Abstract
Some new α-aminomethylenephosphonic acids 1–11 were synthesised and characterised by 1H, 13C, 31P NMR, IR spectroscopy and elemental analysis. The potencies of these compounds to inhibit human erythrocyte acetylcholinesterase (hAChE, EC 3.1.1.7) were studied by a modified Ellman’s method. In addition, the log P values were computed by Hyperchem software. Here, alendronate was used as a reference inhibitor. Results showed that the IC50 values ranged from 9.11 to 28.72 mM. The half maximal inhibitory concentration (IC50) value decreased with an increasing number of carbon atoms of the amine group in compounds 1–5. Also, in most cases, increasing the number of carbon atoms led to enhancement of the toxicity as predicted by the log P values. Using Lineweaver-Burk and Dixon analysis, it was indicated that compounds 1–10 are mixed inhibitors while compound 11 is a coupling or uncompetitive inhibitor. The results showed that the electronic changes have ignorable effects, steric influence is important in some cases, but the lipophilicity parameter is the most significant factor in hAChE inhibition by bisphosphonates.
Introduction
Aminoalkylphosphonic acids, a class of bisphosphonates are structural analogues of amino acids in which the carboxylic group is substituted by a phosphonic or related moiety. Aminobisphosphonic acids and their derivatives have received considerable attention because of their potential biological activity in treating various human diseases such as osteoporosis [Citation1–6], cancer [Citation7–10], Alzheimer’s [Citation11,Citation12], and HIV [Citation13]. Moreover, there are several significant applications for these compounds as plant growth regulators [Citation14], antiparasitics [Citation15], herbicides [Citation16–18], pesticides [Citation19], and antiviral agents [Citation20]. The effect of cholesterol-lowering bisphosphonate drugs (e.g. alendronate, ) on the activity of cholinesterases (ChE) in rat brain and blood has been reported [Citation21]. Also, it has been shown that cholesterol-modifying drugs modulate AChE activity and it is better to use a blood-brain barrier (BBB) penetrating drug [Citation21]. Additionally, the effect of alendronate on lowering cholesterol levels in the central nervous system of rats has been investigated [Citation22].
We have previously evaluated the inhibition potency of many phosphoramidate compounds on human erythrocyte AChE activity [Citation23–26]. The existence of the phosphoryl group in such molecules is important for biological activity. Acetylcholinesterase inhibitors represent the standard treatment of Alzheimer’s disease and cholesterol plays a key role in the development of this disease. Since the cholesterol synthesis may be inhibited by bisphosphonates, investigations on these types of compounds have been developed [Citation21]. The differences in the inhibition potencies of organophosphorus agents are a manifestation of the differing molecular properties of the inhibitors involved in the interaction with the active site of the enzyme. To study these parameters, we have prepared and characterised a series of new α-aminomethylenebisphosphonic acids 1–11 in which the electronic properties of the phosphorus atom and the hydrophobicity of the surrounding substituents have been changed. It should be noted that molecules 1–5 had been reported earlier, but they were only characterised by melting point and CHN elemental analysis [Citation27]. Here, full characterisations were made by 1H, 13C, 31P NMR, IR spectroscopy and elemental analysis. Also, the inhibitory potencies of these compounds were determined using a modified Ellman’s method [Citation28]. Furthermore, the inhibition mechanisms of these compounds were evaluated by obtaining the Lineweaver-Burk and Dixon plots.
Experimental
Materials and methods
All of the compounds were commercial products (for synthesis) from Merck (Tehran, Iran); purified human plasma erythrocyte acetylcholinesterase (hAChE, EC 3.1.1.7) (50 units / 785μl) from Sigma (Tehran, Iran), acetylthiocholine (ATCh) and 5,5′-dithio bis(2-nitrobenzoic acid) (DTNB) from Fluka (Tehran, Iran). 1H, 13C and 31P NMR spectra were recorded on a Bruker (Avance DRS, Germany) 500 MHz spectrometer (Tehran, Iran). 1H, 13C and 31P chemical shifts were obtained in D2O relative to TMS and 85% H3PO4 (as external standard), respectively. IR spectra were obtained using KBr pellets in the range 400-4000 cm−;1 on a Shimadzu (Japan) IR-60 model spectrometer (Tehran, Iran). Elemental analyses were performed using a Heraeus CHNO RAPID instrument (Tehran, Iran) and UV measurements were obtained with a Shimadzu UV-2100 (Japan) spectrophotometer (Tehran, Iran).
Synthesis
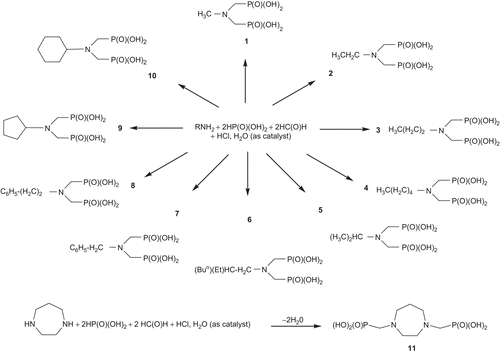
Compounds 1–11 were prepared by a Mannich type reaction according to the procedure previously described [Citation26]. The corresponding amine (1 mmol) was mixed with 37% hydrochloric acid (5 mL), deionised water (5 mL) and phosphorous acid (3 mmol). The mixture was allowed to reflux at 100–120°C for 1.5 h, then paraformaldehyde (4 mmol) was added in small portions over a period of 1 h, and the mixture was refluxed for an additional hour. Removal of the solvents afforded a white powder with a product yield of 89.2%. Its purity was confirmed by NMR measurements and elemental analysis.
