Abstract
A series of chromonyl-2,4-thiazolidinediones/imidazolidinediones/2-thioxo-imidazolidine-4-ones (IIIa–i, IVa–i) was prepared by Knoevenagel reaction of 2,4-thiazolidinedione/2,4-imidazolidinedione/2-thioxo-imidazolidine-4-one (IIa–c) with 2/3-formyl chromone (Ia–b) and then alkylation with methyl/ethyl iodide. The prepared compounds were tested for their insulinotropic activities in INS-1 cells. Compounds ıVb and ıVc (at lower concentration, 1 μg/mL) were able to increase insulin release in the presence of 5.6 mmol/L glucose.” should be written as “Compounds IVb and IVc (at lower concentration, 1 µg/mL) and also IIId and IIIg (at higher concentration) were able to increase insulin release in the presence of 5.6 mmol/L glucose. Compounds ıVb and ıVc (at lower concentration, 1 μg/mL) were able to increase insulin release in the presence of 5.6 mmol/L glucose.
Introduction
Diabetes mellitus (DM) is a common chronic metabolic disease characterized by high levels of glucose in the blood and associated with serious long-term complications, such as neuropathy, nephropathy, retinopathy, cataracts, accelerated atherosclerosis and increased risk of myocardial infarction, stroke, and amputationCitation1. 2,4-Thiazolidinediones (2,4-TZDs) are a new class of antidiabetic agents that increase insulin sensitivity, enhance glucose control, improve the lipid profile, suppress inflammatory cytokines, and improve vascular structure and functionCitation2. Chromones, occurring widely throughout the plant kingdom, are one of the most representative families of plant secondary metabolites and display a remarkable spectrum of biological activities such as anticancer, antimicrobial, and antidiabetic activitiesCitation3–6.
In our previous studies, we synthesized novel furochromonyl-TZDsCitation5 and chromonyl-TZDsCitation6 and tested for their antidiabetic activity. In this study, we describe further modifications of the 2,4-TZD derivatives containing the chromone ring. The synthesized compounds were tested for their insulinotropic activities in INS-1 cells.
Materials and methods
Chemical analysis
Melting points were measured on an electrothermal 9100 type apparatus (electrothermal Engineering Ltd., Essex, UK) and are uncorrected. All instrumental analyses were performed in the Central Laboratory of the Faculty of Pharmacy of Ankara University. Infrared (IR) spectra were recorded as potassium bromide disks with a Jasco FT/IR-420 spectrometer (Easton, MD, USA). 1H nuclear magnetic resonance (NMR) spectra were measured with a Varian Mercury 400 MHz FT-NMR spectrometer (Varian Inc., Palo Alto, CA, USA) in dimethylsulfoxide (DMSO)-d6 and CDCl3; all chemical shifts are reported as δ (ppm) values. Mass spectra were recorded on a Waters ZQ Micromass LC-MS spectrometer (Waters Corporation, Milford, MA, USA) using the electrospray ionization (ESI) (+) method. Elementary analyses were performed on a Leco 932 CHNS analyzer (Leco-932, St. Joseph, MI, USA), and satisfactory results within ±0.4% of calculated values (C, H, N) were obtained. For the chromatographic analysis, Merck silica gel 60 (230–400 mesh ASTM (American Society for Testing and Materials)) was used. The chemical reagents used in the synthesis were purchased from E. Merck (Darmstadt, Germany), Fluka (Buchs, Switzerland), and Aldrich (Milwaukee, MI, USA). 2,4-TZD (II)Citation7, o-acetoxy acetophenoneCitation8, α-acetyl-2-hydroxy acetophenoneCitation9, 2-methyl chromoneCitation10, and 2-formyl chromoneCitation11 were synthesized according to the literature.
Chemical synthesis
General synthesis of compounds IIIa–c, IVa–c
A mixture of 2/3-formyl chromone (Ia–b) (0.001 mol) and thiazolidine-2,4-dione/imidazolidine-2,4-dione/2-thioxo-imidazolidine-4-one (IIa–c) (0.001 mol) was refluxed in the presence of 1 mL glacial acetic acid and sodium acetate (0.001 mol). The crude product was crystallized from dimethylformamide (DMF).
