Abstract
Serum paraoxonase (aryldialkylphosphatase, EC 3.1.8.1., PON1) is an esterase protein synthesised by the liver and released into the serum, where it is associated with HDL lipoproteins. In this study, we have determined the in vitro effects of the following antibiotics: sodium ampicillin, ciprofloxacin, Rifamycin SV and clindamiycin phosphate, on human hepatoma (HepG2) cells (liver hPON1). All the antibiotics caused a dose-dependent and time-dependent decrease in the paraoxonase activity while Rifamycin SV was the most effective antibiotic due to its low 50% inhibition concentration (IC50) value. Liver hPON1 activity was determined using paraoxon as a substrate. The IC50 values of the drugs were calculated from graphs of hydratase activity (%) by plotting concentration of the drugs that showed an inhibition effect.
Keywords::
Introduction
Paraoxonase-1 (PON1) is a high-density lipoprotein (HDL)–associated serum enzyme whose primary physiological role is to protect low-density lipoproteins (LDL) from oxidative modifications [Citation1]. It is a member of a three gene family consisting of PON1, PON2 and PON3 that are located on human chromosome 7 [Citation2]. The three PON genes show a high similarity at the amino acid level between the mammalian species PON1 and PON3, which are expressed primarily in the liver. In contrast PON2 is widely expressed in a number of tissues including brain, liver, kidney and testis and it may have multiple mRNA forms [Citation3,Citation4]. Unlike PON2 and PON3, PON1 is an efficient esterase towards many OP (organophosphate) compounds including paraoxon, the insecticides parathion and chlorpyriphos as well as the nerve agents sarin and soman [Citation5]. The enzyme derives its name from its ability to hydrolyse paraoxon into p-nitrophenol and diethylphosphate, a reaction that was first demonstrated by Aldridge in 1953 [Citation6]. The ability to hydrolyse paraoxon is routinely used for measuring PON1 activity in vitro in serum samples. Furthermore the enzyme inhibits atherogenesis by preventing the oxidation of HDL and low-density lipoprotein (LDL). PON1 hydrolyses aliphatic lactones such as dihydrocoumarin, γ -butyrolactone and homocysteine thiolactone. Therefore, PON1 prevents protein homocysteinylation which is the process involved in atherogenesis [Citation7,Citation8].
More recently, in addition to its important roles in a broad range of fields, PON1 has been shown to play a critical role in the metabolism of pharmaceutical drugs [Citation3]. Paraoxonase should be studied more given its physiological and metabolic importance on medically important drugs that are commonly used in therapies such as antibiotics. There are only a few reports that have determined the changes in paraoxonase enzyme activities by antibiotics [Citation9,Citation10,Citation11]. In our previous study, we reported that different classes of antibiotics differentially affect the PON1 enzyme activity from mouse liver, mouse serum and human serum in vitro. These differential inhibitions on different cells or models imply that the PON1 enzyme inhibition may be distinct in human cells. However, there is no information available about the inhibition of this enzyme in human hepatoma cells which are important cells for the metabolism of drugs in the body.
Therefore in the present study, we aimed to evaluate the effects of penicillin, chinolon, antimicobacterial and macrolid derived antibiotics on the paraoxonase enzyme activity of HepG2 cells, in vitro. The antibiotics were chosen from different classes. Ampicillin is a beta-lactam antibiotic that is active against both Gram-positive and Gram-negative bacteria and is widely used for the treatment of infections [Citation12]. Ciprofloxacin is a broad-spectrum antibiotic belonging to the fluoroquinolone class. Today, fluoroquinolones are the most commonly-prescribed antimicrobial agents. Ciprofloxacin is considered a benchmark for comparing the efficacy of new fluoroquinolones and it is also active against both Gram-positive and Gram-negative bacteria [Citation13]. Rifamycins are a group of antibiotics which are synthesised either naturally by the bacterium Amycolatopsis mediterranei, or artificially. Rifamycins are particularly effective against mycobacteria and are therefore used to treat tuberculosis, leprosy, and Mycobacterium avium complex (MAC) infections [Citation14]. Clindamycin is a lincosamide antibiotic and it prevents the protein synthesis of bacteria, causing the cells to die [Citation10] ().
Materials and methods
Materials
The cell culture reagents and chemicals were obtained from Sigma (Germany). All other chemicals used were of analytical grade and obtained from either Sigma or Merck (Germany). Medical drugs were provided by the local pharmacy.
