Abstract
Context: The antidiabetic drug metformin exhibits antiproliferative and pro-apoptotic effects in various cells, suggesting its potential to treat a variety of malignant and non-malignant hyperplastic diseases. Clinical studies indicate that psoriasis patients with metformin treatment have a better response than those without metformin.
Objective: The present study evaluates the antiproliferative activity and anti-inflammatory responses of metformin in human keratinocytes in vitro and explores the underlying mechanisms.
Materials and methods: HaCaT cells were incubated with metformin at 0, 25, 50, and 100 mM for 48 h. Antiproliferative activity was evaluated by MTT and apoptotic response was examined by flow cytometry. ELISA was used to detect IL-6, TNF-α, and VEGF protein expression. Western blot was used to investigate the expression of the mammalian target of rapamycin (mTOR) and its downstream effectors p70 ribosomal S6 kinase (p70S6K).
Results: The survival rates of HaCaT cells treated with metformin at 50 mM were reduced to 75.6, 59.4, and 30.3% at 24, 48, and 72 h, respectively. The number of apoptotic HaCaT cells was significantly increased at 50 mM metformin after 48 h treatment. Metformin can exert an anti-inflammatory effect by direct inhibition of IL-6, TNF-α, and VEGF. Metformin at 50 mM significantly reduced the phosphorylation of mTOR and p70S6K, by 49.0 and 62.1%, respectively.
Discussion and conclusion: Metformin treatment significantly inhibited proliferation and proinflammatory responses in HaCaT cells by a mechanism associated with inhibition of the mTOR signaling pathway. The results indicate that metformin may be used as a potential therapeutic agent for psoriasis.
Introduction
Psoriasis is a common chronic inflammatory disease of the skin affecting 2–3% of the population in the world (Nestle et al., Citation2009). Physical illness, emotional pressure, and financial burden caused by the disease significantly affect the patient's quality of life. The pathophysiology of psoriasis is complex and has not been completely elucidated. Current studies show that abnormal proliferation and differentiation of keratinocytes as well as prominent immune cell infiltration contribute to psoriasis development (Abdou & Hanout, Citation2008). Consistently, inhibition of the excessive proliferation of keratinocytes and proinflammatory responses has proved to be efficient methods for psoriasis treatment.
Metformin is the first drug recommended by the American Diabetes Association for newly diagnosed type 2 diabetic patients for its hypoglycemic effects and ability to reduce cardiovascular morbidity and mortality (Kahn et al., Citation2005). Recent clinical and experimental studies have revealed that metformin treatment can reduce cancer risk and improve the prognosis of cancer patients (Leone et al., Citation2014). Clinical studies have shown that psoriasis patients on metformin have a better treatment response than those not on metformin (Brauchli et al., Citation2008). The mechanism underlying this phenomenon is thought to be through the activation of AMP-activated protein kinase (AMPK), which is a master sensor and regulator of energy homeostasis in mammalian cells and also a therapeutic target for metabolic disorders (Choi & Park, Citation2013). AMPK activation strongly inhibits cell growth and proliferation in both malignant and non-malignant cells through inhibition of mTOR signaling and stimulation of the p53-p21 axis (Storozhuk et al., Citation2013). In addition, the cell growth regulatory effects of metformin were associated with a reduction in mTOR signaling independent of AMPK (Ben et al., Citation2011).
The mTOR pathway is conserved in organisms from yeasts to humans. The central protein, mTOR, is an atypical serine/threonine protein kinase that belongs to the phosphoinositide 3-kinase (PI3K)-related kinase family. The mTOR pathway has been identified as a key intracellular signaling pathway for important cellular functions. It regulates cell growth and proliferation largely by promoting key anabolic processes, sensing nutrition levels and growth factors, as well as responding to various environmental cues (Wang & Zhang, Citation2014). mTOR has been implicated in the development and progression of various skin diseases including melanoma, acne vulgaris, and psoriasis.