N-Methyliminobis(methylenephosphonic acid) 1
Yield 89.2%; mp 223°C; Anal. Calc. for. C3H11NO6P2: C, 16.45; H, 5.06; N, 6.39; found: C, 16.44; H, 5.07; N, 6.39%; 31PNMR (202.46 MHz, D2O), δ (ppm) = 7.81 (t, 2J(P,H) = 12.6 Hz); 1H NMR (500.13 MHz, D2O), δ (ppm) = 3.08 (s, 3 H, CH3), 3.47 (d, 4H, 2J(P,H) = 12.6 Hz, CH2-N); 13C NMR (125.77 MHz, D2O), δ (ppm) = 44.08 (s), 53.82 (dd, 3J(P,C) = 5.082 Hz, 1J(P,C) = 137.5 Hz); IR (KBr), ν (cm−;1): 3425 (w, NH+), 3065 (m, CH), 2850 (m, CH), 2735(m, P-OH), 2335 (m, C-N), 1084-1235 (s, νasPO3), 956-1045 (s, νsPO3), 749 (s, P-C), 540 (s, δP-O), 465 (m), 434 (m).
N-Ethyliminobis(methylenephosphonic acid) 2
Yield 87.5%; mp 205°C; Anal. Calc. for C4H13NO6P2: C, 20.61; H, 5.62; N, 6.01; found: C, 20.60; H, 5.61; N, 6.00%; 31PNMR (202.46 MHz, D2O), δ (ppm) = 8.15 (t, 2J(P,H) = 12.1 Hz); 1H NMR (500.13 MHz, D2O), δ (ppm) = 1.22 (m, 3H, CH3), 3.42 (d, 6H, 2J(P,H) = 12.1 Hz, -CH2-N); 13C NMR (125.77 MHz, D2O), δ (ppm) = 5.67 (s), 47.81 (d, 1J(P,C) = 131.5 Hz, CH2), 49.84 (s); IR (KBr), ν (cm−1): 3450 (m, NH+), 3040 (m, CH), 2755 (m, P-OH), 2585 (m), 1278 (m, C-N), 1107-1221 (s, νasPO3), 972-1038 (s, νsPO3), 709 (m, P-C), 587 (m, δP-O), 527 (w), 416 (m).
N-Propyliminobis(methylenephosphonic acid) 3
Yield 86.3%; mp 183°C; Anal. Calc. for C5H15NO6P2: C, 24.30; H, 6.12; N, 5.67; found: C, 24.29; H, 6.11; N, 5.66%; 31P NMR (202.46 MHz, D2O), δ = 8.14 (t, 2J(P,H) = 12.5 Hz); 1H NMR (500.13 MHz, D2O), δ (ppm) = 0.78 (t, 3H, CH3), 1.61 (m, 2H, CH2), 3.28 (m,2H, CH2), 3.40 (d, 4H, 2J(P,H) = 12.5 Hz, CH2); 13C NMR (125.77 MHz, D2O), δ (ppm) = 7.09 (s), 14.14 (s), 48.5 ( d, 1J(P,C) = 138.00 Hz,CH2), 55.84 (s); IR (KBr), ν (cm−1): 3415 (w, NH+), 3005 (m, CH), 2885 (m, CH), 2765 (m, P-OH), 2640 (m), 1283 (m, C-N), 1145-1230 (s, νasPO3), 945-1014 (s, νsPO3), 771 (w, P-C), 583(s, δP-O), 506(m), 433(m).
N-Pentyliminobis(methylenephosphonic acid) 4
Yield 88.1%; mp 205°C; Anal. Calc. for C7H19NO6P2: C, 30.55; H, 6.96; N, 5.09; found: C, 30.54; H, 6.95; N, 5.09%; 31P NMR (202.46MHz, D2O), δ (ppm) = 8.13 (t, 2J(P,H) = 12.7 Hz); 1H NMR (500.13 MHz, D2O), δ (ppm) = 0.706 (t, 3H, CH3), 1.17 (m, 4H, CH2), 1.60 (m, 2H, CH2), 3.31 (t, 2H, CH2), 3.41 (d, 4H, 2J(P,H) = 12.7 Hz, CH2); 13C NMR (125.77 MHz, D2O), δ (ppm) = 10.26 (s), 18.65 (s), 20.02 (s), 24.86(s), 48.30 (dd, 3J(P,C) = 4.00 Hz, 1J(P,C) = 138.00 Hz,CH2), 54.40 (s); IR (KBr), ν (cm−1): 3420 (w, NH+), 2950 (m, CH), 2750 (m, P-OH), 2640 (m), 2500 (m), 1271 (m, C-N), 1150-1212 (s, νasPO3), 940-1014 (s, νsPO3), 775 (w, P-C), 583 (s, δP-O), 517 (m), 484 (m).
