They prepared theophylline tablets by waxy maize starch gels and investigated the effect of retrogradation on the release of theophylline. They found that both the air cell sizes and the gel pore sizes decreased by increasing the duration of 4 °C-retrogradation. Moreover, the cell walls were attenuated. The retrogradation-induced results enhanced the resistance to enzymatic erosion and decreased the ability of swelling in aqueous matrix. They also found that the temperature-cycled retrogradation under 4/30 °C cycles yielded a more compact matrix structure with lower swelling ability, which is the premise of resisting to enzymatic hydrolysis. Therefore, the temperature-cycled retrogradation effectively extended the release of theophylline by forming a stable amorphous network.
Enzymatically modified starch
The molecular weight distributions of starch macromolecules together with the chain lengths of enzymatically modified starches affect the properties of starches such as viscosity and texture, which also reveal the enzymatic processes beneath starch synthesis (Castro et al., Citation2005). Pullulanase and isoamylase enzymatically hydrolyze the α-1,6 glycosidic bonds of amylopectin selectively. The digestibility of debranched products is related to the molecular and crystal structures as well as morphology during the debranching and crystallization of native starches (Cai et al., Citation2010; Cai & Shi, Citation2010). Linear short chain amylose (amylodextrin) is produced due to debranching enzyme, followed by precipitation, filtration and ethanol washing. The enzymatically modified starch extended the release of drugs at an almost constant rate. Moreover, this product is featured in the high-specific surface area owing to ethanol washing, which augmented the compactibility of starch products following an almost zero-order sustained drug release (Wierik et al., Citation1997a,Citationb).
The tablets containing the new linear short-chain starch product did not disintegrate and functioned following an almost constant sustained drug release. During swelling, a solvent front slowly penetrated into the tablets, and the delivery from these tablets followed a swelling-controlled solvent-activated mechanism. The incorporation of magnesium stearate into the tablets or α-amylase in the dissolution medium did not influence drug release. Tablets containing the starch with a lower specific surface area show an increased and less constant release rate and higher standard deviations. When the specific surface area is lower than 1.0 m2/g, the tablets will rapidly disintegrate and show a faster drug release. A specific surface area of 1.5 m2/g is required in order to obtain a zero-order release (Wierik et al., Citation1997a,Citationb).
Our research group recently investigated the extended-release properties of amorphous debranched starch (ADBS) derived from pullulanase enzymatic modification (Liu et al., Citation2013). The results showed the enzymatically modified starch-based tablets could effectively retard drug release for more than 12 h. Different factors (e.g. pH, pancreatin and ionic strength) of dissolution medium for ADBS-based tablets were evaluated. We found the drug release, which was accelerated by decreased pH and pancreatin, was barely affected by ionic strength. The drug release data were best fitted to the Higuchi equation, and the drug release process was dominated by Fickian diffusion. We confirm ADBS is able to be used in oral tablets to extend drug release.
Substituted starches
Substituted starches are prepared by esterifying and etherifying the hydroxyl groups of glucose units, such as acetylated starch (Ali & Hasnain, Citation2011; Han et al., Citation2012), carboxymethyl starch (CMS) (Tatongjai & Lumdubwong, Citation2010; Bhandari & Hanna, Citation2011), hydroxyethyl starch (Liu et al., Citation2003), hydroxypropylated starch (Chuenkamol et al., Citation2007) and phosphate starch (Park et al., Citation2004). The substituted starches are prepared in aqueous, organic or water-miscible organic media. Organic media allow higher substitution, but residues such as by-products and salts remain in the finial products (Assaad & Mateescu, Citation2010).
Carboxymethyl starch
CMS is a pH-sensitive tablet excipient that modulates drug release according to the physiological pH values. Until now, high amylose starch (more than 70% amylose) has been used as the reaction substrate in most cases of CMS preparation. An oral dosage form can be readily prepared by dry-mixing and directly compressing drugs and CMS.
