Abstract
The inhibitory effects of hinokitiol, a constituent of the woody oils isolated from Cupressaceae heartwood, on mushroom tyrosinase and melanin formation in B16 melanoma cells as well as its antimicrobial activity were investigated. Our results showed that hinokitiol could strongly inhibit both monophenolase activity and diphenolase activity of the enzyme and the inhibition was reversible. The IC50 values were estimated as 9.67 μM for monophenolase activity and 0.21 μM for diphenolase activity. The lag time of the monophenolase activity was not obviously lengthened by the compound. Kinetic analyses showed that the inhibition mechanism of hinokitiol was a mixed-type inhibition of the diphenolase activity. Hinokitiol effectively inhibited both cellular tyrosinase activity and melanin biosynthesis in B16 melanoma cells with significant cytotoxicity. Furthermore, it was found that hinokitiol could inhibit the proliferation of Salmonella enteritidis, Escherichia coli, Bacillus subtilis, Staphyloccocus aureus, Klebsiella pneumoniae, and Ralstonia solanacearum to different extents. This research may widen the use of hinokitiol in the fields of food preservation, depigmentation, and insecticide use.
| Abbreviations: | ||
| DMSO, | = | dimethylsulfoxide; |
| l-DOPA, | = | l-3,4-dihydroxyphenylalanine; |
| l-Tyr, | = | l-tyrosine; |
| MTT, | = | 3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium bromide; |
| IC50, | = | inhibitor concentrations leading to 50% activity lost; |
| MIC, | = | minimum inhibitory concentration; |
| MBC, | = | minimum bactericidal concentration; |
| Kmapp, | = | apparent Michaelis–Menten constant; |
| Vmapp, | = | apparent maximum velocity; |
| KI, | = | equilibrium constant of inhibitor combining with free enzyme; |
| KIS, | = | equilibrium constant of inhibitor combining with enzyme–substrate complex. |
Introduction
Tyrosinase (EC 1.14.18.1), which is also referred to as polyphenol oxidase (PPO), is a copper-containing monooxygenase that is considered to be a key enzyme in melanin synthesisCitation1,Citation2. The enzyme catalyzes two distinct reactions involving molecular oxygen: the hydroxylation of monophenols to o-diphenols (monophenolase activity) and the oxidation of the o-diphenols to o-quinones (diphenolase activity). Quinones are highly reactive compounds and can polymerize spontaneously to form high molecular-weight compounds or brown pigments (melanins), or react with amino acids and proteins that enhance the brown color producedCitation3.
In the food industry, tyrosinase is a very important enzyme in controlling the quality and economics of fruit and vegetable storage and processing, including fruit pulp manufacturingCitation4,Citation5. Tyrosinase catalyzes the oxidation of phenolic compounds to the corresponding quinone, and is responsible for the enzymatic browning of fruits and vegetablesCitation5. Tyrosinase is responsible for not only browning in plants, but also melanization in animals. Melanin is essential for protecting human skin against radiation, but the accumulation of abnormal melanin induces pigmentation disorders, such as melasma, freckles, and senile lentiginesCitation6. Melanogenesis is conducted in the melanocytes, located in the basal layer of the epidermis, and controlled by tyrosinase. Thus, in cosmetic, medicinal, and food products, tyrosinase inhibitors have become increasingly important to prevent hyperpigmentation and enzymatic browningCitation7. Hence, tyrosinase inhibitors should have broad applications.
Hinokitiol (4-isopropyl tropolone, β-thujaplicin) is a constituent of the woody oils isolated from Cupressaceae heartwood, and is known to have antifungal and antibacterial activitiesCitation8. In melanocytes, hinokitiol was found to reduce melanin content, and has been reported to be a potent tyrosinase inhibitorCitation9,Citation10. However, its inhibitory mechanisms have not been clearly studied. The aim of this present experiment was, therefore, to carry out a kinetic study of the inhibition of the monophenolase and o-diphenolase activity of tyrosinase by hinokitiol, with an evaluation of the kinetic parameters and constants characterizing the system. In addition, its inhibitory effect on melanin formation in B16 melanoma cells and its antimicrobial activity were also studied in order to present a potential use of hinokitiol as an antibrowning and antimicrobial cosmetic and food additive.