N-Isopropyliminobis(methylenephosphonic acid) 5
Yield 89.8%; mp 292°C (decomposed); Anal. Calc. for C5H15NO6P2: C, 24.30; H, 6.12; N, 5.67; found: C, 24.30; H, 6.12; N, 5.68%; 31P NMR (202.46 MHz, D2O), δ (ppm) = 8.55 (t, 2J(P,H) = 13.0 Hz); 1H NMR (500.13 MHz, D2O), δ (ppm) = 1.21 (d, 6 H, 3J(P,H) = 6.1Hz, CH3), 3.98 (m, -CH), 3.34 (d, 4H, 2J(P,H) = 13.0 Hz, -CH2-N); 13C NMR (125.77 MHz, D2O), δ (ppm) =13.05 (s), 45.62 (dd, 3J(P,C) = 4.1 Hz, 1J(P,C) = 136.8 Hz, CH2), 56.63 (s); IR (KBr), ν (cm−1): 3420 (w,NH+), 3010 (m,CH), 2730 (m, P-OH), 2605 (m), 1258 (m, C-N), 1120-1220 (s, νasPO3), 948-1004 (s, νs PO3), 781 (w, P-C), 555 (m, δP-O), 504 (m), 453 (w).
N-2-Ethyl hexyliminobis(methylenephosphonic acid) 6
Yield 88.2%; mp 216°C; Anal. Calc. for C10H25NO6P2: C, 37.86; H, 7.94; N, 4.42; found: C, 37.85; H, 7.94; N, 4.41%; 31PNMR (202.46 MHz, D2O), δ (ppm) = 7.59 (t, 2J(P,H) = 12.4 Hz); 1H NMR (500.13 MHz, D2O), δ (ppm) = 0.88 (t, 6H, J(P,H) = 12.6Hz, CH3), 1.29 (m, 4H, CH2),1.40 (m, 4H, CH2), 1.87 (m, 1H, CH), 3.46 (m, 2H, CH2), 3.54 (d, 4H, 2J(P,H)= 12.4Hz, CH2 ); 13C NMR (125.77 MHz, D2O), δ = 8.73 (s), 12.81 (s), 21.75 (s), 22.31(s), 26.87(s), 28.80 (s), 33.81 (s), 52.01 (d, 1J(P,C) = 135.2 Hz, CH2), 61.02 (s); IR (KBr), ν (cm−1): 3405 (w, NH+), 2930 (s, CH), 2735 (m, P-OH), 2585 (m), 2295 (m), 1254 (m,C-N), 1134-1225 (s, νasPO3), 933-1009 (s, νsPO3), 763 (w, P-C), 570 (s, δP-O), 508 (m), 444 (m).
N-Benzyliminobis(methylenephosphonic acid) 7
Yield 88.8%; mp 247°C (decomposed); Anal. Calc. for C9H15NO6P2: C, 36.62; H, 5.12; N, 4.74; found: C, 36.61; H, 5.12; N, 4.73%; 31PMR (202.46MHz, D2O), δ (ppm) = 6.20 (t, 2J(P,H) = 12.1 Hz); 1H NMR (500.13 MHz, D2O), δ (ppm) = 3.06 (d, 4H, 2J(P,H) = 12.1 Hz, CH2), 4.20 (s, 2H, CH2), 7.31-7.48 (m, 5H, C6H5); 13C NMR (125.77MHz,D2O), δ (ppm) = 50.61 (dd, 3J(P,C) = 5.1 Hz, 1J(P,C) = 143.6 Hz, CH2), 59.99 (s), 127.93 (s),128.25 (s),130.06 (s), 135.18 (s); IR (KBr), ν (cm−1): 3405 (w, NH+), 2965 (s, CH), 2845 (s, CH),2720 (s, P-OH), 2545 (s), 1284 (m, C-N), 1166-1229 (s, νasPO3), 935-1008 (s, νsPO3), 746 (s, P-C), 573(s, δP-O), 485 (m), 421 (m).
N-2-Phenyl ethyliminobis(methylenephosphonic acid) 8
Yield 89.6%; mp 255°C (decomposed); Anal. Calc. for C10H17NO6P2: C, 38.85; H, 5.54; N, 4.53; found: C, 38.84; H, 5.54; N, 4.52%; 31P NMR (202.46 MHz, D2O), δ (ppm) = 7.27 (t, 2J(P,H) = 12.5 Hz); 1H NMR (500.13 MHz, D2O), δ (ppm) = 3.07 (t, 2H, CH2 ), 3.52 (d, 4H, 2J(P,H) = 12.5 Hz, CH2), 3.68 (d, 2H, 2J(P,H) = 13.0 Hz, CH2), 7.29-7.33(m, 5H, C6H5); 13C NMR(125.77 MHz, D2O), δ (ppm) = 26.7 (s), 48.7 (d, 1J(P,C) = 136.00 Hz,CH2), 55.46 (s), 124.62 (s), 126.25 (s), 126.33 (s), 133.15 (s); IR (KBr), ν (cm−1): 3415 (w, NH+), 3040 (m, CH), 2870 (m, CH), 2775 (m, P-OH), 2590 (m), 1450 (w), 1237 (m,C-N), 1161-1200 (s, νasPO3), 940-1004 (s, νsPO3), 744 (w, P-C), 575 (m, δP-O), 493 (m), 405 (m).
