Abstract
Novel bis cyclohexenone ester derivatives 14–19 were synthesized and characterized by their spectral data. In vitro microbiological evaluations were carried out for all the novel compounds 14–19 against clinically isolated bacterial and fungal strains. Compounds 15, 16, 18 against Staphylococcus aureus, 14, 15 against β-Haemolytic streptococcus, 15, 19 against Micrococcus luteus, 17, 18 against Salmonella typhii, 14, 17 against Shigella flexneri, 15 against Escherichia coli, 16 against Pseudomonas aeruginosa, 15, 18, 19 against Klebsiella pneumonia exhibited potent antibacterial activity at an minimum inhibitory concentration (MIC) value of 6.25 μg/ml, whereas compound 16 against Aspergillus flavus, 17 against A. niger, 16, 18 against Mucor indicus, 15, 17–19 against Microsporum gypseum revealed excellent antifungal activity at an MIC value of 6.25 μg/ml.
Introduction
Literature survey reveals the value of chalcones as potent biologically active compounds. An important feature of chalcones from the chemical point of view is the ability to act as activated unsaturated systems in conjugated addition reactions of carbanions in the presence of basic catalystsCitation1,Citation2. This type of reaction may be exploited for the preparation of 3,5-diaryl-6-carbethoxycyclohexenones via Michael addition of ethyl acetoacetate. The mentioned cyclohexenones are effective synthons in some projected synthesis of structurally diverse heterocyclesCitation3–5. The motive for the preparation of cyclic chalcones is because they are excellent carriers of different types of biological activityCitation6,Citation7. Cyclohexenone carboxylates have known to possess effective biological activity, such as anti-HIVCitation8,Citation9, anticancerCitation10, antifungalCitation11,Citation12, antitumorCitation13, anticonvulsantCitation14,Citation15, and antitubercularCitation16 activity. Novel cyclohexenoic long chain fatty alcohols are used in the treatment of neurological disordersCitation17. Ambuic acid, a highly functionalized cyclohexenone exhibits antifungal activityCitation18.
The synthesis of 6-acetyl-5-aryl-2-cyclohexenones, 5-aryl-6-carbethoxy-2-cyclohexenones substituted in position 4 with a 2-thienyl moiety, 5-aryl-6-carbethoxy-3-(2-thienyl)-2-cyclohexenonesCitation19,Citation20 is already described. In recent years, there has been a great deal of interest in exploiting more than one proximal functional group for designing novel structures capable of performing a variety of functions. In view of the above and as part of the ongoing research on antimicrobialsCitation3,Citation21–23, we planned to synthesize highly functionalized bis cyclohexenone ester derivatives, whose various chemical functions commend them as valuable intermediates in subsequent reactions and to study their biopotentiality against clinically isolated bacterial and fungal strains.
Experimental
Chemistry
The progress of the reaction is monitored by thin layer chromatography (TLC) analysis. All the reported melting points are taken in open capillaries and are uncorrected. IR spectra are recorded in KBr (pellet forms) on a Thermo Nicolet-Avatar-330 FT-IR spectrophotometer and important absorption values (cm−1) alone are listed. 1H and 13C NMR spectra are recorded at 400 and 100 MHz, respectively, on Bruker Avance II 400 NMR spectrometer using DMSO-d6 as solvent. Two-dimensional HOMOCOR and HSQC spectra are recorded at Bruker DRX 500 NMR spectrometer. The ESI +ve MS spectra are recorded on a Bruker Daltonics LC-MS spectrometer. Satisfactory microanalysis data are obtained on Carlo Erba 1106 CHN analyzer.
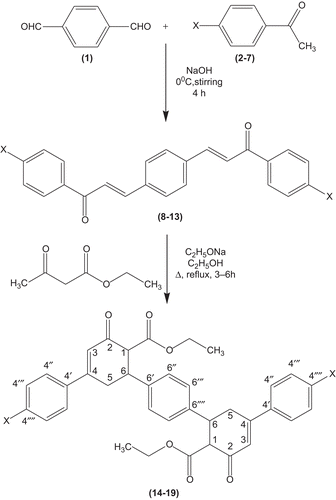
By adopting the literature precedentCitation24, bis chalcone derivatives 8–13 are prepared.