Materials and methods
Reagents
Tyrosinase (EC 1.14.18.1) from mushrooms was the product of Sigma Chemical Co. (St. Louis, MO, USA). The specific activity of the enzyme is 6680 U/mg. l-Tyrosine (l-Tyr) and l-3,4-dihydroxyphenylalanine (l-DOPA) were obtained from Aldrich (St. Louis, MO), 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA), 10,000 U/mL penicillin G, 10,000 μg/mL streptomycin, and RPMI 1640 medium from Gibco (Grand Island, NY), and Triton X-10, 3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and dimethylsulfoxide (DMSO) from Amresco (Dallas, TX). Hinokitiol was acquired from Shanghai Kaikule Medicine Science Ltd. Salmonella enteritidis, Escherichia coli, Bacillus subtilis, Staphyloccocus aureus, Klebsiella pneumoniae, and Ralstonia solanacearum were collected from the colony preserved at −80°C in the Fujian Academy of Agricultural Sciences. All other reagents were local and of analytical grade. The water used was redistilled and ion-free.
Enzyme assay
The enzymatic activity assay was performed as reported by Chen et al.Citation11. We used l-Tyr as the substrate for the monophenolase activity assay, and l-DOPA as the substrate for the diphenolase activity assay. The reaction medium (3 mL) for the activity assay contained 2.0 mM l-Tyr or 0.5 mM l-DOPA in 50 mM Na2HPO4–NaH2PO4 buffer (pH 6.8). The final concentration of mushroom tyrosinase was 33.3 μg/mL for monophenolase activity and 6.67 μg/mL for diphenolase activity. The reaction was carried out at a constant temperature of 30°C. Hinokitiol was first dissolved in DMSO and then added to the reaction medium. The final concentration of DMSO in the test solution was 3.3%. Thus, 3.3% DMSO without inhibitor was used as control. All the above measurements were performed on a Beckman UV-650 spectrophotometer. The extent of inhibition by addition of the sample was expressed as the percentage necessary for 50% inhibition (IC50). The inhibition type was assayed by Lineweaver–Burk plot, and the inhibition constant was determined by the second plot of apparent Km/Vm or 1/Vm vs. concentration of the inhibitor.
Cell culture
B16 melanoma cells were acquired from the Institute of Biochemistry and Cell Biology, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). B16 cells were maintained in RPMI 1640 medium (Gibco) containing 10% heat-inactivated fetal bovine serum (FBS), 100 U/mg penicillin G, and 100 μg/mL streptomycin, and cultured at 37°C in a humidified atmosphere with 5% CO2. Afterward, the cytotoxicity levels of the compound on melanoma B16 cells were assessed via the MTT method, and its effects on the tyrosinase activity and melanin content assays were determined.
Cell viability
Cell viability was quantified by a colorimetric MTT assay that measures mitochondrial activity in living cellsCitation12. Cells were cultured to 90–95% confluent monolayers and collected by trypsinization with 0.25% trypsin-EDTA. The cells were cultured in flat-bottomed 96-well plates (1 × 104 cells/well) overnight, and then treated with various concentrations of hinokitiol for another 72 h. Cells were rewashed with phosphate buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) twice, and then 10 μL of MTT solution (0.5 mg/mL) and 90 μL fresh RPMI 1640 medium were added and the mixture incubated for 4 h. Finally, the culture supernatant was discarded and 200 μL DMSO was added to solubilize the formed formazan salt. The amount of formazan salt was quantified by measuring the absorbance at 570 nm using a microplate reader (Orange Scientific, Belgium). Relative cell viability was determined by the quantity of MTT converted into formazan salt. The viability of the cells was quantified as a percentage compared to the control: [(absorbance of treated cells – absorbance of blank)/(absorbance of control – absorbance of blank) × 100%].
Assay of cellular tyrosinase activity
Tyrosinase activity in B16 cells was examined by measuring the rate of oxidation of l-DOPACitation12. After incubation in the presence of hinokitiol for 72 h, B16 cells were washed with PBS twice to remove non-adherent dead cells. Ninety microliters of PBS containing 1% Triton X-100 and 10 μL l-DOPA (1.0 mg/mL) were added to each well of the 96-well plate and sonicated for 30 s. The samples were inoculated at 30°C for 30 min, and then measured at 475 nm. Relative remaining tyrosinase activity was calculated by the equation as follows: tyrosinase activity (OD475)/cell viability (OD570) × 100%.
