Abstract
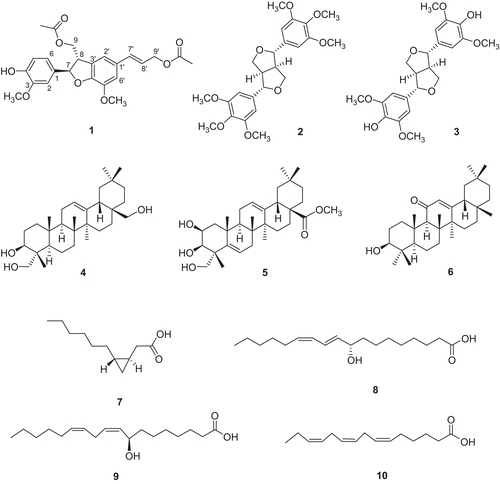
The ethyl acetate (EtOAc) soluble fraction of the 85% ethanol (EtOH) extract of the dried bark of Limonia acidissima potently inhibited nitric oxide (NO) production in lipopolysaccharide (LPS) activated BV-2 cells, a microglial cell line. Bioassay-guided column chromatography separation afforded a new stereoisomer of neolignan, (7’E)-(7R,8S)-4-hydroxy-3,5’-dimethoxy-4’,7-epoxy-8,3’-neolig-7’-en-9,9’-diyil diacetate (1), together with two known lignans, (+)-yangambin (2) and (+)-syringaresinol (3), three known triterpenoids, hederatriol (4), basic acid methyl ester (5), and 3β-hydroxyolean-12-en-11-one (6), and four known fatty acid derivatives, cascarillic acid (7), (+)-α-dimorphecolic acid (8), 8(R)-hydroxylinoleic acid (9), and (6Z,9Z,12Z)-pentadecatrienoic acid (10). The structure of the new compound 1 was elucidated by detailed analysis of spectroscopic data and circular dichroism (CD) spectroscopy. Compounds 1, 3-6, and 8-10 isolated from L. acidissima significantly reduced NO production in LPS-stimulated BV-2 microglia cells.
Introduction
Microglia are the primary immune cells in the central nervous system and mediate inflammation-mediated neurotoxicity [Citation1]. Under normal conditions microglia act as immune surveillance; however, activated microglia secrete excessive pro-inflammatory substances such as nitric oxide (NO), tumour necrosis factor-α, interleukin-1β, and prostaglandins [Citation2,Citation3]. The NO produced by inducible nitric oxide synthase is a primary pro-inflammatory mediator and plays an important role in neuroinflammatory diseases [Citation4].
Thus, in the course of our continuing search for biologically active compounds from natural medicinal sources, we investigated the inhibitory constituents from dried bark of Limonia acidissima L. on NO production in lipopolysaccharide (LPS)-stimulated microglia cells, as the EtOAc-soluble fraction of the 85% EtOH extract of this herb inhibited NO production.
L. acidissima, or wood apple, belongs to the family of Rutaceae. Various parts of this plant have medicinal properties; the fruit pulp has been used for a remedy for insect bites [Citation5] and thanaka, a root paste made from its pulp, is used as a facial cosmetic to remove small spots and lesions [Citation6]. Various constituents, including coumarins, steroids, triterpenoids, benzoquinones, and tyramine derivatives have been isolated from different parts of this plant [Citation7–12]. We also reported that benzamide derivatives and coumarins from L. acidissima inhibit NO production [Citation13,Citation14].
In the present study, a bioassay-guided column chromatographic separation of L. acidissima led to the isolation of a new stereoisomer of neolignan (1), (7’E)-(7R,8S)-4-hydroxy-3,5'-dimethoxy-4’,7-epoxy-8,3'-neolig-7'-en-9,9'-diyil diacetate, together with nine known compounds (2-10). Furthermore, the ability of all ten isolated compounds (1-10) to inhibit NO production was evaluated in LPS-activated BV-2 cells, a microglial cell line.