5-(4-Oxo-4H-chromen-2-yl methylene)-thiazolidine-2,4-dione) (IIIa)
Yield: 57.36%, mp: 316°C, 1H NMR (DMSO-d6): δ = 6.93 (s, 1H, =CH), 7.53 (t, 1H, Jo = 7.60, 6-H), 7.64 (s, 1H, 3-H), 7.75 (d, 1H, J8,7 = 8.80 Hz, 8-H), 7.86 (t, 1H, Jo = 7.20, 7-H), 8.03 (d, 1H, J5,6 = 7.60 Hz, 5-H), 12.82 (s, 1H, NH), MS (ESI+) m/z (rel intensity): 274 (M + 1, 100), Anal. for C13H7NO4S·1.5H2O: Calc. C, 52.00; H, 3.33; N, 4.67; S, 10.67%. Found C, 52.03; H, 3.23; N, 5.09; S, 10.04%.
5-(4-Oxo-4H-chromen-2-yl methylene)-imidazolidine-2,4-dione) (IIIb)
Yield: 30.81%, mp: 333°C, 1H NMR (DMSO-d6): δ = 6.26 (s, 1H, =CH), 6.66 (s, 1H, 3-H), 7.47 (ψt, 1H, Jo = 7.20 and 7.60 Hz, 6-H), 7.81 (ψt, 1H, Jo = 8.00 and 7.60 Hz, 7-H), 7.99 (d, 1H, J8,7 = 7.60 Hz, 8-H), 8.24 (d, 1H, J5,6 = 8.80 Hz, 5-H), 10.99 (s, 1H, NH), 11.60 (s, 1H, NH), MS (ESI+) m/z (rel intensity): 257 (M + 1, 100), Anal. for C13H8N2O4·0.9H2O: Calc. C, 57.31; H, 3.60; N, 10.29%. Found C, 57.60; H, 3.51; N, 9.95%.
5-(4-Oxo-4H-chromen-2-yl methylene)-2-thioxo-imidazolidine-4-one) (IIIc)
Yield: 56.50%, mp: 339°C, 1H NMR (DMSO-d6): δ = 6.29 (s, 1H, =CH), 6.74 (s, 1H, 3-H), 7.45 (ψt, 1H, Jo = 7.20 and 7.60 Hz, 6-H), 7.80 (td, 1H, Jo = 6.80 and 8.80 Hz, Jm = 2.00 Hz, 7-H), 7.96 (dd, 1H, J8,7 = 7.60 Hz, J8,6 = 1.60 Hz, 8-H), 8.18 (d, 1H, J5,6 = 8.40 Hz, 5-H), 12.42 (s, 1H, NH), 12.69 (s, 1H, NH), MS (ESI+) m/z (rel intensity): 273 (M + 1, 100), Anal. for C13H8N203S·H2O: Calc. C, 53.79; H, 3.45; N, 9.66; S, 11.03%. Found C, 53.80; H, 3.45; N, 9.83; S, 11.21%.
5-(4-Oxo-4H-chromen-3-yl methylene)-thiazolidine-2,4-dione) (IVa) Yield: 87.19%, mp: 311°C (Ref. 12 mp: 271°C), 1H NMR (DMSO-d6): δ = 7.56 (ψt, 1H, Jo = 7.20 and 7.80 Hz, 6-H), 7.61 (s, 1H, =CH), 7.73 (d, 1H, J8,7 = 8.40 Hz, 8-H), 7.88 (td, 1H, Jo = 7.80 and 8.00 Hz, Jm = 1.60 Hz, 7-H), 8.13 (dd, 1H, J5,6 = 8.40 Hz, J5,7 = 1.60 Hz, 5-H), 8.85 (s, 1H, 2-H), 12.45 (s, 1H, NH), MS (ESI+) m/z (rel intensity): 274 (M + 1, 100), Anal. for C13H7NO4S: Calc. C, 57.14; H, 2.58; N, 5.13; S, 11.73%. Found C, 57.30; H, 2.08; N, 5.19; S, 11.61%.
5-(4-Oxo-4H-chromen-3-yl methylene)-imidazolidine-2,4-dione) (IVb)
Yield: 86.17%, mp: 363°C (Ref. 13 mp: 345–348°C), 1H NMR (DMSO-d6): δ = 6.35 (s, 1H, =CH), 7.55 (ψt, 1H, Jo = 7.20 and 7.80 Hz, 6-H), 7.74 (d, 1H, J8,7 = 7.60 Hz, 8-H), 7.87 (td, 1H, Jo = 7.60 and 8.00 Hz, Jm = 1.60 Hz, 7-H), 8.14 (dd, 1H, J5,6 = 8.00, J5,7 = 1.60 Hz, 5-H), 8.76 (s, 1H, 2-H), 10.40 (s, 1H, NH), 11.30 (s, 1H, NH), MS (ESI+) m/z (rel intensity): 257 (M + 1, 100), Anal. for C13H8N2O4·0.1H2O: Calc. C, 60.51; H, 3.18; N, 10.86%. Found C, 60.46; H, 3.11; N, 11.05%.