General procedure for the synthesis of bis cyclohexenone ester derivatives 14–19
To a solution of sodium ethoxide (0.001 mol) in 30 ml of absolute ethanol, freshly distilled ethyl acetoacetate (0.01 mol) and respective bis chalcones 8–13 (0.01 mol) in absolute ethanol (40 ml) was mixed. This mixture was refluxed in a water bath for 3–6 h by maintaining the temperature around (70–80°C). The reaction mixture was allowed to cool and filtered. Then the crude product was recrystallized from absolute ethanol to afford bis cyclohexenone ester derivatives 14–19.
Bis cyclohexenone ester 14: IR (KBr) ν (cm−1): 3052, 2980, 2924, 2854, 1663, 1738, 1607, 757, 694; 1H NMR (δ ppm), (J Hz): 0.93 (6H, t, CH2CH3 at C-1, J=5.2), 2.99–2.95 (2H, H5a, m), 3.14–3.00 (2H, H5a, m), 3.68–3.61 (1H, H6, m), 3.95–3.87 (4H, m, CH2CH3 at C-1), 4.11 (1H, H1, d, J=13.6), 6.54 (1H, d, H3, J=2.0), 7.72–7.37 (14H, m, Harom.); 13C NMR (δ ppm): 13.79 CH2CH3 at C-1, 35.28 C-5, 43.46 C-6, 59.89 CH2CH3 at C-1, 58.67 C-1, 122.89 C-3, 159.29 C-4, 169.31 C=O at C-1, 194.27 C-2, 130.10-124.16 -Carom., 140.28, 139.86, 138.00, 137.32 ipso-Cs.
Bis cyclohexenone ester 15: IR (KBr) ν (cm−1): 3063, 2986, 2925, 1664, 1738, 1600, 832, 756; 1H NMR (δ ppm), (J Hz): 0.92 (6H, t, CH2CH3 at C-1, J=7.2), 2.99–2.94 (2H, H5a, m), 3.12–3.05 (2H, H5a, m), 3.67–3.59 (1H, H6, m), 3.95–3.85 (4H, m, CH2CH3 at C-1), 4.09 (1H, H1, d, J=14.3), 6.52 (1H, s, H3), 7.81–7.18 (12H, m, Harom.); 13C NMR (δ ppm): 13.79 CH2CH3 at C-1, 35.27 C-5, 43.39 C-6, 59.89 CH2CH3 at C-1, 58.59 C-1, 122.84 C-3, 158.07 C-4, 169.15 C=O at C-1, 194.19 C-2, 128.91-115.64 -Carom., 162.11, 140.25, 133.80, 129.00 ipso-C’s.
Bis cyclohexenone ester 16: IR (KBr) ν (cm−1): 3030, 2977, 2926, 2854, 1658, 1738, 1601, 811, 756; 1H NMR (δ ppm), (J Hz): 0.92 (6H, m, CH2CH3 at C-1, J=7.0), 2.33 (6H, s, CH3 at phenyl rings), 2.99–2.95 (2H, H5a, m), 3.13–3.04 (2H, H5a, m), 3.68–3.57 (1H, H6, m), 3.92–3.90 (4H, m, CH2CH3 at C-1), 4.09 (1H, H1, d, J=14.3), 6.52 (1H, s, H3), 7.63–7.24 (12H, m, Harom.); 13C NMR (δ ppm): 14.30 CH2CH3 at C-1, 21.32, 21.34 CH3 at phenyl rings, 35.66, 35.56 C-5, 43.96, 44.08 C-6, 60.40, 60.37 CH2CH3 at C-1, 59.17 C-1, 123.88, 122.55 C-3, 159.63, 158.93 C-4, 169.85, 169.73 C=O at C-1, 194.71 C-2, 129.93-126.78 -Carom., 143.02, 141.00, 140.83, 140.53, 135.54, 134.87 ipso-C’s.