Determination of melanin biosynthesis in B16 melanoma cells
Melanin content was used as an index of melanin biosynthesis in the present study. To measure the cellular melanin contents, the experiment was conducted in accordance with the procedure described by Huang et al.Citation13. The B16 cells were seeded at a density of 2 × 104 cells per well in 9 cm culture plates and then incubated for 24 h. The cells were treated with various concentrations of hinokitiol (1.25–10 μM) for 72 h. The cells were washed twice in PBS and dissolved in 1N NaOH (in 10% DMSO) by 2 h of boiling (80°C). The lysates were centrifuged for 10 min at 1000g, and then the absorbance value of the supernatant was measured at 405 nm. The relative melanin content of the cells was estimated as: melanin content (OD405)/cell viability (OD570) × 100%.
Antimicrobial assay
The antimicrobial assay was carried out in tryptone beef extract agar, at pH 7.2, with an inoculum of 1–2 × 105 cells/mL. The antimicrobial activity of hinokitiol was determined using the agar well diffusion method following the published procedure with slight modificationCitation14,Citation15. Briefly, culture medium was inoculated with the given microorganism by spreading the bacterial inoculum in the medium. Wells (7 mm diameter) were punched in the agar and filled with hinokitiol at different concentrations. Control wells, containing neat DMSO (negative control) and standard antibiotic streptomycin sulfate (1000 U/mL) for the tested bacteria, were also run parallel in the same plate. Bacterials were incubated at 37°C for 24 h. The antimicrobial activity was assessed by measuring the diameter of the zone of inhibition for the respective drug. The minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) were tested by broth macrodilution methods according to Kubo et al.Citation14.
Statistical analysis
All data are expressed as the mean (standard deviation (SD)) value of three independent experiments.
Results
Inhibitory effects of hinokitiol on monophenolase activity of mushroom tyrosinase
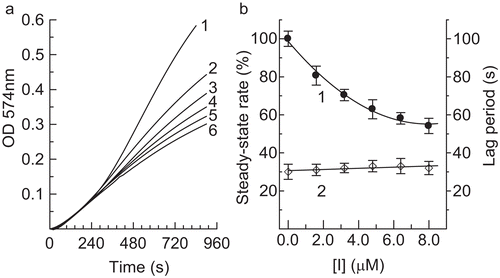
Tyrosinase can catalyze the hydroxylation of monophenols (monophenolase activity) and the oxidation of o-diphenols to o-quinones (diphenolase activity), both depending on molecular oxygenCitation15. The o-quinones evolve non-enzymatically to yield several unstable intermediates, which then polymerize to give melaninsCitation16. Thus, 2.0 mM l-Tyr was used as a substrate to assay the effect of hinokitiol on the monophenolase activity of mushroom tyrosinase. The kinetic courses of oxidation of the substrate in the presence of hinokitiol at different concentrations are shown in . A marked lag period, characteristic of monophenolase activity, was observed simultaneously with the appearance of the first stable product, dopachrome ()Citation17. The system reached a constant rate after the lag period, which was estimated by extrapolation of the linear portion of the product accumulation curve to the abscissa. After the reaction system reached steady state, the curve of the product increased linearly with increasing reaction time; the slope of the line denoted the steady-state rate. In the presence of different concentrations of hinokitiol, the lag time and the steady-state rate were determined and the results are shown in . The results indicated that the lag time did not change obviously with increasing concentration of the compound. However, the steady-state rate decreased distinctly. The remaining enzyme activity was found to be 54.1% with the concentration of compound at 8.0 μM, which indicated that the inhibitory effect of hinokitiol on monophenolase was concentration-dependent. The IC50 value for the monophenolase activity inhibited by hinokitiol was 9.67 μM.
Figure 1. Inhibition effects of hinokitiol on monophenolase activity of mushroom tyrosinase. (a) Progress curves for oxidation of l-Tyr by the enzyme. Concentrations of hinokitiol for curves 1–6 were 0, 1.6, 3.2, 4.0, 4.8, 6.4, and 8.0 μM, respectively. (b) Effects of hinokitiol on steady-state rate of monophenolase activity (curve 1) and lag period of mushroom tyrosinase (curve 2) for oxidation of tyrosine. Concentrations of enzyme and substrate (l-Tyr) were 6.67 μg/mL and 0.5 mM, respectively.