Materials and methods
General experimental procedures
Optical rotations were measured on a Jasco P-1020 polarimeter (Jasco, Easton, MD, USA) in MeOH. Circular dichroism (CD) spectra were measured on a JASCO J-715 spectropolarimeter (Bruker, Karlsruhe, Germany). IR spectra were recorded on a Bruker IFS-66/S FT-IR spectrometer (Shimadzu, Tokyo, Japan). UV spectra were recorded using a Shimadzu UV-1601 UV-Visible spectrophotometer (JEOL, Peabody, MA, USA). Fast atom bombardment (FAB) and high resolution fast atom bombardment (HRFAB) mass spectra were obtained on a JEOL JMS700 mass spectrometer (JEOL, Peabody, MA, USA). Nuclear magnetic resonance (NMR) spectra, including 1H-1H correlation spectroscopy (COSY), heteronuclear multiple quantum coherence (HMQC), heteronuclear multiple bond correlation (HMBC), and nuclear Overhauser effect spectroscopy (NOESY) experiments, were recorded on a Varian UNITY INOVA 500 NMR spectrometer (Varian, Palo Alto, CA, USA) operating at 500 MHz (1H) and 125 MHz (13C), with chemical shifts given in ppm (δ). Preparative HPLC was conducted using a Gilson 306 pump (Gilson, Middleton, WI, USA) with a Shodex refractive index detector (Shodex, New York, NY, USA) and an Apollo Silica 5μ column (250 × 10 mm; Alltech, Nicholasville, KY, USA). Silica gel 60 (Merck, Darmstadt, Germany; 70-230 mesh and 230-400 mesh) was used for column chromatography. The packing material for molecular sieve column chromatography was Sephadex LH-20 (Pharmacia, Uppsala, Sweden). Spots were detected on thin layer chromatography (TLC) under UV light or by heating after spraying with 10% H2SO4 in C2H5OH (v/v).
Plant materials
The dried bark of L. acidissima was imported from Yangon, Union of Myanmar, in October 2006, and identified by Dr W. Bae, researcher of the R & D Institute, Miwon Commercial (Ansan, Korea) and a voucher specimen (SKKU 2006-10) was deposited at the R & D Institute, Miwon Commercial, Co., Ltd, Ansan, Korea.
Extraction and isolation
The dried bark (3 kg) of L. acidissima was extracted with 85% EtOH three times at 85°C. The resulting ethanol extract (250 g) was suspended in distilled water (7.2 L) and then partitioned with EtOAc, yielding an EtOAc soluble extract (50 g). The EtOAc-soluble fraction (50 g) was separated over a silica gel column (800 g, 10 × 80 cm), eluted with a gradient of n-hexane-EtOAc (10:1, 5:1, 1:1, and 0:1, v/v) to afford 12 fractions (fractions A to L). The active fraction H (810 mg) was separated further over a silica gel column (80 g, 3 × 40 cm) using CHCl3-MeOH (17:1, v/v) to give three sub-fractions (fractions H1 to H3). Fraction H2 (450 mg) was separated over a Sephadex LH-20 column (Pharmacia, 150 g, 3 × 80 cm), using a solvent system of CH2Cl2-MeOH (1:1, v/v) to yield three sub-fractions (fractions H21 to H23). Fraction H22 (70 mg) was separated by preparative HPLC, over 30 min at a flow rate of 2 mL/min (Apollo Silica 5μ column; 250 × 10 mm; 5μ particle size; Shodex refractive index detector; n-hexane-EtOAc, 1:1, v/v) to obtain compound 1 (8 mg, retention times (Rt) = 17.5). Compounds 5 (5 mg, n-hexane-EtOAc, 1:1, v/v), 6 (18 mg, n-hexane-EtOAc, 1:1, v/v), and 7 (5 mg, n-hexane-EtOAc, 1:1, v/v) were isolated from fraction H22, and compound 8 (5 mg, MeOH-H2O, 4:1, v/v) was isolated from fraction H3 through HPLC purification. The active fraction I (760 mg) was separated over a silica gel column (80 g, 3 × 40 cm) using CHCl3-MeOH (15:1, v/v) to give three subfractions (fractions I1 to I3). Compound 2 (8 mg, MeOH-H2O, 1:1, v/v) was isolated from fraction I2 and compounds 4 (7 mg, MeOH-H2O, 1:0, v/v), 9 (4 mg, MeOH-H2O, 19:1, v/v), and 10 (3 mg, MeOH-H2O, 19:1, v/v) were isolated from fraction I3 through HPLC purification. Compound 3 (10 mg) was isolated from subfraction of active fraction J (2.7 g) by HPLC purification using MeOH-H2O (9:11, v/v).