N-Cyclopentyliminobis(methylenephosphonic acid) 9
Yield 88.7%; Anal. calcd for C7H17NO6P2: C, 30.78; H, 6.27; N, 5.13; found: C, 30.77; H, 6.27; N, 5.12%; 31PNMR (202.46MHz, D2O): δ (ppm) = 8.60 (t, 2J(P,H) = 13 Hz); 1H NMR (500.13MHz, D2O), δ (ppm) = 1.54 (m, 2H, CH2), 1.66 (m, 4H, CH2), 2.07 (m, 2H, CH2), 3.51 (d, 4H, 2J(P,H) = 13 Hz, CH2), 4.1 (m, 1H, CH); 13CNMR (125.77MHz, D2O), δ (ppm) = 21.09 (s), 25.12 (s), 46.72 (d, 1J(P,C) = 137.4 Hz,CH2), 65.97 (s); IR (KBr), ν (cm−;1): 3435 (w, NH+), 2980 (m, CH), 2755 (m, P-OH), 2295 (m), 1274 (m, C-N), 1078–1192 (s, νasPO3), 913-1015 (s, νsPO3), 759 (w, P-C), 521 (s, δP-O), 461 (m), 411 (m).
N-Cyclohexyliminobis(methylenephosphonic acid) 10
Yield 89.0%; mp 240°C (decomposed); Anal. Calc. for C8H19NO6P2: C, 33.46; H, 6.67; N, 4.88; found: C, 33.45; H, 6.66; N, 4.88%; 31P NMR (202.46 MHz, D2O), δ = 8.47 (t, 2J(P,H) = 13.0 Hz); 1H NMR (500.13MHz, D2O), δ (ppm) = 1.31-1.48 (m, 6H, CH2), 1.87-2.02 (m, 4H, -CH2), 3.46(d, 2J(P,H) = 13.0 Hz, CH2), 3.56 (m, 1H, CH). 13C NMR (125.77MHz, D2O), δ (ppm) = 21.62 (s), 21.79(s), 23.60 (s), 46.22 (dd, 3J(P,C) = 4.00 Hz, 1J(P,C) = 136.00 Hz, CH2), 63.86; IR (KBr), ν (cm−1): 3395 (w, NH+), 3000 (m, CH), 2870 (m, CH), 2735 (m, P-OH), 2570 (m), 1420 (w), 1259 (m, C-N), 1173-1231 (s, νasPO3), 928-1000 (s, νsPO3), 784 (w, P-C), 595 (m), 552 (m), 509 (m).
Homopiperazine-1,4-bis(methylenephosphonic acid) 11
Yield 89.2%; mp 236°C (decomposed); Anal. Calc. for C7H18N2O6P2: C, 29.17; H, 6.30; N, 9.72; found: C, 29.16; H, 6.31; N, 9.71%; 31P NMR (202.46 MHz, D2O), δ (ppm) = 6.80 (t, 2J(P,H) = 12.6 Hz); 1H NMR (500.13 MHz, D2O), δ = 2.27 (s, 2H, CH2), 3.38 (d, 4H, 2J(P,H) = 12.6 Hz, CH2), 3. 63 (s, 4H, CH2), 3.90 (s, 4H, CH2); 13C NMR (125.77 MHz, D2O), δ (ppm) = 19.81 (s), 49.74 (s), 53.21 (d, 1J(P,C) = 136.0 Hz, CH2), 55.23; IR (KBr), ν (cm−1): 3420 (s, NH+), 2985 (s, CH), 2875 (s, CH), 2750 (s, P-OH), 2625 (s), 1461 (m), 1235 (m, C-N), 1167 (s, νasPO3), 938-1067 (s, νsPO3), 780 (m, P-C), 537 (m), 451 (m).
Measurement of AChE activity
The activity of hAChE was determined by a modified Ellman’s method [Citation28], the methodology described by Ellman follows the hydrolysis of acetylthiocholine, by indirect means, that is, the rate of increase of absorbance at 412 nm, after chemical reaction between the residue of thiocholine (resulting from hydrolysis) and the DTNB particle in the solution. This method is based on evaluation of the rate of enzymatic hydrolysis of acetylthiocholine substrate with 5,5′-dithiobis-(2-nitrobenzoic) acid as the thiol group indicator (interaction of this acid with thiocholine results in the formation of 5-mercapto-2-nitrobenzoic acid with maximum absorption at 412 nm). The reaction was carried out at 37°C in 70 mM phosphate buffer (Na2HPO4/NaH2PO4, pH 7.4) containing the enzyme (20 µl volume, diluted 100 times in phosphate buffer, pH 7.4), DTNB (5,5-dithiobis(2-nitrobenzoic acid)) (10−;4M concentration) and ATCh (1.35 × 10−;4M concentration). The absorbance change at 37°C was monitored with the spectrophotometer at 412 nm for 3 min. and three replicates were run in each experiment. In the absence of inhibitor, the absorbance change was directly proportional to the enzyme activity.
Human acetylcholinesterase inhibition experiments
The reaction mixtures for determination of the half maximal inhibitory concentration (IC50) values, the median inhibitory concentration, Consisted of DTNB solution, 50μl; × μl (5–250); acetylthiocholine (ATCh) solution, 30 μl; phosphate buffer (890-x) μl; hAChE solution, 30 μl. The final concentrations (M) of DTNB, ATCh, and inhibitors 1–11 were: 10−;4, 1.35 × 10−;4, (0.91 × 10−;3≤x≤22.8 × 10−;3) 1, (4.3 × 10−;3≤x≤17.2 × 10−;3) 2, (4.0 × 10−;3≤x≤20.2 × 10−;3) 3, (3.6 × 10−;3≤x≤16.7 × 10−;3) 4, (4.9 × 10−;3≤x≤24.3 × 10−;3) 5, (3.2 × 10−;3≤x≤15.8 × 10−;3) 6, (1.4 × 10−;3≤x≤13.6 × 10−;3) 7, (2.6 × 10−;3≤x≤16.2 × 10−;3) 8, (2.9 × 10−;3≤x≤18.3 × 10−;3) 9, (1.6 × 10−;3≤x≤13.1 × 10−;3) 10, (1.4 × 10−;3≤x≤20.8 × 10−;3) 11, respectively. The reaction mixtures for determination of the inhibition mechanism were: DTNB (the same as above) and ATCh substrate (10≤x≤40 μl); a solution of enzyme plus inhibitors (inhibitors concentration were adjusted to give about 50% of hAChE inhibition) 150 μl; phosphate buffer, (820-x) μl. A control solution containing all of above materials except inhibitor was used to determine the activity of the enzyme.