5-(4-Oxo-4H-chromen-3-yl methylene)-2-thioxo-imidazolidine-4-one) (IVc)
Yield: 91.78%, mp: 326°C (Ref. 14 mp: 292–295°C), 1H NMR (DMSO-d6): δ = 6.37 (s, 1H, =CH), 7.57 (ψt, 1H, Jo = 7.40 and 7.60 Hz, 6-H), 7.75 (d, 1H, J8,7 = 8.00 Hz, 8-H), 7.88 (td, 1H, Jo = 7.60 and 8.00 Hz, Jm = 1.60 Hz, 7-H), 8.17 (dd, 1H, J5,6 = 8.00 Hz, J5,7 = 1.60 Hz, 5-H), 8.92 (s, 1H, 2-H), 11.81 (s, 1H, NH), 12.43 (s, 1H, NH), MS (ESI+) m/z (rel intensity): 273 (M + 1, 100), Anal. for C13H8N203S: Calc. C, 57.35; H, 2.96; N, 10.29; S, 11.78%. Found C, 57.22; H, 2.93; N, 10.43; S, 11.90%.
General synthesis of compounds IIId, IIIf, IIIh, IVd, IVf, IVh
Compounds IIIa–c/IVa–c (0.0004 mmol) and anhydrous sodium carbonate (0.0004 mmol) were dissolved in 3 mL DMF. Methyl iodide (0.0008 mmol) was added to this mixture and stirred at 40°C. The reaction mixture was poured on to ice. The residue was filtered off. The filtrate was purified by column chromatography using silica gel 60 (230–400 mesh ASTM) as the adsorbent and petroleum ether:chloroform (1:1) as the eluent.
3-Methyl-5-(4-oxo-4H-chromen-2-yl methylene)-thiazolidine-2,4-dione) (IIId)
Yield: 41.85%, mp: 275°C, 1H NMR (DMSO-d6): δ = 3.12 (s, 3H, -CH3), 6.97 (s, 1H, =CH), 7.54 (ψt, 1H, Jo = 7.20 and 7.60 Hz, 6-H), 7.77 (s, 1H, 3-H), 7.78 (d, 1H, J8,7 = 8.40 Hz, 8-H), 7.87 (ψt, 1H, Jo = 8.40 and 8.80 Hz, 7-H), 8.04 (d, 1H, J5,6 = 8.00 Hz, 5-H), MS (ESI+) m/z (rel intensity): 288 (M + 1, 100), Anal. for C14H9NO4S: Calc. C, 58.53; H, 3.16; N, 4.88; S, 11.16%. Found C, 58.59; H, 3.13; N, 4.92; S, 10.85%.
1,3-Dimethyl-5-(4-oxo-4H-chromen-2-yl methylene)-imidazolidine-2,4-dione) (IIIf)
Yield: 16.23%, mp: 208°C, 1H NMR (CDCl3): δ = 3.19 (s, 3H, -CH3), 3.56 (s, 3H, -CH3), 6.42 (s, 1H, =CH), 6.45 (s, 1H, 3-H), 7.45 (m, 2H, 6-H and 8-H), 7.72 (td, 1H, Jo = 6.80 and 8.80 Hz, Jm = 1.60 Hz, 7-H), 8.22 (dd, 1H, J5,6 = 8.00 Hz, J5,7 = 1.20 Hz, 5-H), MS (ESI+) m/z (rel intensity): 285 (M + 1, 100), Anal. for C15H12N2O4: Calc. C, 63.38; H, 4.25; N, 9.85%. Found C, 63.06; H, 4.06; N, 9.67%.
3-Methyl-2-(methylthio)-5-(4-Oxo-4H-chromen-2-yl methylene)-imidazolidine-4-one) (IIIh)
Yield: 72.53%, mp: 292°C, 1H NMR (CDCl3): δ = 2.80 (s, 3H, -CH3), 3.19 (s, 3H, -CH3), 6.62 (s, 1H, =CH), 7.39 (ψt, 1H, Jo = 7.20 and 8.00 Hz, 6-H), 7.47 (d, 1H, J8,7 = 8.00 Hz, 8-H), 7.62 (s, 1H, 3-H), 7.67 (td, 1H, Jo = 7.20 and 7.60 Hz, Jm = 1.60 Hz, 7-H), 8.20 (dd, 1H, J5,6 = 8.40 Hz, J5,7 = 1.60 Hz, 5-H), MS (ESI+) m/z (rel intensity): 301 (M + 1, 100), Anal. for C15H12N203S: Calc. C, 59.99; H, 4.03; N, 9.33; S, 10.68%. Found C, 60.30; H, 4.22; N, 9.16; S, 10.35%.