Compound (1): Amorphous gum (8 mg); ![]() -60.5° (C = 0.4, MeOH); IR (KBr) νmax 3729, 3395, 2931, 1656, 1531, 1518, 1240, 1058, 669 cm−1; UV (MeOH) λmax 278 (4.1), 303 (3.5) 345 (3.0) nm; CD (MeOH) [θ]234 +5300, [θ]265 +3200, [θ]287 - 5700; 1H and 13C NMR (see ). HRFABMS (positive mode) m/z 443.1701 [M + H]+ (calcd for C24H27O8, 443.1706).
-60.5° (C = 0.4, MeOH); IR (KBr) νmax 3729, 3395, 2931, 1656, 1531, 1518, 1240, 1058, 669 cm−1; UV (MeOH) λmax 278 (4.1), 303 (3.5) 345 (3.0) nm; CD (MeOH) [θ]234 +5300, [θ]265 +3200, [θ]287 - 5700; 1H and 13C NMR (see ). HRFABMS (positive mode) m/z 443.1701 [M + H]+ (calcd for C24H27O8, 443.1706).
Table 1. 1H and 13C NMR data for compound 1 ( δ in ppm, 500 MHz for 1H and 125 MHz for 13C, in CDCl3).
Measurement of NO production and cell viability in LPS-activated BV-2 cells
BV-2 microglia cells were stimulated with 100 ng/mL LPS in the presence or absence of samples for 24 h. Nitrite in the culture media, a soluble oxidation product of NO, was measured by a Griess reaction. The supernatant (50 μL) was harvested and mixed with an equal volume of Griess reagent (1% sulphanilamide, 0.1% N-1-napthylethylenediamine dihydrochloride in 5% phosphoric acid). After 10 min, the absorbance at 540 nm was measured using a microplate reader (Emax, Molecular Device, Sunnyvale, CA, USA). Sodium nitrite was used as a standard to calculate the nitrite concentration [Citation15]. Cell viability was measured using a 3-[4, 5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay [Citation16]. NG-monomethyl-L-arginine (L-NMMA, Sigma, St. Louis, MO, USA), a well-known NOS inhibitor, was tested as a positive control.
Statistical analysis
The data were analysed using Statistical Analysis System software (PRISM, GraphPad, La Jolla, CA, USA). Data are expressed as mean ± SEM. Statistical comparisons between the different treatments were performed using one-way ANOVA with Tukey’s multiple comparison post test and the data were considered to be statistically significant if the probability had a value of less than 0.05.