Enzymatic measurements
The plot of VI/V0 (VI and V0 are the activity of the enzyme in the presence and absence of inhibitors, respectively) against log [I], where [I] is the inhibitor concentration, gave the IC50 values of compounds 1–11 as shown in and . Km and Vm were obtained in the absence and presence of inhibitor from double reciprocal Lineweaver-Burk plots [Citation29]. The IC50 (mM), Km (M) and Vmax (M min−;1) values are given in .
Table 1. Selected spectroscopic data for compounds 1–11.
Table 2. The enzymatic data for hAChE enzyme, alendranate and compounds 1–11.
Enzyme inhibition mode
The double reciprocal, or Lineweaver-Burk, plot is the most straightforward method of diagnosing inhibitor modality [Citation30]. The kinetic constants Km and Vmax can be determined in the absence and presence of inhibitor from the slope and intercept values of the linear fit of the data in a Lineweaver-Burk and Dixon plot ( and ).
Results and discussion
Spectroscopic study
In this work, we have prepared and characterised some new α-aminomethylphosphonic acids 1–11 () to study their inhibition potencies against hAChE enzyme. The selected spectroscopic data of the compounds are listed in . The 1H NMR spectra of compounds 1-11 showed a doublet peak for methylene moiety with a coupling constant 2J(PCH) = 12.1–13 Hz, which results from the coupling of methylene protons with the phosphorus atom. The 31P{1H} NMR spectra indicated a singlet in the range of 6.20–8.6 ppm for the phosphorus atom, which splits into a triplet in 31P NMR spectra due to coupling of the phosphorus atoms with neighboring CH2 protons. The formation of aminomethylphosphonic acid moieties was signaled by the appearance of the triplet [Citation26]. The 31P NMR spectra exhibited that δ(31P) shifts to down fields from compound 1 to compounds 2–4. Interestingly, in compounds 3 and 5 which have same carbon atoms of alkyl groups, the phosphorus atom in 3 is more negative than in 5, due to the steric effects. Also, the δ(31P) in 7 is at up field relative to that of 8. This trend can also be observed for compounds 9 and 10. These results revealed that in similar compounds, increasing the number of CH2 moieties produced a more positive phosphorus atom. The 13C NMR spectra displayed a doublet peak for the methylene carbon atom with a 1J(P,C) of 131.5–143.6 Hz. Furthermore, a doublet peak appeared for the CH2 carbon atom with a 3J(P,C) of 4–5.1 Hz. IR spectra of the bisphosphonates 1–11 indicated the ν(NH+) in the range of 3395–3450 cm−;1 due to the resonance interaction between nitrogen and oxygen atom of the P(O) group as follows:
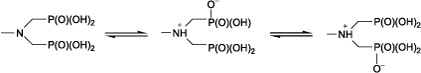
The νs(PO3) values are observed at lower frequencies than νas(PO3) amounts. In compounds 3 and 5 with the same number of carbon atoms in the alkyl chain, ν(P-C) is stronger in compound 5 that has an isopropyl group. A comparison of the analogous compounds 7, 8 and 9, 10 showed that addition of a CH2 moiety enhances the ν(P-C).
Enzymatic study
Lipophilicity effect
One of the most exciting fields of modern enzymology is the application of enzyme inhibitors as drugs in human and veterinary medicine [Citation31]. In the search for potent and selective inhibitors of the enzyme human acetylcholinesterase (hAChE, EC 3.1.1.7), compounds 1–11 were prepared and their ability to inhibit hAChE were examined by a modified Ellman’s method [Citation28]. Selected enzymatic data of hAChE enzyme, alendronate and compounds 1–11 are presented in . Alendronate was used as a reference inhibitor. It has been argued that the inhibitory process of organophosphorus inhibitors is dependent upon the charge on the phosphorous atom, stereochemistry, reactivity, substituents on the phosphorous atom and the leaving group [Citation32]. Thus, we examined the effects of ring size, side chain and number of carbon atoms on enzyme activity.
In addition, the log P(o/w) values, the octanol–water partition coefficient, representing the lipophilicity of a molecule, were computed by Hyperchem 7.0 software (Tehran, Iran). Waterhouse determined the lipophilicity and its use as a predictor of blood–brain barrier (BBB) penetration [Citation33]. He noted that there is often a parabolic relationship between measured lipophilicity and in vivo brain penetration of drugs, where those that are moderate in lipophilicity exhibit the highest uptake.