Paraoxonase enzyme assay
Paraoxonase enzyme activity towards paraoxon was quantified spectrophotometrically by the method described by Gan et al. [Citation15]. The reaction was followed for 2 min at 37°C by monitoring the appearance of p-nitrophenol at 412 nm in a Biotek (USA) automated recording spectrophotometer. The final substrate concentration during the enzyme assay was 2 mM and all rates were determined in duplicate and corrected for the non-enzymatic hydrolysis. PON1 activity (1U/L) was defined as 1 μmol of p-nitrophenol formed per minute.
Cell culture of HepG2 cells and in vitro inhibition kinetic studies
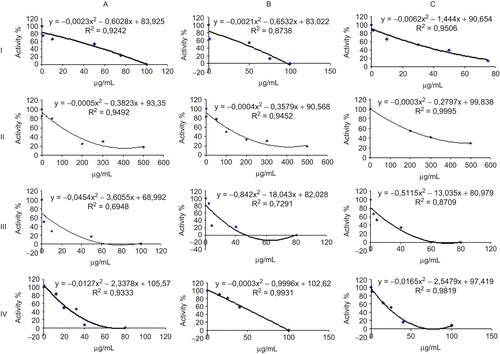
A human hepatoma cell line (HepG2) was used in this study. The cells were seeded at 250 000/well into 12-well plates containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with glutamine (0.2 mM), penicillin and streptomycin (100 U/mL and 100 μg/mL, respectively), and bovine fetal calf serum (10% (v/v). The cells were then incubated at 37°C under 5% (v/v) CO2. After the cells had been incubated for 16 h, different concentrations of antibiotics were added into the medium. The concentrations for sodium ampicillin used were as follows: 1, 10, 30, 50, 75, and 100 μg/mL. For ciprofloxacin: 1, 50, 100, 200, 300 and 500 μg/mL. For Rifamycin SV: 0.5, 1, 5, 15, 25 and 50 μg/mL. Finally for clindamiycin phosphate: 1, 15, 25, 40, 50 and 100 μg/mL. For each drug, cells were lysed with a lysis buffer (10% Triton X_100 and 500 mM potassium phosphate buffer, pH 8) according to the method of Foka et al. [Citation16] and after the 2, 4, and 6 h time points the drug was added. The enzyme activity of the supernatant was determined according to Gan et al. [Citation15]. For each drug, a graph of percentage activity versus drug concentration was plotted for different inhibitor concentrations, and the drug concentrations causing 50% inhibition (IC50) were calculated.
Statistical Analysis
Statistical analysis was performed using a Minitab program (PC version) for Windows, version 10.02. Analysis of variance, ANOVA, was used when more than two groups were compared. Data are presented as mean ± SD and values of p < 0.05 were considered significant.
Results and discussion
Paraoxonase is one of the most important enzymes in lipid metabolism, cardiovascular disease and atherosclerosis. In addition to these roles PON1 has also been shown to play a role in the metabolism of pharmaceutical drugs [Citation18]. It was found that PON1 enzyme activity was increased significantly in rabbits that had been fed a hypercholesterolemic diet with four weeks of atorvastatin application [Citation19]. In another study, patients with established cardiovascular disease with high-density lipoprotein cholesterol were treated with rosuvastatin and this resulted in a significant increment of serum PON-1 activity with increasing dose although this was not observed with atorvastatin [Citation17]. Pravastatin was found to increase serum apolipoprotein A1, HDL cholesterol and PON activity [Citation20]. It has also been reported that mouse liver PON activity decreased with some contraceptives while the serum PON activity increased [Citation21]. Furthermore the PON enzyme hydrolyses the diuretic, spironolactone and hypocholesterolaemic drugs [Citation20,Citation22,Citation23]. The effect of different pharmaceutical drugs on paraoxonase enzyme activity can be used in order to clarify PON1 status in the metabolism. So, given the physiological importance of paraoxonase, the study of the effect of antibiotics on paraoxonase enzyme activity is an increasingly important issue for human health.