Inhibitory effects of hinokitiol on diphenolase activity of mushroom tyrosinase
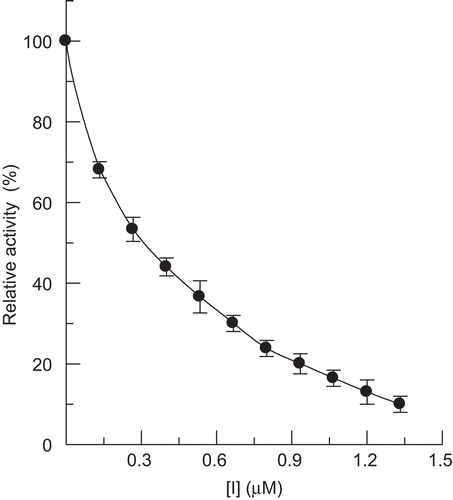
Hinokitiol was used as an effector on the activity of mushroom tyrosinase for the oxidation of l-DOPA. The progress curve of the enzyme reaction was a line passing through the origin without a lag period. The formation of product was in proportion to the reaction time. The value of the slope of the line indicated the diphenolase activity. On increasing the concentration of hinokitiol, the diphenolase activity of mushroom tyrosinase was markedly decreased in a concentration-dependent manner (). The IC50 value of the compound was estimated to be 0.21 μM.
Inhibitory mechanism of hinokitiol on diphenolase activity of mushroom tyrosinase
The inhibition mechanism on the enzyme by hinokitiol for the oxidation of l-DOPA was investigated. The result is shown in . Plots of remaining enzyme activity versus concentration of enzyme in the presence of different concentrations of hinokitiol gave a family of straight lines, which all passed through the origin, indicating that the inhibition of hinokitiol on diphenolase showed a reversible reaction course. The presence of hinokitiol did not reduce the amount of efficient enzyme, but just resulted in descending activity of the enzyme.
Inhibition type and inhibition constants of hinokitiol on diphenolase activity of mushroom tyrosinase
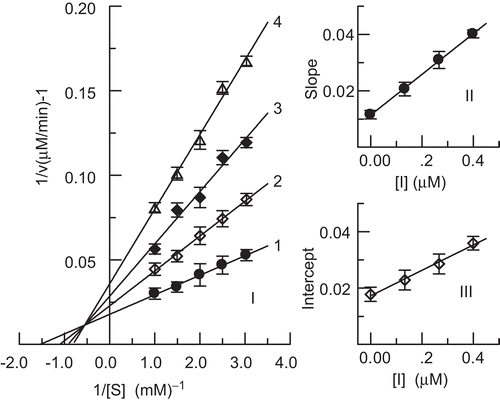
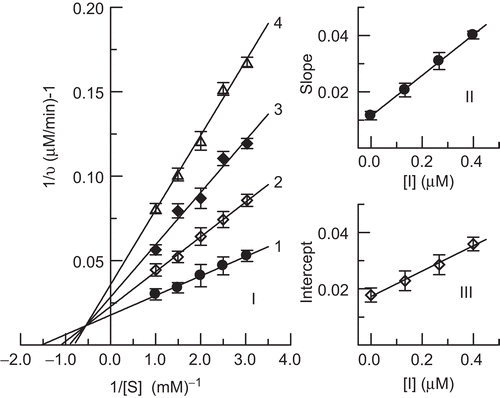
The inhibition kinetics of hinokitiol on the enzyme was studied using Lineweaver–Burk plots. The results are shown in . The double-reciprocal plots yielded a family of lines with different slopes and different intercepts, and they intersected one another in the second quadrant. This behavior indicated that hinokitiol could bind not only with free enzyme, but also with the enzyme–substrate complex, and their equilibrium constants were different; hinokitiol was a mixed-I type inhibitor of the enzyme. The equilibrium constants for its binding with free enzyme, KI, and with the enzyme–substrate complex, KIS, were obtained from the second plots of Kmapp/Vmapp and 1/Vmapp vs. concentration of hinokitiol, respectively. The values of KI and KIS were determined to be 0.16 µM and 0.38 µM, respectively.
Figure 4. Lineweaver–Burk plot for determination of inhibitory mechanism of hinokitiol on mushroom tyrosinase (I). Concentrations of hinokitiol for curves 1–4 were 0, 0.13, 0.26, and 0.40 μM, respectively. (II) and (III) represent plots of slope and intercept vs. concentration of hinokitiol for determining inhibition constants KI and KIS, respectively.