The carboxyl groups of starches are dimerized by hydrogen bonds in acid fluids, and the resultant dimerization and hydrogen bonds enhance the stability of tablets. When the CMS tablets were transferred into a high pH fluid (SIF, pH 6.8), protons started to exchange with cations between the matrix and aqueous medium. This hydration facilitated the swelling, diffusion and erosion of the matrix, as well as the release of bioactive agents (Assaad et al., Citation2011). CMS is lately added to tablets as a pH-sensitive excipient to protect bioactive agents from being destructed by strong acidic gastric fluids. The properties of CMS excipients and the drug release mechanisms concerning monolithic tablets have been frequently referred. Numerous researchers have investigated the influences of the percentage of protonation, the degree of substitution (DS) () and amylose content on the release kinetics of small bioactive agents from CMS matrix tablets () (Lemieux et al., Citation2009; Tatongjai & Lumdubwong, Citation2010; Assaad et al., Citation2011).
Figure 1. Physical appearance of CMS- (high amylose content) based tablets with different DS incubated in SGF for different time (Lemieux et al., Citation2009). (A): High amylose starch (HAS) without carboxymethylation (DS 0); (B): CMS with a DS of 0.09 (DS 0.09); and (C): CMS with a DS of 1.23 (DS 1.23). Reprinted from International Journal of Pharmaceutics, Vol. 382, Lemieux M, Gosselin P, Mateescu MA, Carboxymethyl high amylose starch as excipient for controlled drug release: mechanistic study and the influence of degree of substitution, pp. 172–182, Copyright (2009), with permission from Elsevier.
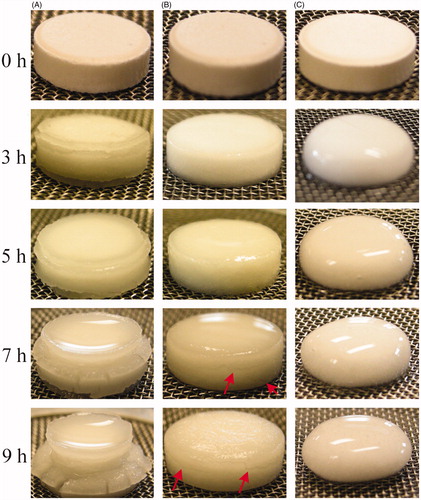
Figure 2. SEM micrographs of radial cross-sections of SA based tablets (Pohja et al., Citation2004). (A) Tablet containing 80% SA (DS 3.0) before the dissolution test. Voids formed after the densification process indicate the incorporation of drug model decreased the compactibility of tablets; (B) tablet containing 84% SA (DS 2.6) after the dissolution test. The drug was released completely, and some pores were formed during drug dissolution (shown by the white arrows.); (C) tablet containing 80% SA (DS 3.0) after the dissolution test. A distinct solvent front formed in the tablet, and the drug in the core of the tablet was not dissolved; (D) tablet containing 75% SA (DS 2.6) after the dissolution test. The white arrows indicate macroscopic cracks formed on the horizontal plane in the middle of the tablets when the SA concentration was lower than a certain degree. Reprinted from Journal of Controlled Release, Vol. 94, Pohja S, Suihko E, Vidgren M, Paronen P, Ketolainen J, Starch acetate as a tablet matrix for sustained drug release, pp. 293–302, Copyright (2004), with permission from Elsevier.
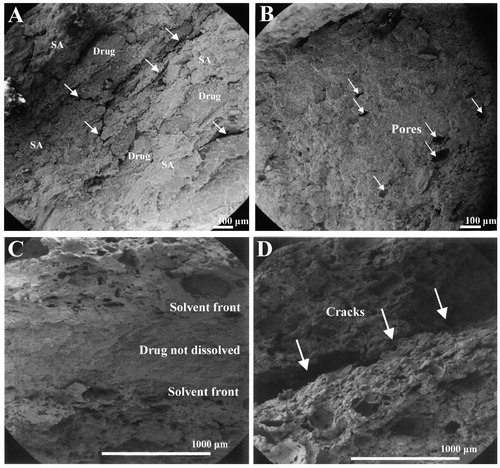
Table 1. Investigations of carboxymethyl starch used in extended-release matrix tablets.