Ghadimi et al. considered three electronic, steric and lipophilicity parameters as significant possible factors affecting the inhibition potencies of phosphoramide compounds, and found that liphophilicity has the main influence on IC50 (in agreement with the QSAR equations) [Citation34]. Here, the greatest IC50 values were observed for compounds 1 and 11 (∼28 mM), while the lowest values were for 5, 6 (∼9 mM). The reference inhibitor (alendronate) has a relatively high IC50 (27.45 mM) with a small logP value (0.1) and it can be seen that its potency is close to those of compounds 1 and 11. That is, the least toxic compounds (that are similar to our reference drug) were 1and 11 while the other inhibitors are much more toxic. Thus, compounds 1 and 11 may be good alternatives for the alendronate drug.
It is interesting that increasing the number of carbon atoms in a linear alkyl chain in the compounds 1–4 leads to a decrease in the IC50 values (and increasing the log P amounts). The IC50 value of compound 7 is greater than that of compound 8 even though they are similar compounds. Therefore, it may be said that addition of one CH2 group to the alkyl chain increases the toxicity. A comparison of compounds 9 and 10 revealed that the IC50 values were dependent on ring size, i.e. by increasing the ring size, the inhibition power is enhanced.
Steric effect
Compounds 3 and 5 with the same number of carbon atoms have nearly the same calculated log P values, but they have different effects on hAChE enzyme (the IC50 value of 5 (3.56 mM) is less than that of 3). This effect is perhaps due to the effect of the alkyl side chain against the linear chain (steric effect). Ashani et al. indicated that inhibition potency is influenced by a combined contribution of the electropositivity of the phosphorous atom, size and charge of the O-alkyl or S-alkyl substituents [Citation35]. Thus, the greatest activity is attributed in part to the hydrophobic moieties that can offer favourable interactions with the enzyme which enables a better alignment of the electropositive phosphonyl centre toward nucleophilic attack by the active site serine [Citation35].
The structure of compound 11 is different from those of 1–10. This molecule has two phosphorus atoms (similar to other inhibitors) but three carbon atoms in an aliphatic ring connected to the CH2 groups. The IC50 value for 11 is the greatest one with the least log P=0.22 and δ(31P)=6.8 ppm. If it is assumed that the interaction of hAChE enzyme active site occurs through the P-O bonds, the electronic effects as well as steric ones must be considered simultaneously. Since δ(31P) of compounds 1–11 do not display significant differences, this effect can be attributed to the steric factors. Thus, the type of substituents, their bulkiness and the dihedral angles are important factors for toxicity. That is, the geometry of molecule 11 is not very suitable for the nucleophilic attack of the serine active site.
Enzyme inhibition mode
The relationship between inhibitory potency and substrate concentration was studied initially in a classical way in order to determine the mode of inhibition [Citation36]. Double reciprocal plots for compounds 1–10 yielded increasing slopes and intercepts, indicating the inhibitors are mixed ones. Mixed inhibition refers to an inhibitor which displays fixed but uneven binding affinity for both the free enzyme and the enzyme-substrate binary complex. The reciprocal form of the velocity equation for evaluating noncompetitive or mixed inhibitors is: 1/ν=Km/Vmax(1+ [I]/Ki)1/[S] + 1/Vmax(1+[I]/αKi), (here α is finite but not equal to 1). As indicated by the above equation, both the slope and the y-intercept of the double-reciprocal plot will be affected by the presence of a noncompetitive inhibitor. The pattern of lines revealed that the plots for varying inhibitor concentrations depend on the value of α. In compounds 7–10, the lines intersect at a value of 1/[S] less than zero and a value of 1/ν of greater than zero () and α exceeds 1 (α>1). In alendronate and compounds 1–6, the lines intersect below the x and y axes at negative values of 1/[S] and 1/ν, α<1 ( and ). Bukowska and Hutnik revealed mixed inhibition mechanism for the inhibition of erythrocyte acetylcholinesterase by 2,4-dichlorophenoxyacetic acid [Citation37].
In compound 11, the slope of the double-reciprocal plot is independent of inhibitor concentration and the y-intercept increases steadily with an increasing inhibitor concentration (). The straight line pattern is the characteristic signature of a coupling or uncompetitive inhibitor. Uncompetitive inhibitors bind exclusively to the ES complex, rather than to the free enzyme. The apparent effect of an uncompetitive inhibitor is to decrease Vmax and to actually decrease Km. The reciprocal form of the velocity equation for an uncompetitive inhibitor is given by 1/ν=(Km/Vmax× 1/[S]) + 1/Vmax(1+[I]/αKi).
In and , the y-intercept = 1/Vm, the x-intercept = -1/Km and the regression coefficients were between 0.96 and 0.99. The Km and Vmax values of the bisphosphonates and hAChE were obtained under experimental conditions (). The Kmapp values were between 0.035 and 0.057 mol L−;1 and the Vmaxapp values ranged from 7.38 to 14.39 mol L−;1 min−;1. Also, the Km and Vmax values for enzyme were 0.098 mol L−;1 and 15.77 mol L−;1min−;1, respectively.
Conclusion
In summary, a series of new α-aminomethylene-bisphosphonates 1–11 were synthesised and characterised to study their inhibitory potencies against the hAChE enzyme. Our observations confirm the importance of lipophilicity, steric and electronic factors as significant determinants of the inhibitory potency. These factors can be experimentally estimated from the log P, bulk of molecule and δ(31P) values. It is deduced from the results that the electronic changes have ignorable effects, steric influence is important in some cases, but the lipophilicity parameter is the most significant factor in hAChE inhibition by bisphosphonates. That is, in most of the cases, increasing the number of carbon atoms leads to increasing the toxicity of compounds, as predicted by the log P values.