Results and discussion
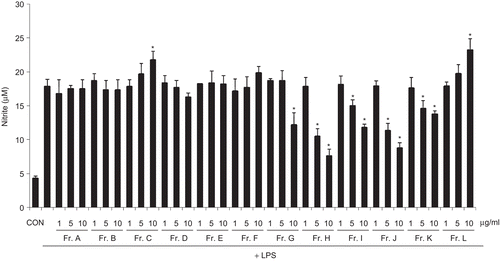
The dried bark of L. acidissima was extracted with 85% EtOH and the EtOH extract was partitioned with EtOAc. The EtOAc-soluble fraction inhibited LPS-induced NO production and was subjected to silica gel open-column chromatography to afford 12 fractions (fractions A to L). Fractions H, I, and J inhibited LPS-induced NO production in a concentration-dependent manner (). Isolation from these three fractions afforded a new stereoisomer of neolignan (1), (7’E)-(7R,8S)-4-hydroxy-3,5’-dimethoxy-4’,7-epoxy-8,3’-neolig-7’-en-9,9’-diyil diacetate, together with nine known compounds (2-10) (). Two known lignans, (+)-yangambin (2) [Citation17] and (+)-syringaresinol (3) [Citation18], three known triterpenoids, hederatriol (4) [Citation19], bassic acid methyl ester (5) [Citation20], and 3β-hydroxyolean-12-en-11-one (6) [Citation21], and four known fatty acid derivatives, cascarillic acid (7) [Citation22], (+)-α-dimorphecolic acid (8) [Citation23], 8(R)-hydroxylinoleic acid (9) [Citation24], and (6Z,9Z,12Z)-pentadecatrienoic acid (10) [Citation25] were identified by comparison with published data. This is the first report of these compounds in this plant, except for compound 3.
Figure 1. Effects of Limonia acidissima fractions (Fr. A to Fr. L) on LPS-induced NO production in BV-2 microglia cells. BV-2 cells were incubated in the absence (CON, control) or presence of LPS (100 ng/mL). Cells were pre-treated with the fractions (1, 5 and 10 μg/mL) for 30 min, and then stimulated with LPS (100 ng/mL) for 24 h. The culture medium was then collected for a nitrite assay. Nitrate was measured using a Griess reaction and sodium nitrite was used as a standard. All data are presented as the mean ± SEM of three independent experiments. *p <0.05 indicates significant difference compared to LPS alone.
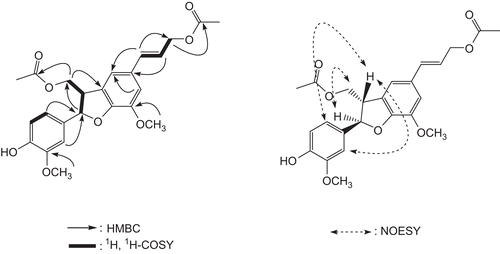
Compound 1 was obtained as an amorphous gum with optical rotation; ![]() -60.5° (C = 0.4, MeOH). The molecular formula was determined to be C24H26O8 by high-resolution positive fast atom bombardment ionisation mass spectroscopy (HRFABMS) at m/z 443.1701 [M + H]+ (calcd for C24H27O8, 443.1706), together with the 1H and 13C NMR data (). The IR spectrum exhibited the presence of hydroxyl (3395 cm−1), carboxyl (1656 cm−1) and aromatic (1531 and 1518 cm−1) groups. The UV spectrum of 1 showed absorption maxima at 278, 303, and 345 nm in MeOH. The 1H NMR spectrum of 1 () displayed signals for the presence of a 3,4-disubstituted aromatic ring [δH 6.85 (1H, dipole-dipole (dd), J = 1.5, 8.0 Hz), 6.86 (1H, d, J = 8.0 Hz), and 6.88 (1H, d, J = 1.5 Hz)] and a set of meta-coupled signals at δH 6.86 (1H, s) and 6.87 (1H, s). The 1H NMR and 1H-1H COSY spectrum also showed signals attributable to trans olefinic protons [δH 6.16 (1H, dd, J = 6.5, 15.5 Hz), and 6.58 (1H, d, J = 15.5 Hz)], two acetoxy-methylenes [δH 4.28 (1H, dd, J = 7.5, 11.5 Hz), 4.42 (1H, dd, J = 5.5, 11.5 Hz), and 4.71 (2H, d, J = 6.5 Hz], two methines [δH 3.78 (1H, m), and 5.47 (1H, d, J = 7.5 Hz)], and two methoxy groups [δH 3.87 (3H, s), and 3.90 (3H, s)]. In addition, the signals of two additional acetyl protons at δH 2.02 (3H, s), and 2.10 (3H, s) were observed. The 13C NMR spectrum also contained 18 skeletal carbon resonances. These spectral features indicated that 1 was a dihydro[b]benzofuran-type neolignan formed by two coniferyl alcohol acetate molecules [Citation26,Citation27]. Detailed analysis of COSY and HMBC correlations () reconfirmed the skeletal structure of 1. The methoxy groups were determined to be at C-3 and C-3’, which was confirmed by the HMBC correlations of the methoxy protons at δH 3.90 with C-3 (δC 56.1), and at δH 3.87 with C-3’ (δC 56.2). The HMBC correlations of acetoxymethylene protons at δH 4.28/4.42 (H-9) with an acetyl group (δC 171), and at δH 4.71 (H-9’) with an acetyl group (δC 171.1) indicated the two acetyl groups were at C-9 and C-9’.