Bis cyclohexenone ester 17: IR (KBr) ν (cm−1): 3052, 2980, 2927, 1665, 1738, 1609, 825, 679; 1H NMR (δ ppm), (J Hz): 0.92 (6H, t, CH2CH3 at C-1, J=7.0), 2.97-2.93 (2H, H5a, m), 3.07–3.00 (2H, H5a, m), 3.66–3.63 (1H, H6, m), 3.93–3.87 (4H, m, CH2CH3 at C-1), 4.11 (1H, H1, d, J=15.6), 6.56 (1H, d, H3, J=2.0), 7.76–7.38 (12H, m, Harom.); 13C NMR (δ ppm): 13.79 CH2CH3 at C-1, 35.20 C-5, 43.35 C-6, 59.91 CH2CH3 at C-1, 58.60 C-1, 123.28 C-3, 157.89 C-4, 169.09 C=O at C-1, 194.21 C-2, 129.78–127.61 -Carom., 140.22, 136.17, 135.14 ipso-C’s.
Bis cyclohexenone ester 18: IR (KBr) ν (cm−1): 3063, 2974, 2923, 2849, 1664, 1737, 1607, 825, 756; 1H NMR (δ ppm), (J Hz): 0.85–0.97 (6H, m, CH2CH3 at C-1), 2.99–2.92 (2H, H5a, m), 3.08–3.00 (2H, H5a, m), 3.68–3.56 (1H, H6, m), 3.94–3.86 (4H, m, CH2CH3 at C-1), 4.03 (1H, H1, d, J=14.5), 6.58 (1H, d, H3, J=3.0), 7.67–7.28 (12H, m, Harom.); 13C NMR (δ ppm): 14.31 CH2CH3 at C-1, 35.44 C-5, 43.90 C-6, 60.51 CH2CH3 at C-1, 59.22 C-1, 123.78 C-3, 158.41 C-4, 169.82 C=O at C-1, 194.59 C-2, 128.23–124.46 -Carom., 137.04, 132.22, 129.07 ipso-C’s.
Bis cyclohexenone ester 19: IR (KBr) ν (cm−1): 3030, 2958, 2924, 2850, 1653, 1737, 1601, 831, 756; 1H NMR (δ ppm), (J Hz): 0.94 (6H, m, CH2CH3 at C-1, J=7.2), 3.02–2.94 (2H, H5a, m), 3.09–3.03 (2H, H5a, m), 3.64–3.60 (1H, H6, m), 3.81 (6H, s, OCH3 at phenyl rings), 3.92–3.90 (4H, m, CH2CH3 at C-1), 4.06 (1H, H1, d, J=14.5), 6.51 (1H, d, H3, J=1.5), 7.71–6.99 (12H, m, Harom.); 13C NMR (δ ppm): 14.30 CH2CH3 at C-1, 35.40 C-5, 43.95 C-6, 60.34 CH2CH3 at C-1, 59.17 C-1, 55.85 OCH3 at phenyl rings, 121.53 C-3, 159.11 C-4, 169.78 C=O at C-1, 194.50 C-2, 128.70-114.68 -Carom., 161.74, 140.88, 129.74 ipso-C’s.
Microbiology
Materials
All the clinically isolated bacterial strains namely Staphylococcus aureus, β-Haemolytic streptococcus, Micrococcus luteus, Bacillus subtilis, Salmonella typhii, Shigella felxneri, Vibreo cholerae, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia and fungal strains namely Aspergillus flavus, Aspergillus niger, Mucor indicus, Rhizopus arrhizus and Microsporum gypseum are obtained from Faculty of Medicine, Annamalai University, Annamalainagar-608 002, Tamil Nadu, India.
In vitro antibacterial and antifungal activity by disc diffusion method
The in vitro activities of the compounds are tested in Sabourauds dextrose broth (SDB) (Hi-media, Mumbai) for fungi and nutrient broth (NB) (Hi-media, Mumbai) for bacteria by the disc diffusion method following the reported methodCitation25. The respective hydrochlorides of the test compounds 14–19 are dissolved in water to obtain 1 mg ml−1 stock solution and the different concentrations (100, 200, 500 ppm) were prepared from the stock solution. Seeded broth (broth containing microbial spores) is prepared in NB from 24-h-old bacterial cultures on nutrient agar (Hi-media, Mumbai) at 37 ± 1°C while fungal spores from 1- to 7-day-old Sabourauds agar (Hi-media, Mumbai) slant cultures are suspended in SDB. Sterile paper disc of 5 mm diameter is saturated with the three different concentrations and such discs are placed in each seeded agar plates. The petri plates are incubated in BOD incubator at 37°C for bacteria and at 28°C for fungi. The zone of inhibition is recorded by visual observations after 24 h of inhibition for bacteria and after 72–96 h of inhibition for fungi. Moreover, the zone of inhibition is measured by excluding the diameter of the paper disc. Ciprofloxacin is used as standards for bacteria and fluconazole as standard for fungi under analogous conditions.