Inhibitory effect of hinokitiol on cell viability, cellular tyrosinase activity, and melanin biosynthesis
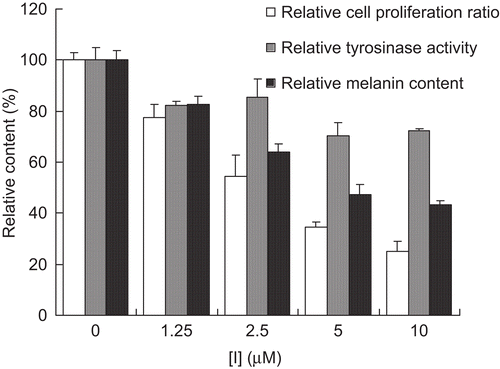
In this study, we also attempted to confirm whether hinokitiol could inhibit melanin biosynthesis in melanoma cells. We evaluated the effects of hinokitiol on melanin synthesis in B16 cells. The cell viability of the compounds was determined via a 3-day MTT assay. As shown in , the compound exerted a cytotoxic effect on melanoma cells and inhibition of tyrosinase activity and melanin content in the concentration range of 1.25–10 μM. When the concentration of inhibitor reached 10 μM, cell viability, enzyme activity, and melanin content were inhibited to 25.1%, 72.4%, and 43.2%, respectively. The cell viability and melanin content of hinokitiol-treated cells were reduced significantly in a dose-dependent manner. As a result, hinokitiol was shown to have cytotoxicity to the cells and function as a potent inhibitor on melanin production in B16 melanoma cells.
Antimicrobial activity of hinokitiol
The antibacterial activity of hinokitiol on S. enteritidis, E. coli, B. subtilis, S. aureus, K. pneumoniae, and R. solanacearum was investigated and the results are shown in and . In the test, the bacteriostatic activities were assayed by taking 2000 U/mL streptomycin sulfate as control. DMSO had no obvious inhibition on the proliferation of these six different kinds of bacteria. It was found that hinokitiol could inhibit the proliferation of these six different kinds of microbials to different extents.
Figure 6. Antimicrobial activity of hinokitiol at different concentrations. Concentrations of hinokitiol for wells 1–5 were 10, 5, 2.5, 1.25, and 0.625 mg/mL, respectively. a, positive control with 2000 U/mL of streptomycin sulfate for bacterium; b, negative control with DMSO.
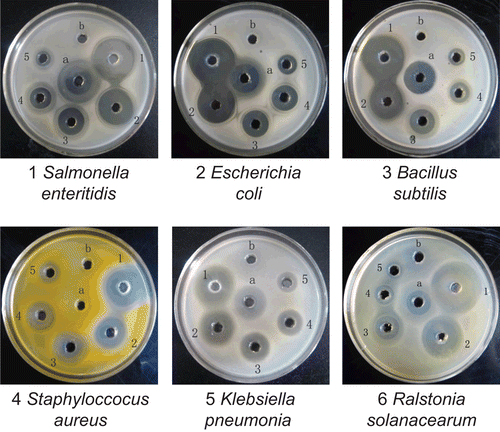
Table 1. Antimicrobial activity of hinokitiol.
A broth dilution method was also used to test the antimicrobial activities of hinokitiol against the six bacteria mentioned above. The results obtained are listed in . Hinokitiol was effective against S. enteritidis, E. coli, B. subtilis, and R. solanacearum, among which the antimicrobial activity against S. enteritidis, E. coli, and B. subtilis was more effective, with the same MIC of 50 μg/mL and the same MBC of 50 μg/mL, while the MIC and MBC against R. solanacearum were 50 μg/mL and 200 μg/mL, respectively.
Table 2. MIC and MBC (μg/mL) of hinokitiol.
Discussion
Tyrosinase is a key enzyme of melanin biosynthesis, which is responsible for browning in plants and is considered to be deleterious to the color quality of plant-derived foods and beverages. This unfavorable darkening from enzymatic oxidation generally results in a loss of nutritional and market values and has been of great concernCitation18. Besides, abnormal melanin pigmentation such as melasma, freckles, and senile lentigines is a serious esthetic problemCitation6. Melanin pigments are also found in many disease states. In the human brain, tyrosinase plays an important role in neuromelanin formation, which could be central to dopamine neurotoxicity as well as contribute to the neurodegeneration associated with Parkinson’s diseaseCitation19. Tyrosinase also plays an important role in the developmental and defensive functions of insects, which involve melanogenesis, wound healing, parasite encapsulation, and sclerotizationCitation5.