Starch acetate
Starch acetate (SA) is a film-forming polymer produced by acetylating native starch (Tarvainen et al., Citation2002). Unlike other modified starches, SA is less hydrophilic due to the hydrophobic nature of acetoxy substituent (Pohja et al., Citation2004).
Highly substituted SA has been introduced for directly compressed tablets as a pharmaceutical excipient (Korhonen et al., Citation2004). Highly substituted SA (DS 2–3) is soluble in acetone, chloroform and other organic solvents. Moreover, the thermoplastic property of high acetyl-substituted starch and the above properties are in need of an in-depth study (Singh et al., Citation2011). The acetylation of native starch considerably overcomes the substantial swelling and rapid enzymatic degradation in biological fluids, which can be ascribed to the raised hydrophobicity and steric bulkiness of the modified starch owing to the acetyl groups of SA (Tuovinen et al., Citation2003).
Drugs are released from the SA matrix monolithic tablets via diffusion in most cases. Many factors, such as SA properties, the porosity and compaction of tablets, the concentration ratio of drug to SA in the formulation, the physicochemical nature of drugs, remarkably affect the drug release behaviors. The drug release profile is governed by taking all the above variables into consideration () (Korhonen et al., Citation2004).
Table 2. Evaluations of starch acetate used as an excipient.
Cross-linked starch
Cross-linked high amylose starch (CLHAS) was introduced in the early 1990s as a tablet excipient for drug controlled release with the brand name Contramid® (Lenaerts et al., Citation1991, Citation1998; Dumoulin et al., Citation1998). CLHAS is generally derived from the cross-linking of high amylose starch (70% amylose, 30% amylopectin) with epichlorohydrin or phosphate (Rahmouni et al., Citation2001). CLHAS tablets are of high drug loading and quasi zero-order release profiles. Compared to other excipients, CLHAS is more suitable for hydrophilic matrices preparation. The water uptake rate, drug release rate and equilibrium swelling are elevated with increasing cross-linking degree (cld), which is ascribed to the unique characteristics of CLHAS tablets, such as erosion resistance and swelling restriction (Lenaerts et al., Citation1998).
CLHAS tablets swell in aqueous medium, yielding an elastic gel layer on the surface of tablets (Ispas-Szabo et al., Citation2000). Hydrogel, which functions superbly as a pharmaceutical excipient due to the appropriate swelling resistance and drug release control, forms a gel network upon drug captures and thus hinders the release to the surrounding medium (Onofre et al., Citation2009). Cross-linked amylose (CLA) with low cld shows intriguing mechanical and sustained release properties. Unlike other polymeric matrices, CLA tablets can no longer control drug release with increasing cld. High-cld CLA excipient outweighs others as a binder or disintegrant (Dumoulin et al., Citation1998), which is closely related to the network organization. The result is consistent with the hypothesis that network organization and water availability dominate in drug release kinetics. The matrix cohesion and sustained release properties of tablets are determined by covalent linkages and inter-chain hydrogen bonds (). Particularly structured low-cld CLHAS matrix is conducive to controlling the release behaviors. The crystal morphology of low-cld CLHAS is transformed from B-type double helix to V-type single helix, leading to the intriguing dissolution kinetics and mechanical hardness of dry tablets (Ispas-Szabo et al., Citation2000). Nevertheless, the network organization of high-cld CLHAS entraps more water molecules, which promotes the swelling of tablets and accelerates the release of drugs. The investigations were exhaustively described in .
Figure 3. Schematic representation of the covalent and hydrogen bonding stabilization of CLA (Dumoulin et al., Citation1998). (A) CLA with low-moderate cld and (B) CLA with high cld. Reprinted from Carbohydrate Polymers, Vol. 37, Y. Dumoulin, Alex S, Szabo P, Cross-linked amylose as matrix for drug controlled release. X-ray and FT-IR structural analysis, pp. 361–370, Copyright (1998), with permission from Elsevier.