Recent findings have suggested that the mTOR pathway may play an important role in the pathogenesis of psoriasis by mediating epidermal hyperplasia, immune response, and/or angiogenesis in psoriasis (Huang et al., Citation2014). Inhibition of the mTOR pathway can inhibit multiple proinflammatory cytokines including IL-6, TNF-α, and VEGF (Kirsch et al., Citation2012), which play important roles in the pathogenesis of psoriasis. Our previous study showed that metformin could inhibit the proliferation of human keratinocytes in association with the activation of the AMPK signaling pathway (Li et al., Citation2014). However, whether metformin suppresses human keratinocyte proliferation by specifically targeting the mTOR signaling pathway has not been studied.
Human immortalized keratinocyte (HaCaT) is a spontaneously immortalized human epithelial cell line that maintains full epidermal differentiation capacity and has been widely used as an in vitro model for the study of psoriasis (Boukamp et al., Citation1988; Stein et al., Citation1997). In the present study, the effects of metformin on HaCaT cells proliferation, apoptosis, and regulation of proinflammatory cytokines were evaluated.
Materials and methods
Cells and reagents
HaCaT cells were purchased from the American Type Culture Collection (Manassas, VA). Metformin hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO). MTT Cell Viability and Proliferation Assay was purchased from Sciencell (8028, Carlsbad, CA). FITC Annexin V Apoptosis Detection Kit was purchased from BD Pharmingen (San Diego, CA). The terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) in situ apoptosis detection kit was from Roche (Mannheim, Germany). The ELISA kit was from R&D systems (Minneapolis, MN). mTOR, p-mTOR(ser2448), p70S6K, and p-p70S6k antibodies were purchased from Abcam (Cambridge, MA). The quantitative automatic microplate reader (Model no., 2010) was purchased from Anthos Labtec Co., Ltd. (Salzburg, Austria). The study was approved by the ethics committee of Shandong University (Jinan, China).
Metformin treatment
HaCaT cells were cultured in DMEM with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a 5% CO2 humidified environment. HaCaT cells were collected and inoculated in 96-well (1 × 104 cells/well) or 6-well plates (2 × 105 cells/well) during the logarithmic growth phase. After 24 h of inoculation, the culture media in the metformin groups were replaced with DMEM containing 3% fetal bovine serum and 25, 50, and 100 mM of metformin. An equal volume of DMEM containing 3% fetal bovine serum was added to the control group.
Cell proliferation assay (MTT)
HaCaT cells were seeded at a density of 1 × 104 cells per well into 96-well plates and incubated for 24 h at 37 °C. Cell culture media (100 μl DMEM) and 100 μl metformin at various concentrations (25, 50, and 100 mM) were added to the cells. The cells were cultured at 37 °C in 5% CO2 for 24, 48, 72, and 96 h. Following 24 h of various concentrations of metformin treatment, the morphology of HaCaT cells was observed under an inverted microscope (Olympus BX-51; Olympus optical Co., Ltd., Tokyo, Japan). HaCaT cell proliferation was measured by the MTT assay performed according to standardized protocol. The OD values at 490 nm of each group were measured and the cell viability was calculated. Cell viability (%) = (ODmetformin/ODcontrol) × 100.
Annexin V/PI staining assay
Cells of 2 × 105 HaCaT were cultured in 6-well plates and incubated with metformin (0, 25, 50, and 100 mM) for 48 h at 37 °C. Cells were washed twice with cold PBS and resuspended in binding buffer. The solution (100 μl) was then transferred to a 5 ml culture tube. Apoptotic cells were detected by double staining with fluoresceinisothiocyanate (FITC)-conjugated annexin V and propidium iodide (PI) according to the instructions of the manufacturer (BD Pharmingen, San Diego, CA). The samples were analyzed by flow cytometry.
TUNEL assay
TUNEL assay was performed to detect apoptosis via DNA fragmentation. HaCaT cells (1 × 104 cells/well) were seeded into 96-well plates and incubated for 24 h at 37 °C. Cell culture media (100 μl DMEM) and 100 μl metformin (50 mM) were added to the cells. The cells were then cultured at 37 °C in 5% CO2 for 48 h, fixed with 4% paraformaldehyde for 30 min, and permeabilized with PBS containing 0.4% Triton X-100 for 15 min. After blocking with 4% BSA, the cells were incubated in the TUNEL kit according to the instructions of the manufacturer. TUNEL positive cells were counted as apoptotic cells.