3-Methyl-5-(4-oxo-4H-chromen-3-yl methylene)-thiazolidine-2,4-dione) (IVd)
Yield: 78.95%, mp: 249°C, 1H NMR (DMSO-d6): δ = 3.09 (s, 3H, -CH3), 7.58 (ψt, 1H, Jo = 7.20 and 8.00 Hz, 6-H), 7.72 (s, 1H, =CH), 7.75 (d, 1H, J8,7 = 8.80 Hz, 8-H), 7.89 (ψt, 1H, Jo = 7.60 and 8.00 Hz, 7-H), 8.14 (dd, 1H, J5,6 = 7.60 Hz, J5,7 = 1.60 Hz, 5-H), 8.93 (s, 1H, 2-H), MS (ESI+) m/z (rel intensity): 288 (M + 1, 25), 310 (100), Anal. for C14H9NO4S: Calc. C, 58.53; H, 3.16; N, 4.88; S, 11.16%. Found C, 58.63; H, 3.39; N, 4.87; S, 11.06%.
1,3-Dimethyl-5-(4-oxo-4H-chromen-3-yl methylene)-imidazolidine-2,4-dione) (IVf)
Yield: 49.58%, mp: 162°C, 1H NMR (DMSO-d6): δ = 2.96 (s, 3H, -CH3), 2.99 (s, 3H, -CH3), 6.35 (s, 1H, =CH), 7.51 (ψt, 1H, Jo = 7.20 and 7.40 Hz, 6-H), 7.68 (d, 1H, J8,7 = 8.00 Hz, 8-H), 7.82 (td, 1H, Jo = 7.80 and 7.80 Hz, Jm = 1.20 Hz, 7-H), 8.08 (dd, 1H, J5,6 = 8.40 Hz, J5,7 = 1.20 Hz, 5-H), 8.56 (s, 1H, 2-H), MS (ESI+) m/z (rel intensity): 285 (M + 1, 35), 307 (100), Anal. for C15H12N2O4: Calc. C, 63.38; H, 4.25; N, 9.85%. Found C, 63.24; H, 4.56; N, 9.75%.
3-Methyl-2-(methylthio)-5-(4-oxo-4H-chromen-3-yl methylene)-imidazolidine-4-one) (IVh)
Yield: 58.48%, mp: 277°C, 1H NMR (DMSO-d6): δ = 2.74 (s, 3H, -CH3), 3.09 (s, 3H, -CH3), 7.01 (s, 1H, =CH), 7.56 (ψt, 1H, Jo = 6.80 and 8.00 Hz, 6-H), 7.73 (d, 1H, J8,7 = 8.40 Hz, 8-H), 7.87 (ψt, 1H, Jo = 7.20 and 7.60 Hz, 7-H), 8.15 (d, 1H, J5,6 = 7.60 Hz, 5-H), 9.69 (s, 1H, 2-H), MS (ESI+) m/z (rel intensity): 301 (M + 1, 35), 323 (100), Anal. for C15H12N203S: Calc. C, 59.99; H, 4.03; N, 9.33; S, 10.68%. Found C, 60.01; H, 4.19; N, 9.15; S, 10.49%.
General synthesis of compounds IIIe, IIIg, IIIi, IVe, IVg, IVi
Compounds IIIa–c/IVa–c (0.0008 mmol) and anhydrous sodium carbonate (0.0016 mmol) were dissolved in 5 mL DMF. Ethyl iodide (0.0032 mmol) was added to this mixture and stirred at 40°C. The reaction mixture was poured on to ice. The residue was filtered off. The filtrate was purified by column chromatography using silica gel 60 (230–400 mesh ASTM) as the adsorbent and petroleum ether:chloroform (1:1) as the eluent.
3-Ethyl-5-(4-oxo-4H-chromen-2-yl methylene)-thiazolidine-2,4-dione) (IIIe)
Yield: 49.88%, mp: 268°C, 1H NMR (DMSO-d6): δ = 1.18 (ψt, 3H, J = 6.80 and 7.20 Hz, -CH3), 3.70 (q, 2H, J = 7.40 Hz and 7.60 Hz, -CH2-), 6.97 (s, 1H, =CH) 7.54 (ψt, 1H, Jo = 7.20 and 7.60 Hz, 6-H), 7.78 (m, 2H, 3-H and 8-H), 7.87 (ψt, 1H, Jo = 7.40 and 8.20 Hz, 7-H), 8.04 (d, 1H, J5,6 = 8.00 Hz, 5-H), MS (ESI+) m/z (rel intensity): 302 (M + 1, 100), Anal. for C15H11NO4S: Calc. C, 59.79; H, 3.68; N, 4.65; S, 10.64%. Found C, 59.93; H, 3.66; N, 4.72; S, 10.16%.