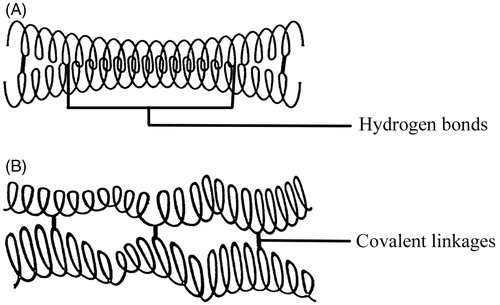
Table 3. Investigations of cross-linked starch used in extended-release matrix tablets.
Grafted starch
Modifying natural polymers chemically by grafting has attracted considerable attraction due to the integrated advantages of natural and synthetic polymers (Ochubiojo & Rodrigues, Citation2012). Modifying a host polymer by the graft copolymerization of guest monomers, such as ethyl methacrylate (Marinich et al., Citation2009, Citation2012), acrylamide (Singh & Nath, Citation2012a), methacrylic acid (Silva et al., Citation2011) and acrylic acid (Geresh et al., Citation2004; Eid, Citation2008), brings about innovative and desirable properties. In recent years, starch-based graft copolymers are of great significance in designing controlled release systems. Several researchers have investigated the influence of different factors on drug release of grafted starch-based tablets ().
Table 4. Investigations of grafted starch used in extended-release matrix tablets.
Graft copolymerization offers novel and eligible properties (Fares et al., Citation2003), during which 3D gel network structures are produced due to the participation of hydrophilic or hydrophobic groups in the guest monomer (Qiu & Park, Citation2001). Starch-based grafted copolymers are rapidly released from corresponding matrix tablets with high equilibrium swelling coefficients. Graft copolymerization primarily takes place in the amorphous regions of starch chains, generating numerous diffusion paths inside the copolymer through which drugs are able to permeate. Hence, the grafted starch is highly swollen in water (Al-Karawi & Al-Daraji, Citation2010). Meanwhile, grafted starch, which is a hydrophilic matrix, swells in aqueous media and is appropriate for preparing oral extended release dosage forms (Singh & Nath, Citation2012b).
Conclusions
Starches have been subjected to physical (retrograded starch) or chemical modification (cross-linked starch (CLS), SA, CMS and grafted starch) and enzymatic hydrolysis (linear short chain amylose) to control drug release as the excipients. A detailed comparison and contrast of different starch derivatives used as extended-release tablets excipients was presented ().
Table 5. Comparison and contrast of different starch derivatives used as extended-release tablets excipients.
Influence factors, like crystalline/amorphous structure, amylose/amylopectin content, DS and drug loading, play important roles in drug release properties from modified starch-based tablets. For starch excipients, good gel-forming capability is essential to retard drug release from tablets matrix. Compared to the other starch derivatives, more cracks formed on the central horizontal plane of SA and enzymatic debranched starch-based tablets. Cracks on the surface of tablets can effectively maintain the drug release at a similar rate and improve the bioavailability of drugs. In summary, a proper modification to native starch is feasible to prepare hydrophilic materials used as tablets excipients. To these starch derivatives, both gel-forming ability and digestibility are prerequisites for extending drug release from tablets.
Present aspects and prospects
For chemically modified starches, like CLS, SA, CMS, as well as grafted starch, high amylose starch is essential to obtain a better extended drug release profile. However, common starch can be used as material during enzymatic modification. Compared to chemical reagents used in chemical modification, pullulanase, as well as isoamylase, is safer to human health. As a personal point of view, enzymatically modified starch as extended-release excipient in oral tablets has a good application prospect.
There are an increasing number of papers about starch-based excipients used in extended-release tablets published in different journals. For most researches, too much attention was paid on the physicochemical properties of excipients and drug release study. However, fewer papers focused on the internal properties of the matrix. Personally speaking, the properties of matrix are the most important aspect, while drug release and physicochemical properties are secondary. Drug release property depends on several factors, like drug loading, compact force and it can be completely changed during hydration.