-60.5° (C = 0.4, MeOH). The molecular formula was determined to be C24H26O8 by high-resolution positive fast atom bombardment ionisation mass spectroscopy (HRFABMS) at m/z 443.1701 [M + H]+ (calcd for C24H27O8, 443.1706), together with the 1H and 13C NMR data (). The IR spectrum exhibited the presence of hydroxyl (3395 cm−1), carboxyl (1656 cm−1) and aromatic (1531 and 1518 cm−1) groups. The UV spectrum of 1 showed absorption maxima at 278, 303, and 345 nm in MeOH. The 1H NMR spectrum of 1 () displayed signals for the presence of a 3,4-disubstituted aromatic ring [δH 6.85 (1H, dipole-dipole (dd), J = 1.5, 8.0 Hz), 6.86 (1H, d, J = 8.0 Hz), and 6.88 (1H, d, J = 1.5 Hz)] and a set of meta-coupled signals at δH 6.86 (1H, s) and 6.87 (1H, s). The 1H NMR and 1H-1H COSY spectrum also showed signals attributable to trans olefinic protons [δH 6.16 (1H, dd, J = 6.5, 15.5 Hz), and 6.58 (1H, d, J = 15.5 Hz)], two acetoxy-methylenes [δH 4.28 (1H, dd, J = 7.5, 11.5 Hz), 4.42 (1H, dd, J = 5.5, 11.5 Hz), and 4.71 (2H, d, J = 6.5 Hz], two methines [δH 3.78 (1H, m), and 5.47 (1H, d, J = 7.5 Hz)], and two methoxy groups [δH 3.87 (3H, s), and 3.90 (3H, s)]. In addition, the signals of two additional acetyl protons at δH 2.02 (3H, s), and 2.10 (3H, s) were observed. The 13C NMR spectrum also contained 18 skeletal carbon resonances. These spectral features indicated that 1 was a dihydro[b]benzofuran-type neolignan formed by two coniferyl alcohol acetate molecules [Citation26,Citation27]. Detailed analysis of COSY and HMBC correlations () reconfirmed the skeletal structure of 1. The methoxy groups were determined to be at C-3 and C-3’, which was confirmed by the HMBC correlations of the methoxy protons at δH 3.90 with C-3 (δC 56.1), and at δH 3.87 with C-3’ (δC 56.2). The HMBC correlations of acetoxymethylene protons at δH 4.28/4.42 (H-9) with an acetyl group (δC 171), and at δH 4.71 (H-9’) with an acetyl group (δC 171.1) indicated the two acetyl groups were at C-9 and C-9’.
A coupling constant (7.5 Hz) between H-7 and H-8 implied the trans-configuration between these protons [Citation26]. This arrangement was verified by a lack of NOESY correlations between these protons but NOESY correlations between H-7 and H-9 and between H-8 and H-2/H-6 (). The absolute configuration of C-7 and C-8 were confirmed as 7R and 8S, respectively, by the CD spectrum showing the positive Cotton effect at 234 nm, the positive Cotton effect at 265 nm, and the negative Cotton effect at 287 nm, and the optical rotation (![]() -60.5°) value [Citation27,Citation28]. For biogenesis of 8-5’ neolignans, ring closure will always proceed the formation of the trans-form due to the more stable trans configuration of the C-7/C-8 bond [Citation29]. Accordingly, compound 1 was identified as (7’E)-(7R,8S)-4-hydroxy-3,5’-dimethoxy-4’,7-epoxy-8,3’-neolig-7’-en-9,9’-diyil diacetate. The diastereoisomer of 1 was reported from the roots of Lasiolaena morii [Citation30], which was reassigned to β form for H-7 (7S) and α form for H-8 (8R) as relative-configuration by Li et al. [Citation29]. But the absolute stereochemistry of 1 was assigned by CD data, and 1 was the first example isolated as the 7R, 8S-form in nature.