Acknowledgement
The financial support of this work by Research Council of Tarbiat Modares University is gratefully acknowledged.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science 2000; 289:1508–1514.
- Hirabayashi H, Sawamoto T, Fujisaki J, Tokunaga Y, Kimura S, Hata T. Dose-dependent pharmacokinetics and disposition of bisphosphonic prodrug of diclofenac based on osteotropic drug delivery system (ODDS). Biopharm Drug Dispos 2002; 23:307–315.
- Vieillard MH, Maes JM, Penel G, Facon T, Magro L, Bonneterre J, Cortet B. Thirteen cases of jaw osteonecrosis in patients on bisphosphonate therapy. Joint Bone Spine 2008; 75:34–40.
- Dannemann C, Grätz KW, Riener MO, Zwahlen RA. Jaw osteonecrosis related to bisphosphonate therapy: A severe secondary disorder. Bone 2007; 40:828–834.
- Widler L, Jaeggi KA, Glatt M, Muller K, Bachmann R, Bisping M, Born A-R Cortesi, R, Guiglia G, Jeker H, Klein R, Ramseier U, Schmid J, Schreiber G, Seltenmeyer Y, Green JR.Highly Potent Geminal Bisphosphonates. From Pamidronate Disodium (Aredia) to Zoledronic Acid (Zometa). J Med Chem 2002; 45:3721–3738.
- Houghton TJ, Tanaka KSE, Kang T, Dietrich E, Lafontaine Y, Delorme D, Ferreira SS, Viens F, Arhin FF, Sarmiento I, Lehoux D, Fadhil I, Laquerre K, Liu J, Ostiguy V, Poirier H, Moeck G, Parr Jr TR, Far AR. Linking bisphosphonates to the free amino groups in fluoroquinolones: preparation of osteotropic prodrugs for the prevention of osteomyelitis. J Med Chem 2008; 51:6955–6969.
- Coleman RE. Optimising treatment of bone metastases by aredia and zometa. Breast Cancer 2000; 7:361–369.
- Simoni D, Gebbia N, Invidiata FP, Eleopra M, Marchetti P, Rondanin R, Baruchello R, Provera S, Marchioro C, Tolomeo M, Marinelli L, Limongelli V, Novellino E, Kwaasi A, Dunford J, Buccheri S, Caccamo N, Dieli F. Design, synthesis, and biological evaluation of novel aminobisphosphonates possessing an in vivo antitumor activity through a γδ-T lymphocytes-mediated activation mechanism. J Med Chem 2008; 51:6800–6807.
- Das H, Wang L, Kamath A, Bukowski JF. V{gamma}2V{delta}2 T-cell receptor-mediated recognition of aminobisphosphonates. Blood 2001; 98:1616–1618.
- Kiran YB, Reddy CD, Gunasekar D, Reddy CS, Leon A, Barbosa LCA. Synthesis and anticancer activity of new class of bisphosphonates/phosphanamidates. European J Med Chem 2008; 43:885–892.
- Makhaeva GF, Aksinenko AY, Sokolov VB, Serebryakova OG, Richardson RJ. Synthesis of organophosphates with fluorine-containing leaving groups as serine esterase inhibitors with potential for Alzheimer disease therapeutics. Bioorganic & Medicinal Chemistry Letters 2009; 19:5528–5530.
- Grutzendler J, Morris JC. Cholinesterase Inhibitors for Alzheimer’s Disease. Drugs 2001; 61:41–52.
- McKenna CE, Kashemirov BA, Rozé CN. Carbonylbisphosphonate and (diazomethylene)bisphosphonate analogues of AZT 5′-diphosphate. Bioorg Chem 2002; 30:383–395.
- Kafarski P, Lejczak B, Forlani G, Gancarz R, Torreilles C, Grembecka J, Ryczek A, Wieczorek PJ.Herbicidal derivatives of sminomethylenebisphosphonic acid. Part III. Structure-Activity Relationship. Plant Growth Regul 1997; 16:153–158.
- Martin MB, Sanders JM, Kendrick H, de Luca-Fradley K, Lewis JC, Grimley JS, Van Brussel EM, Olsen JR, Meints GA, Burzynska A, Kafarski P, Croft SL, Oldfield E.Activity of bisphosphonates against Trypanosoma brucei rhodesiense. J Med Chem 2002; 45:2904–2914.
- Obojska A, Berlicki L, Kafarski P, Lejczak B, Chicca M, Forlani G.Herbicidal pyridyl derivatives of aminomethylene-bisphosphonic acid inhibit plant glutamine synthetase. J Agric Food Chem 2004; 52:3337–3344.
- Kishore GM, Shah DM. Amino acid biosynthesis inhibitors as herbicides. Annu Rev Biochem 1988; 57:627–663.
- Mori I, Iwasaki G, Hayakawa KJ. Rational design of a new herbicide by inhibition of histidine biosynthesis Design and synthesis of inhibitors of imidazole glycerol phosphate dehydratase (IGPD). Yuki Gosei Kagaku Kyokaishi/ J Synth Org Chem Jpn 1996; 54:62–72.