With the development of technology, several high-tech instruments have been introduced into the study of internal properties of matrix during hydration. Zhu, X.’s research group innovatively used nuclear magnetic resonance imaging in the study of internal properties of hydrating CLHAS tablets (Thérien-Aubin et al., Citation2008; Wang et al., Citation2010; Thérien-Aubin & Zhu, Citation2009). X-ray microtomography has been used to investigate the dynamic behavior of swelling tablets (Laity & Cameron, Citation2010; Laity et al., Citation2010). With the help of these novel technologies, we can investigate the internal properties of hydrating tablets more easily. In the near future, increasing investigators will do correlated researches and further insight into the swelling behavior of starch-based tablets will be obtained.
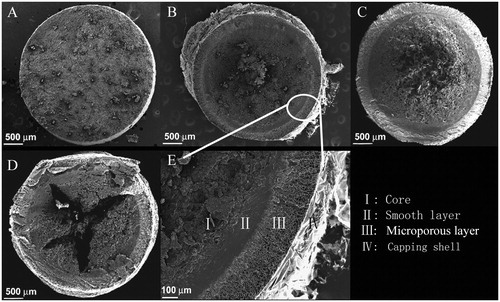
Figure 4. SEM top view micrograph of radial cross-sections of CLHAS-based tablets (Chauve et al., Citation2007). (A) 0-min swelling; (B) 15-min swelling; (C) 60-min swelling; (D) 150-min swelling; and (E) a close view of the 15-min swelling tablet. Four concentric domains are seen indicating a distinct evidence of membrane formation. Reprinted from Carbohydrate Polymers, Vol. 70, Chauve G, Ravenelle F, Marchessault RH, Comparative imaging of a slow-release starch excipient tablet: evidence of membrane formation, pp. 61–67, Copyright (2007), with permission from Elsevier.
Declaration of interest
The authors report no declarations of interest.
This research was supported by National Key Technologies 356 R&D Program of China (2012BAD37B01), Research Fund for the Doctoral Program 357 of Higher Education of China (20100093110001), 111 Project-B07029 and PCSIRT0627.
References
- Al-Karawi AJM, Al-Daraji AHR. (2010). Preparation and using of acrylamide grafted starch as polymer drug carrier. Carbohyd Polym 79:769–74
- Ali TM, Hasnain A. (2011). Functional and morphological characterization of low-substituted acetylated white sorghum (Sorghum bicolor) starch. Int J Polym Anal Ch 16:187–98
- Alias J, Goñi I, Gurruchaga M. (2007). Enzymatic and anaerobic degradation of amylose based acrylic copolymers, for use as matrices for drug release. Polym Degrad Stabil 92:658–66
- Assaad E, Mateescu MA. (2010). The influence of protonation ratio on properties of carboxymethyl starch excipient at various substitution degrees: structural insights and drug release kinetics. Int J Pharm 394:75–84
- Assaad E, Wang YJ, Zhu XX, Mateescu MA. (2011). Polyelectrolyte complex of carboxymethyl starch and chitosan as drug carrier for oral administration. Carbohyd Polym 84:1399–407
- Bhandari PN, Hanna MA. (2011). Continuous solvent less extrusion process for producing sodium carboxymethyl starch suitable for disintegrant applications in solid dosage forms. Ind Eng Chem Res 50:12784–9
- Cai L, Shi Y-C. (2010). Structure and digestibility of crystalline short-chain amylose from debranched waxy wheat, waxy maize, and waxy potato starches. Carbohyd Polym 79:1117–23
- Cai L, Shi Y-C, Rong L, Hsiao BS. (2010). Debranching and crystallization of waxy maize starch in relation to enzyme digestibility. Carbohyd Polym 81:385–93
- Casas M, Ferrero C, De Paz MV, Jiménez-Castellanos MR. (2009). Synthesis and characterization of new copolymers of ethyl methacrylate grafted on tapioca starch as novel excipients for direct compression matrix tablets. Eur Polym J 45:1765–76
- Castro JV, Ward RM, Gilbert RG, Fitzgerald MA. (2005). Measurement of the molecular weight distribution of debranched starch. Biomacromolecules 6:2260–70