In vitro antibacterial and antifungal activity by two-fold serial dilution method
Minimum inhibitory concentration (MIC) in μg/ml values is carried out by two-fold serial dilution methodCitation26. The respective test compounds (14–19) are dissolved in dimethyl sulfoxide (DMSO) to obtain 1 mg ml−1 stock solution. Seeded broth (broth containing microbial spores) is prepared in NB from 24-h-old bacterial cultures on nutrient agar (Hi-media, Mumbai) at 37 ± 1°C, while fungal spores from 1- to 7-day-old Sabourauds agar (Hi-media, Mumbai) slant cultures were suspended in SDB. The colony-forming units (cfu) of the seeded broth are determined by plating technique and adjusted in the range of 104–105 cfu/ml. The final inoculum size was 105cfu/ml for antibacterial assay and 1.1–1.5 × 102 cfu/ml for antifungal assay. Testing is performed at pH 7.4 ± 0.2 for bacteria (NB) and at a pH of 5.6 for fungi (SDB). Exactly 0.4 ml of the solution of test compound was added to 1.6 ml of seeded broth to form the first dilution. One millilitre of this is diluted with a further 1 ml of seeded broth to give the second dilution and so on till six such dilutions are obtained. A set of assay tubes containing only seeded broth is kept as control. The tubes are incubated in BOD incubators at 37 ± 1°C for bacteria and 28 ± 1°C for fungi. The MICs are recorded by visual observations after 24 h (for bacteria) and 72–96 h (for fungi) of incubation. Ciprofloxacin is used as standard for bacterial studies and fluconazole is used as standard for fungal studies.
Results and discussion
Chemistry
The conventional approach for the synthesis of target molecules diethyl 6,6′-(1,4-phenylene)bis(4-aryl-2-oxo-cyclohex-3-enecarboxylates) 14–19 are as follows: Bis chalcones 8–13 are synthesized by the Claisen-Schmidt condensation of terephthaldehyde 1 and respective substituted acetophenones 2–7 in the presence of alcoholic sodium hydroxide. Treatment of bis chalcones 8–13 with ethyl acetoacetate in the presence of sodium ethoxide in refluxing ethanol ( and ) afford target molecules, bis cyclohexanone ester derivatives 14–19. The structures of all the synthesized bis cyclohexanone ester derivatives 14–19 are confirmed by FT-IR, MS, 1H NMR, and 13C NMR spectral studies and elemental analysis. Moreover, compound 16 is also characterized by two-dimensional 1H-1H HOMOCOR and 1H-13C HSQC spectral studies. The reaction mechanism involves the formation of Michael addition product by ethyl acetoacetate with bis chalcones 8–13 in the presence of base, sodium ethoxide. Later, the addition product undergoes intramolecular aldol reaction in the presence of sodium ethoxide base to give the title compounds 14–19.
Table 1. Physical and analytical data of compounds 14–19.
To discuss the spectral data of the synthesized compounds, methyl substituted compound 16 is chosen as the representative compound.
Analysis of FT-IR spectrum of diethyl 6,6′-(1,4-phenylene)bis(2-oxo-4-p-tolylcyclohex-3-enecarboxylate) 16
FT-IR spectrum of diethyl 6,6′-(1,4-phenylene)bis(2-oxo-4-p-tolylcyclohex-3-enecarboxylate) 16 shows two strong characteristic absorptions at 1738 and 1658 cm−1 are due to ester carbonyl and ketone functional groups, respectively. The band at 1601 cm−1 is due to the presence of C=C stretching frequency. The absorption frequency at 3030 and 2977 cm−1 is assigned to aromatic C-H stretching vibration and the absorption frequencies at 2926 and 2854 cm−1 is assigned to aliphatic C-H stretching vibration. The observed ester carbonyl, ketone, and C=C stretching vibrational bands are supporting evidence for the formation of synthesized compound 16.