Enzyme-linked immunosorbent assay
HaCaT cells were treated with metformin (0, 25, 50, and 100 mM) for 48 h. The culture supernatant was used for cytokine examination. Protein levels of IL-6, TNF-α, and VEGF were quantified using an ELISA kit (R&D Systems, Minneapolis, MN) according to the instruction of the manufacturer. The OD values at 450 nm of each group were recorded and the concentrations of the target protein were read according to the standard curve. Results were expressed as pg/mg protein.
Western blot analysis
HaCaT cells grown in 60 mm plates were treated with metformin (0, 50, and 100 mM) for 48 h. Total protein extracts (20 μg) were electrophoresed on 10% SDS polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat dried milk in Tris-buffered saline and incubated for 2 h at room temperature with appropriate primary antibodies against mTOR, p-mTOR (ser2448), p70S6K, and p-p70S6K. After washing with TBST, the membranes were incubated for 1 h with appropriate horseradish peroxidase-conjugated secondary antibody and the immune-complex was detected with chemiluminescent substrate. The developed films were scanned using the AlphaImager gel imaging systems (AlphaImager, Santa Clara, CA). The western blot images were analyzed using Quantity One software (Bio-Rad Laboratories, Hercules, CA). β-Actin was used as an internal control.
Statistical analysis
SPSS statistical software (v13.0; SPSS, Inc., Chicago, IL) was used for analysis. One-way analysis of variance was used for mean comparisons. Data were analyzed using Student's t-test and Chi-square statistics. Data are expressed as the mean ± SD. p values of < 0.05 were considered to be statistically significant.
Results
Metformin inhibits the growth of HaCaT cells in a dose-dependent manner
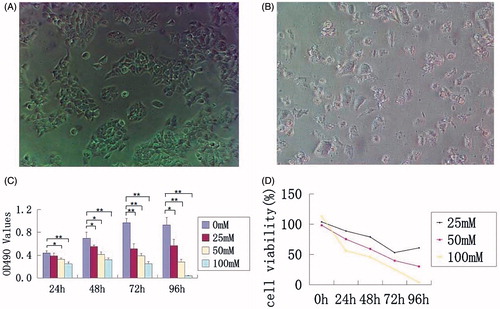
The effects of metformin treatment on the morphology of HaCaT cells were evaluated. Compared with typical healthy HaCaT cells in the untreated group, the cells treated with 50 mM metformin for 48 h became small and deformed, with nuclear pyknosis and fragmentation. With the increase in concentration of metformin, adherent cells appeared to be shrunken and round with reduced cytoplasm. In addition, exfoliated cells increased significantly as shown in . To investigate cell proliferation in response to metformin treatment, the MTT assay was performed. Compared with the non-treated group, inhibition of cell proliferation is closely associated with the concentration of metformin. Metformin at higher concentrations over 50 mM significantly inhibited proliferation of HaCaT cells after 24 h treatment. Cell proliferation inhibition was also observed with 25 mM metformin treatment at 48 h (). Similar to the findings for growth inhibition, cell viability inhibition by metformin was also dose dependent. The survival rates of HaCaT cells treated with metformin at 50 mM were 75.6, 59.4, and 30.3% at 24, 48, and 72 h, respectively ().
Figure 1. HaCaT cells morphology, proliferation and viability analysis following metformin treatment. (A) Untreated HaCaT cells form many colonies. (B) HaCaT cells treated with 50 mM metformin for 48 h form scattered deformed cells. (C) OD value of 490 nm in each group with different concentrations of metformin treatment at 24, 48, and 72 h. (D) Cell viability of each group at 24, 48, and 72 h following metformin treatment. *p < 0.05, **p < 0.001.