Melanin biosynthesis can be inhibited by avoiding ultraviolet (UV) exposure, by the inhibition of tyrosinase, and by the inhibition of melanocyte metabolism and proliferationCitation3. Hinokitiol has been reported to strongly inhibit human and mushroom tyrosinase activity, and reductions in tyrosinase activity are known to be associated with marked reductions in melanin synthesisCitation8. Sakuma et al.Citation20 reported that hinokitiol acted as a representative type of competitive inhibitor and exhibited the most potent inhibition (IC50 = 8.22 μM) followed by hydroquinone, resorcinol, hydroxyhydroquinone, kojic acid, l-ascorbic acid, phloroglucinol, p-nitrophenol, methyl p-hydroxybenzoate, and arbutin, while l-Tyr was used as substrate. Our results showed an approximate IC50 value of 9.67 μM for monophenolase activity inhibited by hinokitiol, when the enzymatic oxidation reaction used l-Tyr as the substrate. High inhibition of the diphenolase activity of mushroom tyrosinase was also found for hinokitiol with an IC50 value of 0.21 μM, lower than that of tropolone, one of the strongest tyrosinase inhibitors reported (IC50 = 0.4 μM)Citation21. However, the inhibition mechanism and type on the enzyme by hinokitiol for oxidation of l-DOPA revealed that the inhibition belonged to mixed-I type, namely, the competitive effect being stronger than the uncompetitive effect, which indicated that this compound inhibited the enzyme–substrate complex more weakly than the free enzymeCitation22.
Choi et al.Citation8 commented that hinokitiol observably inhibited melanin synthesis and also reduced the protein levels of tyrosinase, tyrosinase-related protein 1 (TYRP-1), tyrosinase-related protein 2 (TYRP-2), and microphthalmia-associated transcription factor (MITF). In the present study, we also investigated the inhibitory effect of hinokitiol on cellular tyrosinase activity, which was markedly reduced by hinokitiol in B16 cells. Furthermore, we estimated the cell viability and melanin biosynthesis of B16 cells in the presence of hinokitiol, demonstrating that the cells and melanin content were significantly reduced by hinokitiol in a dose-related manner. The depigmenting effect of hinokitiol was thought to be attributable to a combinative function of repression of tyrosinase activity, cell proliferation, and tyrosinase gene expression.
In addition, hinokitiol has been noted to have a wide spectrum of antimicrobial activities against Gram-positive and -negative bacteria, yeasts, and molds, and is effective as a plant growth stimulator. Of particular note is the fact that hinokitiol does not develop microorganism resistance, in contrast to many antibiotics. The results here confirmed the effective antibacterial activitiesCitation23,Citation24. The inhibition on R. solanacearum, a Gram-negative soil-borne β-proteobacterium that causes bacterial wilt disease in diverse and important food crops such as tomato, potato, banana, and gingerCitation25, is first reported.
In conclusion, the present study showed that hinokitiol is a promising candidate for the development of a food preservative or insecticidal agent which has potent inhibitory effects on tyrosinase activity and melanin biosynthesis as well as significant antimicrobial activity.
Acknowledgements
The present investigation was supported by the National High Technology Research and Development Program (“863” Program) of China (2006AA10A211), the Natural Science Foundation of China (No. 30570408, 20832005), the Science and Technology Foundation of Fujian Province (No. 2007N0051), the Program for New Century Excellent Talents in Fujian Province University, and the Postdoctoral Foundation of China (20080430787).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Iozumi K, Hoganson GE, Pennella R, Everett MA, Fuller BB. Role of tyrosinase as the determinant of pigmentation in cultured human melanocytes. J Invest Dermatol 1993;100:806–11.
- Yoon NY, Eom TK, Kim MM, Kim SK. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J Agric Food Chem 2009;57:4124–9.
- Nerya O, Vaya J, Musa R, Izrael S, Ben-Arie R, Tamir S. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J Agric Food Chem 2003;51:1201–7.
- Lee HS. Tyrosinase inhibitors of Pulsatilla cernua root-derived materials. J Agric Food Chem 2002;50:1400–3.
- Yu L. Inhibitory effects of (S)- and (R)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acids on tyrosinase activity. J Agric Food Chem 2003;51:2344–7.
- Matsuura R, Ukeda H, Sawamura M. Tyrosinase inhibitory activity of citrus essential oils. J Agric Food Chem 2006;54:2309–13.
- Kim YJ, Uyama H. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci 2005;62:1707–23.