-60.5°) value [Citation27,Citation28]. For biogenesis of 8-5’ neolignans, ring closure will always proceed the formation of the trans-form due to the more stable trans configuration of the C-7/C-8 bond [Citation29]. Accordingly, compound 1 was identified as (7’E)-(7R,8S)-4-hydroxy-3,5’-dimethoxy-4’,7-epoxy-8,3’-neolig-7’-en-9,9’-diyil diacetate. The diastereoisomer of 1 was reported from the roots of Lasiolaena morii [Citation30], which was reassigned to β form for H-7 (7S) and α form for H-8 (8R) as relative-configuration by Li et al. [Citation29]. But the absolute stereochemistry of 1 was assigned by CD data, and 1 was the first example isolated as the 7R, 8S-form in nature.
We evaluated whether compounds (1-10) could inhibit NO production in LPS-activated BV-2 microglia cells (). Compounds 1, 3-6, and 8-10 dose-dependently inhibited LPS-stimulated NO production, but compounds 2 and 7 (up to 10 μM) were not active. Compounds 1, 4, 8, 9 and 10 were more active than NG-monomethyl-L-arginine (L-NMMA), a NOS inhibitor. The IC50 values of 1, 4, 8, 9 and 10 were 8.08, 7.28, 8.82, 6.73, and 7.01 μM, respectively. These results suggest that Limonia acidissima and its constituents may be good leads for the development of novel neuroprotective agents.
Table 2. Effects of compounds 1-10 and L-NMMA on LPS-induced NO production in BV-2 microglia cells.
Acknowledgements
The authors would like to thank Do Kyun Kim, Eun Jung Bang, and Jung Ju Seo at the Korea Basic Science Institute for the measurements of NMR and MS spectra.
Declaration of interest
The authors report no conflicts of interest.
References
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther 2003;304:1–7.
- McGeer PL, McGeer EG. The inflammatory response system of brain: Implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Rev 1995;21:195–218.
- Minghetti L, Levi G. Microglia as effector cells in brain damage and repair: Focus on prostanoids and nitric oxide. Prog Neurobiol 1998;54:99–125.
- Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci 1997;20:132–139.
- Kirtikar KR, Basu BD. Indian Medicinal Plants, Vol. 1. India: Lalith Mohan Basu, 1933: 496–498.
- Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants. New Delhi: CSIR, 1956: 154.
- Ghosh P, Sil P, Majumadar SG, Thakur S. A coumarin from Limonia acidissima. Phytochemistry 1982;21:240–241.
- Patra A, Misra SK, Chaudhuri SK. Constituents of Limonia acidissima. Applications of two-dimentional NMR spectroscopy in structure elucidation. J Indian Chem Soc 1988;65:205–208.
- Bandara BMR, Gunatilaka AAL, Wijeratne EMK, Adikaram NKB. Antifungal constituents of Limonia acidissima. Planta Med 1988;54:374–375.
- MacLeod JK, Moeller PDR, Bandara BMR, Gunatilaka AAL, Wijeratne EMK. Acidissimin, a new limonoid from Limonia acidissima. J Nat Prod 1989;52:882–885.
- Ghosh P, Ghosh MK, Thakur S, Datta JD, Akihisa T, Tamura T, Kimura Y. Dihydroxy acidissiminol and acidissiminol epoxide, two tyramine derivatives from Limonia acidissima. Phytochemistry 1994;37:757–760.