Metformin-induced HaCaT cells apoptosis
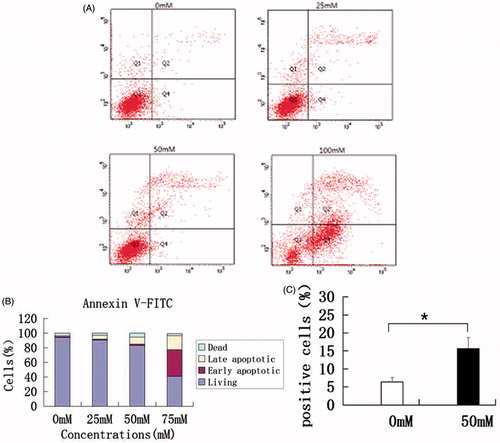
To examine if metformin can induce HaCaT cell apoptosis, we performed flow cytometry and apoptotic analysis. Compared with non-treated cells, the numbers of apoptotic (annexin+) HaCaT cells increased at 25 mM and significantly increased at 50 mM metformin after 48 h treatment. 100 mM metformin treatment induced apoptosis in almost half of the cells (). Furthermore, the effect of metformin (50 mM) on HaCaT cell apoptosis was assessed by TUNEL assay to provide an additional marker of apoptosis. Cell-stained brown are TUNEL positive cells experiencing DNA fragmentation. The percentage of cells positive for TUNEL after exposure to both 0 mM and 50 mM metformin is shown in . The number of apoptotic HaCaT cells was significantly increased at 50 mM metformin treatment (p < 0.05).
Figure 2. Apoptosis analysis by flow cytometric and apoptotic analysis kit and TUNEL assays. (A) Flow cytometric analysis showed that cell apoptotic indices of HaCaT cells increased in a metformin dose-dependent manner. (B) Bar graph quantifying the percentage of dead, living, early-stage apoptotic, and late-stage apoptotic cells of different groups. (C) TUNEL assays of HaCaT cells after 50 mM metformin treatment. Higher rates of apoptosis as indicated by the percentage of TUNEL positive cells were observed at 48 h after 50 mM metformin treatment in HaCaT cells. *p < 0.05.
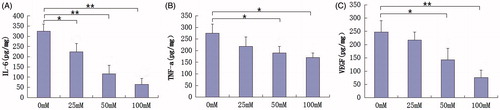
Metformin attenuated IL-6, TNF-α, and VEGF expression
We used ELISA to evaluate changes in inflammation-related cytokine expression in protein levels. Metformin reduced IL-6, TNF-α, and VEGF levels at 48 h. As shown in , the expression of IL-6, TNF-α, and VEGF reduced with the increase in concentration of metformin. The difference between the metformin and control groups was found to be statistically significant.
Inhibition of mTOR pathway contributed to metformin-induced HaCaT cell apoptosis and proliferation arrest
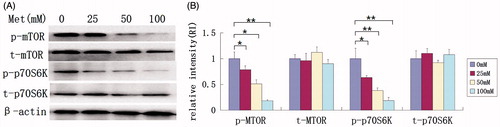
mTOR, a central protein in the mTOR signaling pathway, plays a pivotal role in regulating protein and nucleotide synthesis through its main effectors, p70S6K and eIF4E-binding protein 1 (4E-BP1). We further examined if the effect of metformin on HaCaT cells is through the inhibition of mTOR and p70S6K. Western blot shows that metformin significantly reduced the phosphorylation of mTOR and p70S6K expression at 50 mM (49.0% and 62.1%, respectively) with an amplified reduction at 100 mM with no discrepancy in total mTOR and p70S6K (). The results indicate that down-regulation of mTOR signaling may contribute to the inhibition of metformin-induced proliferation and apoptosis in HaCaT cells.
Figure 4. Analysis of mTOR signaling following metformin treatment. Total protein from cultured HaCaT cells treated with different concentrations of metformin (0, 25, 50, and 100 mM) for 48 h and subjected to western blot analysis. (A) Expressions of p-mTOR and p-p70S6K (but not t-mTOR and t-p70S6K) were significantly decreased following metformin treatment. (B) The relative expression level of each protein by normalization to internal β-actin control. *p < 0.05, **p < 0.001.
Discussion
Metformin is an insulin sensitizer, which is the first-line treatment method for type 2 diabetes. Retrospective epidemiological investigations and basic studies have shown that metformin has anti-tumor effects in humans and experimental animal models of colon, breast, prostate, and lung cancers (Pollak, Citation2010; Taubes, Citation2012). Interestingly, it has been previously reported that diabetics receiving metformin treatment have a lower occurrence of psoriasis than other insulin sensitizing agents (Brauchli et al., Citation2008; Wu et al., Citation2015). One case-control study on 36 702 patients showed that long-term use of metformin was associated with a reduced risk of psoriasis (Brauchli et al., Citation2008).