3-Ethyl-5-(4-oxo-4H-chromen-2-yl methylene)-imidazolidine-2,4-dione) (IIIg)
Yield: 18.03%, mp: 115°C, 1H NMR (CDCl3): δ = 1.26-1.31 (6H, m, -CH3), 3.72 (q, 2H, J = 7.20 and 7.60 Hz, -CH2-), 4.19 (q, 2H, J = 7.20 and 7.60 Hz, -CH2-), 6.40 (s, 1H, =CH), 6.47 (s, 1H, 3-H), 7.45 (m, 1H, 6-H and 8-H), 7.72 (ψt, 1H, Jo = 7.20 and 8.00 Hz, 7-H), 8.23 (d, 1H, J5,6 = 7.20 Hz, 5-H), MS (ESI+) m/z (rel intensity): 313 (M + 1, 100), Anal. for C17H16N2O4: Calc. C, 65.38; H, 5.13; N, 8.97%. Found C, 65.60; H, 5.15; N, 8.55%.
3-Ethyl-2-(ethylthio)-5-(4-oxo-4H-chromen-3-yl methylene)-imidazolidine-4-one) (IIIi)
Yield: 71.87%, mp: 160°C, 1H NMR (DMSO-d6): δ = 1.18 (ψt, 3H, J = 6.80 and 7.20 Hz, -CH3), 1.50 (ψt, 3H, J = 7.20 and 7.60 Hz, -CH3), 3.43 (q, 2H, J = 6.80 and 7.60 Hz, -CH2-), 3.59 (q, 2H, J = 6.80 and 7.60 Hz, -CH2-), 6.49 (s, 1H, =CH), 7.36 (s, 1H, 3-H), 7.50 (ψt, 1H, Jo = 7.20 and 7.60 Hz, 6-H), 7.62 (d, 1H, J8,7 = 8.40 Hz, 8-H), 7.83 (t, 1H, Jo = 7.80, 7-H), 8.03 (d, 1H, J5,6 = 7.60 Hz, 5-H), MS (ESI+) m/z (rel intensity): 329 (M + 1, 100), Anal. for C17H16N2O3S. 0.1H2O: Calc. C, 61.86; H, 4.91; N, 8.49; S, 9.70%. Found C, 61.62; H, 5.06; N, 8.48; S, 9.56%.
3-Ethyl-5-(4-oxo-4H-chromen-3-yl methylene)-thiazolidine-2,4-dione) (IVe)
Yield: 77.10%, mp: 233°C, 1H NMR (DMSO-d6): δ = 1.12 (t, 3H, J = 7.20 Hz, -CH3), 3.63 (q, 2H, J = 7.20 Hz, -CH2-), 7.55 (td, 1H, Jo = 7.60 and 8.00 Hz, Jm = 1.20 Hz, 6-H), 7.69 (s, 1H, =CH), 7.72 (d, 1H, J8,7 = 8.40 Hz, 8-H), 7.86 (td, 1H, Jo = 7.80 and 8.00 Hz, Jm = 1.60 Hz, 7-H), 8.11 (dd, 1H, J5,6 = 8.40 Hz, J5,7 = 1.60 Hz, 5-H), 8.90 (s, 1H, 2-H), MS (ESI+) m/z (rel intensity): 302 (M + 1, 65), 86 (100), Anal. for C15H11NO4S: Calc. C, 59.79; H, 3.68; N, 4.65; S, 10.64%. Found C, 59.83; H, 3.38; N, 4.81; S, 10.80%.
3-Ethyl-5-(4-oxo-4H-chromen-3-yl methylene)-imidazolidine-2,4-dione) (IVg)
Yield: 49.58%, mp: 223°C, 1H NMR (CDCl3): δ = 1.11 (ψt, 3H, J = 6.80 and 7.60 Hz, -CH3), 3.48 (q, 2H, J = 7.20 Hz, -CH2-), 6.42 (s, 1H, =CH), 7.52 (td, 1H, Jo = 7.20 and 7.80 Hz, Jm = 1.20 Hz, 6-H), 7.70 (d, 1H, J8,7 = 8.80 Hz, 8-H), 7.84 (td, 1H, Jo = 7.60 and 8.00 Hz, Jm = 1.60 Hz, 7-H), 8.11 (dd, 1H, J5,6 = 8.40 Hz, J5,7 = 1.60 Hz, 5-H), 8.74 (s, 1H, 2-H), 10.53 (s, 1H, NH), MS (ESI+) m/z (rel intensity): 285 (M + 1, 65), 307 (100), Anal. for C15H12N2O4: Calc. C, 63.38; H, 4.25; N, 9.85%. Found C, 63.42; H, 4.48; N, 9.79%.