The different concentrations of the antibiotics used (sodium ampicillin, clindamiycin phosphate, Rifamycin SV, ciprofloxacin) were applied to the growing HepG2 cells and after 2h, 4h and the 6h incubation the cells were lysed using the lysis buffer. The enzyme activity of the supernatant was determined for each drug and the drug concentrations causing 50% inhibition (IC50) were also calculated (Table I). As seen in Table I, Rifamycin SV is the most effective antibiotic for all time intervals while ciprofloxacin has the least inhibitory effect on the PON1 activity of the HepG2 cells. Sodium ampicillin and clindamiycin phosphate both showed their maximum effect following 6h of incubation.
Table 1. The IC50 values of antibiotics on paraoxonase activity in HepG2 cells (2, 4 and 6 h at time points after the drug application).
There are not many studies evaluating the effects of drugs on human serum PON1 enzyme on cells in vitro. According to the study of Gouedard et al. [Citation24] provastatin, simvastatin and fluvastatin caused decrease in PON1 activity and PON1 mRNA levels in the culture medium of HuH7 human hepatoma cells. In contrast; provastatin, simvastatin and fluvastatin and fenofibric acid caused a 50% and 30% increase in PON1 activity and mRNA, respectively. In another in vitro study on isolated lipoproteins, two oxidised metabolites of atorvastatin and a metabolite of gemfibrozil were found to increase HDL-associated PON1 activity [Citation25].
HepG2 cells were used as a model for evaluating the effects of antibiotics on liver hPON1. Although PON1 has both paraoxonase and lactonase activity, the PON1 lactonase activity is lower than the PON2 and PON3 lactonase activities. Paraoxon is the specific subtrate for PON1 and the best method to determine PON1 spesific activity in crude cell extracts is to use paraoxon as the substrate. All antibiotics caused a decrease in paraoxonase activity in the Hep2G cells. This decrease was in a dose-dependent and time-dependent manner for the antibiotics (). Rifamycin SV was the most effective antibiotic due to its low IC50 value (Table I). In our previous studies, we found that Rifamycin SV inhibits PON mouse liver activity at the 4h time point but it didn’t exhibit any significant inhibition effect for the mouse serum PON1 at any of the time points in vivo. Rifamycin SV also didn’t inhibit the human serum PON1 activity in vitro on the purified enzyme. Sodium ampicillin and clindamiycin phosphate exhibited the most potent inhibitory effect at the 6 h time point, although the ciprofloxacin was the most effective at the 4 h time point. It is also reported that sodium ampicillin, ciprofloxacin and clindamycin phosphate significantly inhibited purified human serum PON1 activity in a dose-dependent fashion. These antibiotics also showed different inhibition effects on mouse serum and liver [Citation10].
Conclusion
Paraoxonase (PON1) is an anti-oxidant enzyme carried on high-density lipoproteins (HDL). Although there are a huge number of studies describing the critical role of PON1 in a variety of diseases specifically cardiovascular disease and atherosclerosis, there is only limited information available about the effects of antibiotics on PON1 activity. In this study, we have evaluated that four different classes of antibiotics namely: sodium ampicillin, ciprofloxacin, Rifamycin SV and clindamiycin phosphate, can cause a dose-dependent and a time-dependent decrease in paraoxonase activity in human hepatoma (HepG2) cells.
Declaration of Interest
This work was supported by Balikesir University Research Project (2003/32). This work was carried out in the Balikesir University Research Center of Applied Sciences (BURCAS).
References
- Durringhton PN, Mackness B, Mackness MJ. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol 2001;21:473–480
- Primo Parmo SL, Sorenson RC, Teiber J, La Du BN. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics 1996;33:498–507.
- La Du BN, Aviram N, Billecke S, Navab M, Primo-Parmo S, Sorenson RC, Standiford TJ. On the physiological role(s) of the paraoxonases. Chem Biol Interact 1999;119–120: 379–388.
- Mochizuki H, Scherer SW, Xi T, Nickle DJ, Majer M, Huizenga JJ, Tsui LC, Prochazka M. Human PON2 gene at 7q21.3: cloning, multiple mRNA forms, and missense polymorphisms in the coding sequence. Gene 1998;213:149–157.
- Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowalla J, Furlong CE. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat Genet 1996;14:334–336.
- Aldridge WN. Serum esterases.II. An enzyme hydrolysing diethyl p-nitrophenyl phosphate (E600) and its identity with the A-esterase of mammalian sera. Biochem J 1953;53:117–124.
- Billecke S, Draganov D, Counsell R, Stetson P, Watson C, Hsu C, La Du BN. Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab Dispos 2000;28:1335–1342.