To study the mechanism of metformin in treating epidermal hyperplastic diseases such as psoriasis, we previously reported that metformin inhibited HaCaT cells proliferation in vitro by upregulating AMPK and Erk pathway (Li et al., Citation2014). In the current study, we found that metformin not only inhibited cell proliferation but also induced HaCaT cell apoptosis. Metformin has further been shown to inhibit the expression of IL-6, TNF-α, and VEGF proteins in HaCaT cells, indicating that metformin may attenuate inflammation in psoriasis patients. The current study reveals insight into the potential underlying antiproliferative and anti-inflammatory mechanisms of metformin, which may contribute to the observed clinical benefits in psoriasis patients.
The mTOR pathway has been identified as a key signaling pathway involved in the regulation of cellular growth, proliferation, lipid synthesis, and protein translation. In epidermal keratinocytes, the mTOR pathway has also been identified as a regulator of proliferation and angiogenesis, both of which contribute to the development of psoriasis (Karar & Maity, Citation2011; Mitra et al., Citation2012). In addition, the mTOR pathway has emerged as a regulator of the immune function. It is well known that mTOR is important in regulating adaptive immune activation and innate immune cell activation (Soliman, Citation2013; Weichhart & Säemann, Citation2008). On the other hand, mTOR seems to constrain full immune cell activation by upregulating the key anti-inflammatory cytokine IL-10 and inhibiting proinflammatory cytokines, including IL-6, TNF-α, and VEGF (Kirsch et al., Citation2012; Weichhart & Säemann, Citation2008; Zhang & Ma, Citation2014). Some of the regulatory actions of the mTOR pathway on the immune system are linked to the immunopathogenesis of psoriasis.
Buerger et al. found that mTOR and its downstream signaling molecule, the ribosomal S6 kinase, were upregulated in lesional psoriatic skin, suggesting a role of mTOR signaling in psoriatic epidermal proliferation (Buerger et al., Citation2013). mTOR inhibitors may offer a range of new therapeutic options for psoriasis patients. Clinical trials indicated that rapamycin, the prototypical mTOR inhibitor could significantly reduce the clinical score of psoriasis when treating psoriasis patients (Ormerod et al., Citation2005; Reitamo et al., Citation2001). Everolimus, a semisynthetic macrolide and a member of the mTOR inhibitors family, could lead to the resolution of recalcitrant psoriatic manifestations (Frigerio et al., Citation2007; Wei & Lai, Citation2015). Our results show that metformin significantly inhibited the phosphorylation of mTOR and its downstream gene p70S6K, indicating that metformin may also contribute to the inhibition of keratinocyte proliferation and inflammation. The inhibition of mTOR activation may provide an additional mechanism for metformin that mediates its apparently beneficial effects in psoriasis patients. However, whether the effects of metformin-induced cell apoptosis and inhibition of cell proliferation and proinflammatory cytokines is through inhibition of the mTOR pathway or more complex interactions among the AMPK, Erk, and mTOR pathways in keratinocytes requires further study.
Conclusions
In summary, clinical data indicated that metformin could alleviate the symptoms of hyperplasic epidermal diseases. We previously reported that metformin inhibited HaCaT cells proliferation through down-regulating the AMPK and Erk signaling pathways. The current study indicates that metformin could inhibit proinflammatory cytokines and induced apoptosis of HaCaT cells possibly through inhibition of mTOR signaling. Our studies provide new evidence for value in testing the clinical efficacy of metformin in treating human psoriasis.
Declaration of interest
This study was supported by the National Natural Science Foundation of China (Grant no. 81402622).