3-Ethyl-2-(ethylthio)-5-(4-oxo-4H-chromen-3-yl methylene)-imidazolidine-4-one) (IVi)
Yield: 70.49%, mp: 195°C, 1H NMR (DMSO-d6): δ = 1.13 (t, 3H, J = 7.20 Hz, -CH3), 1.42 (ψt, 3H, J = 6.80 and 7.60 Hz, -CH3), 3.36 (q, 2H, J = 7.20 and 7.60 Hz, -CH2-), 3.53 (q, 2H, J = 7.20 Hz, -CH2-), 6.97 (s, 1H, =CH), 7.52 (t, 1H, Jo = 8.00 Hz, 6-H), 7.69 (d, 1H, J8,7 = 8.80 Hz, 8-H), 7.84 (td, 1H, Jo = 7.20 Hz, Jm=1.20 Hz, 7-H), 8.12 (dd, 1H, J5,6 = 8.00 Hz, J5,7 = 1.60 Hz, 5-H), 9.61 (s, 1H, 2-H), MS (ESI+) m/z (rel intensity): 329 (M + 1, 90), 351 (100), Anal. for C17H16N2O3S: Calc. C, 62.18; H, 4.91; N, 8.53; S, 9.76%. Found C, 62.16; H, 4.95; N, 8.35; S, 9.40%.
Insulin releasing activity studies
Cell culture of INS-1 cells
INS-1 cells, generously provided by Dr. C. Wollheim, Geneva, SwitzerlandCitation15, were grown in plastic culture bottles or microwells for 4–6 days (half confluence: 1–2 × 106 cells per mL) in RPMI medium supplemented with 10% (v/v) fetal calf serum, 100 U of penicillin per mL, and 0.1 mg of streptomycin per mL. Cells were seeded at a density of 5 × 105 cells/mL. The medium was changed every 5 days, and the cells were detached from the culture flask with trypsin 1 week after seeding, centrifuged, and reseeded as described above. Prior to the experiment cells were washed twice and then incubated in Krebs–Ringer buffer containing 10 mM HEPES and 0.5 % bovine serum albumin (KRBH).
Insulin release
To measure insulin secretion, half-confluent cells in microwells were incubated for 90 min at 37°C in the aforementioned KRBH buffer. Insulin released into the medium was assayed with a radioimmunoassay using rat insulin (Novo Nordisk, Bagsvaerd, Denmark) as a standard, (mono 125I–Tyr ACitation14)-insulin as the labeled compound (Sanofi-Aventis, Germany), and anti-insulin antibodies from Linco (St. Louis, MO, USA). Each compound had been checked for non-interference with the insulin radioimmunoassay. The data were corrected for the effects of solubilizing compounds (ethanol, DMSO).
Results and discussion
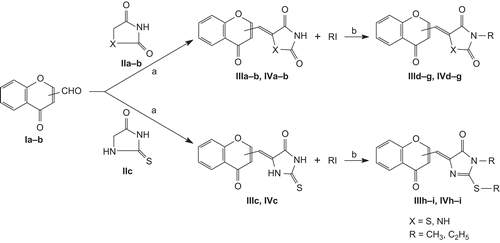
The most common method involves the Baker–Venkataraman rearrangement wherein an o-hydroxyacetophenone is acetylatedCitation8 and the ester is treated with base (pyridine/KOH) to effect an acyl group migration, forming a 1,3-diketoneCitation9. The ensuing diketone is then cyclized under strongly acidic conditions using sulfuric acid to furnish 2-methyl chromoneCitation10. 2,4-TZD (II) was synthesized with ClCH2COOH and thiourea in hot waterCitation7. Compounds IIIa–c, IVa–c were prepared by Knoevenagel reaction of 2,4-thiazolidinedione/2,4-imidazolidinedione/2-thioxo-imidazolidine-4-one (IIa–c) with 2/3-formyl chromone (Ia–b). Compounds IIId–i, IVd–i were synthesized by alkylation of compounds IIIa–c, IVa–c with methyl/ethyl iodide in the presence of anhydrous sodium carbonate/DMF ().