- Chauve G, Ravenelle F, Marchessault RH. (2007). Comparative imaging of a slow-release starch excipient tablet: evidence of membrane formation. Carbohyd Polym 70:61–7
- Chuenkamol B, Puttanlek C, Rungsardthong V, Uttapap D. (2007). Characterization of low-substituted hydroxypropylated canna starch. Food Hydrocolloid 21:1123–32
- Dumoulin Y, Alex S, Szabo P. (1998). Cross-linked amylose as matrix for drug controlled release. X-ray and FT-IR structural analysis. Carbohyd Polym 37:361–70
- Eid M. (2008). In vitro release studies of vitamin B-12 from poly N-vinyl pyrrolidone/starch hydrogels grafted with acrylic acid synthesized by gamma radiation. Nucl Instrum Meth B 266:5020–6
- Fares MM, El-Faqeeh AS, Osman ME. (2003). Graft copolymerization onto starch–I. Synthesis and optimization of starch grafted with N-tert-butylacrylamide copolymer and its hydrogels. J Polym Res 10:119–25
- Geresh S, Gdalevsky GY, Gilboa L, et al. (2004). Bioadhesive grafted starch copolymers as platforms for peroral drug delivery: a study of theophylline release. J Control Release 94:391–9
- Han F, Liu M, Gong H, et al. (2012). Synthesis, characterization and functional properties of low substituted acetylated corn starch. Int J Biol Macromol 50:1026–34
- Ispas-Szabo P, Ravenelle F, Hassan I, et al. (2000). Structure-properties relationship in cross-linked high-amylose starch for use in controlled drug release. Carbohyd Res 323:163–75
- Korhonen O, Kanerva H, Vidgren M, et al. (2004). Evaluation of novel starch acetate-diltiazem controlled release tablets in healthy human volunteers. J Control Release 95:515–20
- Kwon K, Auh JH, Kim JW, et al. (1997). Physicochemical properties and functionality of highly carboxymethylated starch. Starch-Stärke 49:499–505
- Laity PR, Cameron RE. (2010). Synchrotron X-ray microtomographic study of tablet swelling. Eur J Pharm Biopharm 75:263–76
- Laity PR, Mantle MD, Gladden LF, Cameron RE. (2010). Magnetic resonance imaging and X-ray microtomography studies of a gel-forming tablet formulation. Eur J Pharm Biopharm 74:109–19
- Lemieux M, Gosselin P, Mateescu MA. (2009). Carboxymethyl high amylose starch as excipient for controlled drug release: mechanistic study and the influence of degree of substitution. Int J Pharm 382:172–82
- Lenaerts V, Dumoulin Y, Mateescu MA. (1991). Controlled release of theophylline from CLA tablets. J Control Release 15:39–46
- Lenaerts V, Moussa I, Dumoulin Y, et al. (1998). Cross-linked high amylose starch for controlled release of drugs: recent advances. J Control Release 53:225–34
- Liu G, Hong Y, Gu Z. (2013). Evaluation of amorphous debranched starch as extended-release matrices in tablets. Carbohyd Polym 98:995–1001
- Liu YM, Xu GY, He L, et al. (2003). The position investigation of the substituted hydroxyethyl groups of hydroxyethyl starch. Chinese J Anal Chem 31:271–6
- Marinich JA, Ferrero C, Jimenez-Castellanos MR. (2009). Graft copolymers of ethyl methacrylate on waxy maize starch derivatives as novel excipients for matrix tablets: physicochemical and technological characterisation. Eur J Pharm Biopharm 72:138–47
- Marinich JA, Ferrero C, Jimenez-Castellanos MR. (2012). Graft copolymers of ethyl methacrylate on waxy maize starch derivatives as novel excipients for matrix tablets: drug release and fronts movement kinetics. Eur J Pharm Biopharm 80:674–81
- Marsh RDL, Blanshard JMV. (1988). The application of polymer crystal growth theory to the kinetics of formation of the B-amylose polymorph in a 50% wheat-starch gel. Carbohyd Polym 9:301–17
- Nabais T, Brouillet F, Kyriacos S, et al. (2007). High-amylose carboxymethyl starch matrices for oral sustained drug-release: in vitro and in vivo evaluation. Eur J Pharm Biopharm 65:371–8