References
- Abdou AG, Hanout HM. (2008). Evaluation of survivin and NF-kappaB in psoriasis, an immunohistochemical study. J Cutan Pathol 35:445–51
- Ben Sahra I, Regazzetti C, Robert G, et al. (2011). Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res 71:4366–72
- Boukamp P, Petrussevska RT, Breitkreutz D, et al. (1988). Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 106:761–71
- Brauchli YB, Jick SS, Curtin F, et al. (2008). Association between use of thiazolidinediones or other oral antidiabetics and psoriasis: A population based case–control study. J Am Acad Dermatol 58:421–9
- Buerger C, Malisiewicz B, Eiser A, et al. (2013). mTOR and its downstream signalling components are activated in psoriatic skin. Br J Dermatol 169:156–9
- Choi YK, Park KG. (2013). Metabolic roles of AMPK and metformin in cancer cells. Mol Cells 36:279–87
- Frigerio E, Colombo MD, Franchi C. (2007). Severe psoriasis treated with a new macrolide: Everolimus. Br J Dermatol 156:372–4
- Huang T, Lin X, Meng X. (2014). Phosphoinositide-3 kinase/protein kinase-B/mammalian target of rapamycin pathway in psoriasis pathogenesis. A potential therapeutic target? Acta Derm Venereol 94:371–9.
- Kahn BB, Alquier T, Caning D, et al. (2005). AMP activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25
- Karar J, Maity A. (2011). PI3K/AKT/mTOR Pathway in angiogenesis. Front Mol Neurosci 2011;4:51. doi: 10.3389/fnmol.2011.00051
- Kirsch AH, Riegelbauer V, Tagwerker A, et al. (2012). The mTOR-inhibitor rapamycin mediates proteinuria in nephrotoxic serum nephritis by activating the innate immune response. Am J Physiol Renal Physiol 303:569–75
- Leone A, Di Gennaro E, Bruzzese F, et al. (2014). New perspective for an old antidiabetic drug: Metformin as anticancer agent. Cancer Treat Res 159:355–76
- Li W, Ma W, Zhong H, et al. (2014). Metformin inhibits proliferation of human keratinocytes through a mechanism associated with activation of the MAPK signaling pathway. Exp Ther Med 7:389–92
- Mitra A, Raychaudhuri SK, Raychaudhuri SP. (2012). IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine 60:38–42
- Nestle FO, Kaplan DH, Barker J. (2009). Psoriasis. N Engl J Med 361:496–509
- Ormerod AD, Shah SA, Copeland P, et al. (2005). Treatment of psoriasis with topical sirolimus: Preclinical development and a randomized, double-blind trial. Br J Dermatol 152:758–64
- Pollak M. (2010). Metformin and other biguanides in oncology: Advancing the research agenda. Cancer Prev Res (Phila) 3:1060–5
- Reitamo S, Spuls P, Sassolas B, et al. Sirolimu European Psoriasis Study Group. (2001) Efficacy of sirolimus (rapamycin) administered concomitantly with a subtherapeutic dose of cyclosporin in the treatment of severe psoriasis: A randomized controlled trial. Br J Dermatol 145:438–45
- Soliman GA. (2013). The role of mechanistic target of rapamycin (mTOR) complexes signaling in the immune responses. Nutrients 5:2231–57
- Stein M, Bernd A, Ramirez-Bosca A, et al. (1997). Measurement of anti-inflammatory effects of glucocorticoids on human keratinocytes in vitro. Comparison of normal human keratinocytes with the keratinocyte cell line HaCaT. Arzneimittelforschung 47:1266–70
- Storozhuk Y, Hopmans SN, Sanli T, et al. (2013). Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer 108:2021–32
- Taubes G. (2012). Cancer research. Cancer prevention with a diabetes pill? Science. 335:29
- Wang XW, Zhang YJ. (2014). Targeting mTOR network in colorectal cancer therapy. World J Gastroenterolb 20:4178–88
- Wei KC, Lai PC. (2015). Combination of everolimus and tacrolimus: A potentially effective regimen for recalcitrant psoriasis. Dermatol Ther 28:25–7
- Weichhart T, Säemann MD. (2008). The PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic applications. Ann Rheum Dis 67:iii70–4
- Wu CY, Shieh JJ, Shen JL, et al. (2015). Association between antidiabetic drugs and psoriasis risk in diabetic patients: Results from a nationwide nested case-control study in Taiwan. J Am Acad Dermatol 72:123–30
- Zhang J, Ma WY. (2014). Nerve growth factor regulates the expression of vascular endothelial growth factor in human HaCaT keratinocytes via PI3K/mTOR pathway. Genet Mol Res 13:9324–35