The structure of the synthesized chromonyl compounds was elucidated by IR, 1H NMR, mass spectral data, and elementary analysis findings. All spectral data were in accordance with assumed structures. In 1H NMR spectra, chromone protons were observed between 6.45 and 9.69 ppm; methylene protons for chromonyl-2,4-TZDs, chromonyl-2,4-imidiazolidinediones, and chromonyl-2-thioxo-imidazolidine-4-ones were seen at 6.93–7.72 ppm, 6.26–6.42 ppm, and 6.29–7.01 ppm as a singlet, respectively. In mass spectra, the compounds had an M + H ion peak. In the literature it was reported that in reactions using unsubstituted imidazolidinediones and benzaldehydes or 3-substituted thiazolidinedione and 3-formyl chromone in an acidic medium, the main product was the Z isomerCitation16,Citation17. The coupled 13C NMR study of arylidene thiazolidinediones and imidazolidinediones also showed that only the Z isomer was formedCitation18,Citation19. In this study, only one isomer of the compounds was obtained. After the appropriate crystals of compounds IVd, IVg, IVi were obtained, X-ray analysis was performed and the compounds IVd (supplementary material reference number: FL2008), IVg (supplementary material reference number: at2183), and IVi (CCDC deposition number: CCDC-604172) were found to be Z isomersCitation20–22.
Derivatives of chromone were synthesized and their biological activity with respect to insulin release was tested. All the biological results of the tested compounds are given in and . According to their insulinotropic activities in INS-1 cells, compounds IIIg, IVh, IVi (at higher concentration; 10 µg/mL) were able to increase insulin release in the presence of 5.6 mmol/L glucose, but to a lower extent than the reference compound glibenclamide. Compounds IVb, IVc (at lower concentration; 1 µg/mL) showed more potent insulinotropic effect than glibenclamide (1 µg/mL). The effects of compounds IVb and IVc were more pronounced at the lower than at the higher concentrations, which is well known from other insulinotropic compounds. But compounds IIIg, IVh, and IVi had an insulinotropic effect at higher concentrations. Compared to compounds derived from 2-formyl chromone, compounds derived from 3-formyl chromone showed a better insulinotropic effect. Introducing methyl or ethyl groups to 2,4-thiazolidinedione, 2,4-imidazolidinedione, and 2-thioxo-imidazolidine-4-one rings of the synthesized compounds did not improve the insulinotropic effect, except for IIIg.
Table 1. Effects of chromon-2-yl-2,4-thiazolidinediones/imidazoli-dinediones/2-thioxo-imidazolidine-4-ones IIIa–i on glucose-mediated insulin release from INS-1 cells.
Table 2. Effects of chromon-3-yl-2,4-thiazolidinediones/imidazoli-dinediones/2-thioxo-imidazolidine-4-ones IVa–i on glucose-mediated insulin release from INS-1 cells.
It was shown that introducing a 4-oxo-4H-chromen-3-yl methylene group at the fifth position of 2,4-imidazolidinedione and 2-thioxo-imidazolidine-4-one rings increased the insulinotropic effect. Also, it should be pointed out that compounds IVb and IVc are promising molecules, and this kind of structure could be a guide for further investigations on insulinotropic activity studies in INS-1 cells. The effect of these synthesized compounds structurally related to thiazolidinediones may also improve insulin sensitivity, which was not experimentally proven in our experiments.
Acknowledgements
We thank Sanofi-Aventis company for supplying radiolabeled insulin.
Declaration of interest
This work was supported by TUBITAK (No: SBAG-AYD-436).
References
- Bradley C. The glitazones: a new treatment for type 2 diabetes mellitus. Intens Crit Care Nurs 2002;18:189–91.
- Gotoda S, Takahashi N, Nakagawa H, Murakami M, Takechi T, Komura T, et al. Synthesis and fungicidal activity against Pyricularia oryzae of 6-(1,2,4-triazol-4-yl)chromone and -1-thiochromone derivatives. Pestic Sci 1998;52:309–20.
- Wang C-C, Chen L-G, Yang L-L. Inducible nitric oxide synthase inhibitor of the Chinese herb I. Saposhnikovia divaricata (Turcz.) Schischk. Cancer Lett 1999;145:151–7.
- Yu D, Brossi A, Kilgore N, Wild C, Allaway G, Lee K-H.Anti-HIV agents. Part 55: 3′R 4′R-Di-(O)-(−)-camphanoyl-2′,2′-dimethyldihydropyrano[2,3-f]chromone (DCP), a novel anti-HIV agent. Bioorg Med Chem Lett 2003;13:1575–6.