- Jakubowski H. Calcium-dependent human serum homocysteine thiolactone hydrolase. A protective mechanism against protein N-homocysteinylation. J Biol Chem 2000;275:3957–3962.
- Sinan S, Kockar F, Gencer N, Yildirim H, Arslan O. Amphenicol and macrolide derived antibiotics inhibit paraoxonase enzyme activity in human serum and human hepatoma Cells (HepG2) in vitro. Biochemistry (Moscow) 2006;71:46–50.
- Sinan S, Kockar F, Gencer N, Yildirim H, Arslan O. Effects of some antibiotics on paraoxonase from human serum in vitro and from mouse serum and liver in vivo. Biol Pharm Bull 2006;29:1559–1563.
- Sinan S, Kockar F, Arslan O. Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglikozide derived antibiotics. Biochimie 2006;88:565–574.
- Farag SA. Simultaneous liquid chromatographic analysis of the beta_lactam antibiotics cefazolin, cefadroxil, cepalexin, ampicillin, and cephradine in solution. J AOAC Int 1998;81:381–385.
- Sen S, Jaiswal AK, Yanpallewar S, Acharya SB. Anxiogenic potential of ciprofloxacin and norfloxacin in rats. Singapore Med J 2007;48:1028.
- Yu H, Yao Y, Liu Y, Jiao R, Jiang W, Zhao GP. A complex role of Amycolatopsis mediterranei GlnR in nitrogen metabolism and related antibiotics production. Arch Microbiol 2007;188:89–96.
- Gan KN, Smolen A, Eckerson HW, La Du BN. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos 1991;19:100–106.
- Foka P, Irvine SA, Kockar F, Ramji DP. Interleukin-6 represses the transcription of the CCAAT/enhancer binding protein-alpha gene in hepatoma cells by inhibiting its ability to autoactivate the proximal promoter region. Nucleic Acids Res 2003;31:6722–6732.
- Bergheanu SC, Van Tol A, Dallinga-Thie GM, Liem A, Dunselman PH, Van der Bom JG, Jukema JW. Effect of rosuvastatin versus atorvastatin treatment on paraoxonase-1 activity in men with established cardiovascular disease and a low HDL-cholesterol. Curr Med Res Opin 2007;23:2235–2240.
- Tougou K, Nakamura A, Watanabe S, Okuyama Y, Morino A. Paraoxonase has a major role in the hydrolysis of prulifloxacin (NM441), a prodrug of a new antibacterial agent. Drug Metab Dispos 1998;26:355–359.
- Bolayirli IM, Aslan M, Balci H, Altug T, Hacibekiroglu M, Seven A. Effects of atorvastatin therapy on hypercholesterolemic rabbits with respect to oxidative stress, nitric oxide pathway and homocysteine. Life Sciences 2007;81:121–127.
- Malin R, Laaksonen R, Knuuti J, Janatuinen T, Vesalainen R, Nuutila P, Lehtimäki T. Paraoxonase genotype modifies the effect of pravastatin on high-density lipoprotein cholesterol. Pharmacogenetics 2001;11:625–633.
- Kiranoglu S, Sinan S, Gencer N, Kockar F, Arslan O. In Vivo effects of oral contraceptives on paraoxonase, catalase and carbonic anhydrase enzyme activities on mouse. Biol Pharm Bull 2007;30:1048–1051.
- Tomas M, Senti M, Garcia-Faria F, Vila J, Torrents A, Covas M, Marrugat J. Effect of simvastatin therapy on paraoxonase activity and related lipoproteins in familial hypercholesterolemic patients. Arterioscler Thromb Vasc Biol 2000;20:2113–2119.
- Leviev I, James R. Simvastatin increases plasma levels of the antioxidant enzyme paraoxonase by PON1 gene activation. Atherosclerosis 2000;151:41.
- Gouedard C, Koum-Besson N, Barouki R, Morel Y. Opposite regulation of the human paraoxonase-1 gene PON1 by fenofibrate and statins. Mol Pharmacol 2003;63:945.
- Aviram M, Billecke S, Sorenson R, Bisgaier C, Newton R, Rosenblat M, Erogul J, Hsu C, Dunlop C, La Du BN. Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities. Selective action of human paraoxonase allozymes Q and R. Arterioscler Thromb Vasc Biol 1998;18:1617.