- Ghosh P, Sil P, Das S, Kokke WCMC, Kokke WCM, Akihisa T, Shimizu N, Tamura T, Matsumoto T. Tyramine derivatives from the fruit of Limonia acidissima. J Nat Prod 1991;54:1389–1393.
- Kim KH, Lee IK, Kim KR, Ha SK, Kim SY, Lee KR. New benzamide derivatives and NO production inhibitory compounds from Limonia acidissima. Planta Med 2009;75:1146–1151.
- Kim KH, Ha SK, Kim SY, Kim SH, Lee KR. Limodissimin A: A new dimeric coumarin from Limonia acidissima. Bull Korean Chem Soc 2009;30:2135–2137.
- Ha SK, Lee P, Park JA, Oh HR, Lee SY, Park JH, Lee EH, Ryu JH, Lee KR, Kim SY. Apigenin inhibits the production of NO and PGE2 in microglia and inhibits neuronal cell death in a middle cerebral artery occlusion-induced focal ischemia mice model. Neurochem Int 2008;52:878–886.
- Sargent JM, Taylor CG. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br J Cancer 1989;60:206–210.
- Donald Macrae W, Neil Towers GH. Non-alkaloidal constituents of Virola elongate bark. Phytochemistry 1985;24:561–566.
- Nawwar MAM, Buddrus J, Bauer H. Dimeric phenolic constituents from the roots of Tamarix nilotica. Phytochemistry 1982;21:1755–1758.
- Tori K, Seo S, Shimaoka A, Tomita Y. Carbon-13 NMR spectra of olean-12-enes. Full signal assignments including quaternary carbon signals assigned by use of indirect 13C, 1H spen couplings. Tetrahedron Lett 1974;48:4227–4230.
- Yosioka I, Inada A, Kitagawa I. Soil bacterial hydrolysis leading to genuine aglycon. VIII. Structures of a genuine sapogenol protobassic acid and a prosapogenol of seed kernels of Madhuca longifolia. Tetrahedron 1974;30:707–714.
- Herath HMTB, Athukoralage PS. Oleanane triterpenoids from Gordonia ceylanica. Nat Prod Sci 1998;4:253–256.
- Roberts IO, Baird MS, Liu Y. The absolute stereochemistry of cascarillic acid. Tetrahedron Lett 2004;45:8685–8686.
- Naidu SV, Gupta P, Kumar P. Enantioselective syntheses of (-)-pinellic acid, α- and β-dimorphecolic acid. Tetrahedron 2007;63:7624–7633.
- Brodowsky ID, Hamberg M, Oliw EH. A linoleic acid (8R)-dioxygenase and hydroperoxide isomerase of the fungus Gaeumannomyces graminis. Biosynthesis of (8R)-hydroxylinoleic acid and (7S, 8S)-dihydroxylinoleic acid from (8R)-hydroperoxylinoleic acid. J Biol Chem 1992;267:14738–14745.
- Chang HW, Jang KH, Lee D, Kang HR, Kim TY, Lee BH, Choi BW, Kim S, Shin J. Monoglycerides from the brown alga Sargassum sagamianum: Isolation, synthesis, and biological activity. Bioorg Med Chem Lett 2008;18:3589–3592.
- Valcic S, Montenegro G, Timmermann BN. Lignans from Chilean propolis. J Nat Prod 1998;61:771–775.
- Yeo H, Chin YW, Park SY, Kim J. Lignans of Rosa multiflora roots. Arch Pharm Res 2004;27:287–290.
- Hirai N, Okamoto M, Udagawa H, Yamamuro M, Kato M, Koshimizu K. Absolute configuration of dehydrodiconiferyl alcohol. Biosci Biotech Biochem 1994;58:1679–1684.
- Li S, Iliefski T, Lundquist K, Wallis AFA. Reassignment of relative stereochemistry at C-7 and C-8 in arylcoumaran neolignans. Phytochemistry 1997;46:929–934.
- Bohlmann F, Jakupovic J, Schuster J, King RM, Robinson H. Guainolides and homoditerpenes from Lasiolaena morii. Phytochemistry 1982;21:161–165.