- Bozdağ-Dündar O, Verspohl EJ, Waheed A, Ertan R. Synthesis and antidiabetic activity of some new furochromonyl-2,4-thiazolidinediones. Arzneimittelforschung 2003;53:831–6.
- Bozdağ-Dündar O, Ceylan-Ünlüsoy M, Verspohl EJ, Ertan R. Synthesis and antidiabetic activity of some new chromonyl-2,4-thiazolidinediones. Arzneimittelforschung 2007;57:532–6.
- DeLima MCA, Costa DLB, Goes AJS, Galdino SL, Pitta IR, Luu-Duc C. Synthèse et activité antimicrobienne de dérivès chlorobenzyl benzylidène imidazolidinediones et thiazolidinediones substituées. Pharmazie 1992;47:182–4.
- Friedlaender P, Neudörfer J. Ueber das ketocumaran und einige condensationsproducte desselben. Ber Dtsch Chem Ges 1897;30:1077–83.
- Dunne ATM, Gowan JE, Keane J, O’Kelly BM, O’Sullivan D, Roche MM, et al. Thermal cyclization of o-aroyloxyacetoarones. A new synthesis of flavones. J Chem Soc 1950:1252–9.
- Ferrali M, Donati D, Bambagioni S, Fontani M, Giorgi G, Pietrangelo A. 3-Hydroxy-(4H)-benzopyran-4-ones as potential iron chelating agents in vivo. Bioorg Med Chem 2001;9:3041–7.
- Ito K, Nakajima K. Selenium dioxide oxidation of alkylcoumarins and related methyl-substituted heteroaromatics [1]. J Heterocycl Chem 1988;25:511–15.
- Bozdağ-Dündar O, Evranos B, Daş-Evcimen N, Sarıkaya M, Ertan R. Synthesis and aldose reductase inhibitory activity of some new chromonyl-2,4-thiazolidinediones. Eur J Med Chem 2008;43:2412–17.
- Aly YL. 5-Chromonylidene-hydantoins, 2-thiohydantoins, synthesis and reaction with some alkylhalides, some amines and some diazoalkanes. Phosphorus Sulfur Silicon Relat Elem 2005;180:1–18.
- Lecova M, Gasparova R, Loos D, Liptay T, Pronayova N. Effects of microwave irradiation on the condensation of 6-substituted 3-formylchromones with some five-membered heterocyclic compounds. Molecules 2000;5:167–78.
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheinm CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 1992;130:167–78.
- Tan S-F, Ang K-P, Fong Y-F. (Z)-and (E)-5-Arylmethylenehydantoins: spectroscopic properties and configuration assignement. J Chem Soc Perkin Trans 2 1986;12:1941–4.
- Özgen Ö,Ceylan-Ünlüsoy, M,Bozdağ-Dündar O, Ertan R, Kendi E. 3-(4-Chloro-benzyl)-5-(4-oxo-4H-chromen-3-yl-methylene)-thiazolidine-2,4-dione. Acta Crystallogr 2007;E61:2355–6.
- Vögeli U, Von Philipsborn W, Nagarajan K, Nair MD. Carbon-13 NMR spectroscopy, part 19. Structures of addition products of acetylenedicarboxylic acid esters with various dinucleophiles. An application of C, H-spin-coupling constants. Helv Chim Acta 1978;61:607–17.
- Albuquerque JFC, Albuquerque A, Azevedo CC, Thomasson F, Galdino LS, Chantegrel J, et al. Substituted thiazolidinediones and thioxoimidazolidinones: synthesis, structural study and pharmacological activity. Pharmazie 1995;50:387–9.
- Aslantaş M, Ceylan-Ünlüsoy M, Ertan R, Kendi E, Büyükgüngör O. 3-Methyl-5-(4-oxo-4H-chromen-3-yl-methylene)-1,3-thiazolidine-2,4-dione. Acta Crystallogr 2006;E62:o1471–3.
- Aslantaş M, Ceylan-Ünlüsoy M, Ertan R, Kendi E, Büyükgüngör O. 3-Ethyl-5-(4-oxochroman-3-ylmethylene)-1,3-imidazolidine-2,4-dione. Acta Crystallogr 2007;E63:O184–5.
- Aslantaş M, Ceylan-Ünlüsoy M, Ertan R, Kendi E, Büyükgüngör O. 3-Ethyl-2-(ethylthio)-5-(4-oxo-4H-chromen-3-yl-methylene)imidazolidine-4-one. Anal Sci 2007;23:x113–14.