- Choi YG, Bae EJ, Kim DS, Park SH, Kwon SB, Na JI, et al. Differential regulation of melanosomal proteins after hinokitiol treatment. J Dermatol Sci 2006;43:181–8.
- Morita Y, Matsumura E, Okabe T, Fukui T, Ohe T, Ishida N, et al. Biological activity of beta-dolabrin, gamma-thujaplicin, and 4-acetyltropolone, hinokitiol-related compounds. Biol Pharm Bull 2004;27:1666–9.
- Morita Y, Sakagami Y, Okabe T, Ohe T, Inamori Y, Ishida N. The mechanism of the bactericidal activity of hinokitiol. Biocontrol Sci 2007;12:101–10.
- Chen QX, Ke LN, Song KK, Huang H, Liu XD. Inhibitory effects of hexylresorcinol and dodecylresorcinol on mushroom (Agaricus bisporus) tyrosinase. Protein J 2004;23:135–41.
- Zhang X, Hu X, Hou A, Wang H. Inhibitory effect of 2,4,2′,4′-tetrahydroxy-3-(3-methyl-2-butenyl)-chalcone on tyrosinase activity and melanin biosynthesis. Biol Pharm Bull 2009;32:86–90.
- Huang YH, Lee TH, Chan KJ, Hsu FL, Wu YC, Lee MH. Anemonin is a natural bioactive compound that can regulate tyrosinase-related proteins and mRNA in human melanocytes. J Dermatol Sci 2008;49:115–23.
- Kubo I, Fujita K, Kubo A, Nihei K, Ogura T. Antibacterial activity of coriander volatile compounds against Salmonella choleraesuis. J Agric Food Chem 2004;52:3329–32.
- Huang XH, Chen QX, You MS, Wang Q, Song KK, Wang J, et al. Inhibitory effects of fluorobenzaldehydes on the activity of mushroom tyrosinase. J Enzyme Inhib Med Chem 2006;21:413–18.
- Wang Q, Qiu L, Chen XR, Song KK, Shi Y, Chen QX. Inhibitory effects of phloridzin dihydrate on the activity of mushroom (Agaricus bisporus) tyrosinase. Bioorg Med Chem 2007;15:1568–71.
- Fenoll LG, Rodriguez-Lopez JN, Garcia-Sevilla F, Garcia-Ruiz PA, Varon R, Garcia-Canovas F, et al. Analysis and interpretation of the action mechanism of mushroom tyrosinase on monophenols and diphenols generating highly unstable o-quinones. Biochim Biophys Acta 2001;1548:1–22.
- Kubo I, Kinst-Hori I. Tyrosinase inhibitory activity of the olive oil flavor compounds. J Agric Food Chem 1999;47:4574–8.
- Casanola-Martin GM, Marrero-Ponce Y, Khan MT, Ather A, Khan KM, Torrens F, et al. Dragon method for finding novel tyrosinase inhibitors: biosilico identification and experimental in vitro assays. Eur J Med Chem 2007;42:1370–81.
- Sakuma K, Ogawa M, Sugibayashi K, Yamada K, Yamamoto K. Relationship between tyrosinase inhibitory action and oxidation-reduction potential of cosmetic whitening ingredients and phenol derivatives. Arch Pharm Res 1999;22:335–9.
- Ley JP, Bertram HJ. Hydroxy- or methoxy-substituted benzaldoximes and benzaldehyde-o-alkyloximes as tyrosinase inhibitors. Bioorg Med Chem 2001;9:1879–85.
- Song KK, Huang H, Han P, Zhang CL, Shi Y, Chen QX. Inhibitory effects of cis- and trans-isomers of 3,5-dihydroxystilbene on the activity of mushroom tyrosinase. Biochem Biophys Res Commun 2006;342:1147–51.
- Inamori Y, Sakagami Y, Morita Y, Shibata M, Sugiura M, Kumeda Y, et al. Antifungal activity of hinokitiol-related compounds on wood-rotting fungi and their insecticidal activities. Biol Pharm Bull 2000;23:995–7.
- Nomiya K, Yoshizawa A, Kasuga NC, Yokoyama H, Hirakawa S. Synthesis, solid-state characterization and antimicrobial activities of three different polymorphs of a copper(II) complex with 4-isopropyltropolone (hinokitiol). Inorg Chim Acta 2004;357:1168–76.
- Hayward AC. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol 1991;29:65–87.