- Ochubiojo EM, Rodrigues A. (2012). Starch: from food to medicine. In: Valdez B, Schorr M, Zlatev R, eds. Scientific, health and social aspects of the food industry. Rijeka: InTech, 355–80
- Onofre F, Wang Y-J, Mauromoustakos A. (2009). Effects of structure and modification on sustained release properties of starches. Carbohyd Polym 76:541–7
- Park DP, Sung JH, Choi HJ, Jhon MS. (2004). Electroresponsive characteristics of highly substituted phosphate starch. J Mater Sci 39:6083–6
- Pohja S, Suihko E, Vidgren M, et al. (2004). Starch acetate as a tablet matrix for sustained drug release. J Control Release 94:293–302
- Prado HJ, Matulewicz MC, Bonelli PR, Cukierman AL. (2009). Preparation and characterization of a novel starch-based interpolyelectrolyte complex as matrix for controlled drug release. Carbohydr Res 344:1325–31
- Qiu Y, Park K. (2001). Environment-sensitive hydrogels for drug delivery. Adv Drug Deliver Rev 53:321–39
- Rahmouni M, Chouinard F, Nekka F, et al. (2001). Enzymatic degradation of cross-linked high amylose starch tablets and its effect on in vitro release of sodium diclofenac. Eur J Pharm Biopharm 51:191–8
- Silva I, Gurruchaga M, Goni I. (2011). Drug release from microstructured grafted starch monolithic tablets. Starch-starke 63:808–19
- Singh AV, Nath LK. (2012a). Synthesis and evaluation of physicochemical properties of cross-linked sago starch. Int J Biol Macromol 50:14–18
- Singh AV, Nath LK. (2012b). Synthesis, characterization, and compatibility study of acetylated starch with lamivudine. J Therm Anal Calorim 108:307–13
- Singh AV, Nath LK, Guha M. (2011). Synthesis and characterization of highly substituted acetylated moth bean starch. J Polym Mater 28:275–83
- Tako M. (1996). Molecular Origin for the Thermal Stability of Waxy – rice (Kogane) Starch. Starch-Stärke 48:414–17
- Tarvainen M, Sutinen R, Peltonen S, et al. (2002). Starch acetate – a novel film-forming polymer for pharmaceutical coatings. J Pharm Sci 91:282–9
- Tatongjai J, Lumdubwong N. (2010). Physicochemical properties and textile utilization of low- and moderate-substituted carboxymethyl rice starches with various amylose content. Carbohyd Polym 81:377–84
- Thérien-Aubin H, Zhu X. (2009). NMR spectroscopy and imaging studies of pharmaceutical tablets made of starch. Carbohyd Polym 75:369–79
- Thérien-Aubin H, Zhu X, Ravenelle FO, Marchessault RH. (2008). Membrane formation and drug loading effects in high amylose starch tablets studied by NMR imaging. Biomacromolecules 9:1248–54
- Tuovinen L, Peltonen S, Järvinen K. (2003). Drug release from starch-acetate films. J Control Release 91:345–54
- Wang Y, Ravenelle F, Zhu X. (2010). NMR imaging study of cross-linked high-amylose starch tablets – the effect of drug loading. Can J Chem 88:202–7
- Wierik GHPT, Eissens AC, Bergsma J. (1997a). A new generation of starch products as excipient in pharmaceutical tablets. II. High surface area retrograded pregelatinized potato starch products in sustained-release tablets. J Control Release 45:25–33
- Wierik GHPT, Eissens AC, Bergsma J. (1997b). A new generation starch product as excipient in pharmaceutical tablets III. Parameters affecting controlled drug release from tablets based on high surface area retrograded pregelatinized potato starch. Int J Pharm 157:181–7
- Yoon HS, Lee JH, Lim ST. (2009). Utilization of retrograded waxy maize starch gels as tablet matrix for controlled release of theophylline. Carbohyd Polym 76:449–53
- Zhou X, Lim S-T. (2012). Pasting viscosity and in vitro digestibility of retrograded waxy and normal corn starch powders. Carbohyd Polym 87:235–9