- Kafarski PLB, Forlani G. Biodegradation of pesticides containing carbon-to-phosphorus bond. In: Hall JC, Hoagland RE, Zablotowicz RM., Eds. Pesticide Biotransformation in Plants and Microorganisms, American Chemical Society: ACS Symposium Series 777; Washington DC, 2001: 145–163.
- Chen M-H, Chen Z, Song B-A, Bhadury PS, Yang S, Cai X-J, Hu D-Y, Xue W, Zeng S. Synthesis and Antiviral Activities of Chiral Thiourea Derivatives Containing an α-Aminophosphonate Moiety. J Agric Food Chem 2009; 57: 1383–1388.
- Cibičková L, Palička V, Cibiček N, Čermáková E, Mičuda S, Bartošová L, Jun D. Differential effects of statins and alendronate on cholinesterases in serum and brain of rats. Physiol Res 2007; 56:765–770.
- Cibičková Ľ, Hyšpler R, Cibiček N, Čermáková E, Palička V. Alendronate lowers cholesterol synthesis in the central nervous system of rats–a preliminary study. Physiol Res 2009; 58:455–458.
- Golivand K, Abdollahi M, Mojahed F, Madani Alizadegan A, Dehghan G. Acetylcholinesterase/butyrylcholinesterase inhibition activity of some new carbacylamidophosphate deriviatives. J Enzyme Inhib Med Chem 2009; 24:566–576.
- Golivand K, Mojahed F, Salehi M, Naderimanesh H, Khajeh K. Synthesis, characterization and inhibitory potency of two oxono and thiono analogues of phosphoramidate compounds on acetylcholinesterase. J Enzyme Inhib Med Chem 2006; 21:521–525.
- Golivand K, Madani Alizadegan A, Anraki F, Khajeh K, Naderimanesh H, Bijanzadeh H. Anticholinesterase activity of some major intermediates in carbacylamidophosphate synthesis: Preparation, spectral characterization and inhibitory potency determination. J Enzyme Inhib Med Chem 2006; 21:105–111.
- Golivand K, Shariatinia Z, Khajeh K, Naderimanesh H. Syntheses and spectroscopic characterization of some phosphoramidates as reversible inhibitors of human acetylcholinesterase and determination of their potency. J Enzyme Inhib Med Chem 2006; 21:31–35.
- Moedritzer K, Irani RR. The Direct synthesis of α-aminomethylphosphonic acids. Mannich-type reactions with orthophosphorous acid. J Org Chem 1966; 31:1603–1607.
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7:88–90.
- Kitz R, Wilson IB. Esters of methanesulfuric acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem 1962; 237:3245–3249.
- Copeland RA. Enzymes, a Practical Introduction to Structure, Mechanism, and Data Analysis. 2nd ed. New York: John Wiley & Sons, 2000 ; p109–145.
- Ariёns EJ. Drug Design. New York: Academic press, 1971: Vol II, p 129–149.
- Thompson CM, Suarez AI, Rodriguez OP. Synthesis and 31P chemical shift identification of tripeptide active side models that represent human serum acetylcholinesterase covalently modified at serine by certain organophosphates. Chem Res Toxicol 1996; 9:1325–1332.
- Waterhouse RN. Determination of lipophilicity and its use as a predictor of blood–brain barrier penetration of molecular imaging agents. Mol Imaging and Biol 2003; 5:376–389.
- Ghadimi S, Ebrahimi ASV, Pourayoubi M, Samani KA. Structure-activity study of phosphoramido acid esters as acetylcholinesterase inhibitors. J Enzyme Inhib Med Chem 2008; 23:556–561.
- Ashani Y, Segev O, Balan A. The effect of fluoride on the scavenging of organophosphates by human butyrylcholinesterase in buffer solutions and human plasma. Toxicol Appl Pharmacol 2004; 194:90–99.
- Fukuto TR. Mechanism of action of organophosphorus and carbamate insecticides. Environ health persp 1990; 87:245–254.
- Bożena Bukowska Katarzyna, Hutnik. 2,4-D and MCPA and their derivatives: Effect on the activity of membrane erythrocytes acetylcholinesterase (in vitro). Pestic Biochem Phys 2006; 5:174–180.
![Figure 1. The plot of VI/V0 against log ([I], µM) for inhibitors 1–8. VI and V0 are the enzyme activity (OD min−;1) and [I] is the inhibitor concentration (µM).](/cms/asset/183f86a8-813e-41a9-a69a-918964872860/ienz_a_469708_f0002_b.gif)
![Figure 2. The plot of VI/V0 against log ([I], µM) for inhibitors 9–11 and alendronate. VI and V0 are the enzyme activity (OD min−;1) and [I] is the inhibitor concentration (µM).](/cms/asset/0f373882-cca5-41ec-af43-e195e1cc3a92/ienz_a_469708_f0003_b.gif)
![Figure 3. The plot of 1/[V]against 1/[S] for inhibitors 1–5, alendronate and Enzyme activation without inhibitor. [V] is the enzyme activity (OD min−;1) and [S] is the substrate concentration (µM).](/cms/asset/40522644-5f8d-45d9-85b4-0c592524bb3e/ienz_a_469708_f0004_b.gif)
![Figure 4. The plot of 1/[V]against 1/[S] for inhibitors 6-11 and Enzyme activation without inhibitor. [V] is the enzyme activity (OD min−;1) and [S] is the substrate concentration (µM).](/cms/asset/48152e12-b52a-4b5b-a5d7-74840769e2f4/ienz_a_469708_f0005_b.gif)