Analysis of 1H NMR spectrum of bis cyclohexenone ester 16
In the 1H NMR spectrum of 16, a triplet observed at 0.92 ppm (J=7.0 Hz) corresponding to six protons and this signal is due to ester methyl protons at C-1. A multiplet observed at 3.92–3.89 ppm corresponding to four protons, and this signal is due to ester methylene protons at C-1. Three multiplets are obtained in the range 2.99–2.95, 3.13–3.04, and 3.68–3.57 ppm and they are due to H-5a, H-5e, and H-6 protons. The doublet at 4.09 ppm (J=14.3 Hz) has been assigned to H-1 proton. The singlet observed in downfield region at 6.52 ppm is due to H-3 proton. The aromatic protons appeared as a multiplet in the range 7.63–7.24 ppm.
Analysis of 13C NMR spectrum of bis cyclohexenone ester 16
The 13C resonances at 194.71 ppm are assigned to C-2 carbonyl carbon, whereas two resonances observed at 169.85/169.73 ppm are assigned to ester carbonyl carbons. The 13C resonances at 35.66/35.56 and 43.96/44.08 ppm are due to the C-5 and C-6 carbons, respectively. The 13C resonance observed at 60.40/60.37 and 14.30 ppm is assigned to ester methylene and methyl carbons at C-1, respectively. The signal observed at 59.17 ppm is assigned to C-1 carbon, whereas the carbon signal resonates at 123.88/122.55 ppm is assigned to C-3 carbon. The aromatic carbons are observed in the range of 129.93–126.78 ppm. C-4 carbon resonates at 157.89 ppm. The remaining 13C signals at 143.02, 141.00, 140.83, 140.53, 135.54, 134.87 ppm are due to ipso carbons.
Analysis of 1H-1H COSY spectrum of bis cyclohexenone ester 16
In the HOMOCOSY spectrum of 16, the signal at 4.09 ppm shows correlation with the signal at 3.68–3.57 ppm. Similarly, the signal at 3.68–3.57 ppm shows correlation with the signal at 4.09 ppm as well as with the signal at 2.99–2.95 and 3.13–3.04 ppm. Likewise, the signal at 2.99–2.95 ppm shows correlation with the signals at 3.13–3.04 and 3.68–3.57 ppm. Moreover, the signal at 3.13–3.04 ppm shows correlation with the signals at 6.52 ppm, 2.99–2.95 and 3.68–3.57 ppm. Also, the signal at 6.52 ppm shows correlations with the signal at 3.13–3.04 ppm. From the observed correlation, it reveals that three multiplets observed in the range 2.99–2.95, 3.13–3.04, and 3.68–3.57 ppm due to H-5a, H-5e, and H-6 protons, whereas the doublet at 4.09 ppm and the singlet at 6.52 ppm are assigned to H-1 and H-3 proton. One multiplet at 3.92–3.89 shows correlation with the triplet at 0.92 ppm and vice versa. These mutual correlations reveal that the triplet observed at 0.92 ppm is due to ester methyl protons at C-1 and multiplet observed at 3.92–3.89 ppm is due to ester methylene protons at C-1. The methyl protons at aromatic ring observed at 2.33 ppm show correlation with the aromatic protons observed in the range 7.63–7.24 ppm. All the 1H-1H COSY correlations for compound 16 is given in .
Table 2. 1H-1H COSY correlations for 16.
Analysis of 1H-13C HSQC spectrum of bis cyclohexenone ester 16
In the HSQC spectrum of 16, one bond correlation (14.3/0.92 ppm) is observed between ester methyl carbon at C-1 and ester methyl proton at C-1. The two 13C resonances at 60.4 and 60.3 ppm have correlation with the proton signal around 3.92–3.89 ppm, which is obviously due to ester methylene protons at C-1. Another one bond correlation (35.5 & 35.6/32.99–2.95 & 3.13–3.04 ppm) is observed between C-5 and H-5a & H-5e. The 13C resonance at 43.9 & 44.0 ppm has one bond correlation with a multiplet around 3.68–3.57 ppm. Hence, the signal at 43.9 & 44.0 ppm corresponds to C-6 carbon, whereas the multiplet at 3.68–3.57 ppm is assigned to H-6 proton.
Another aliphatic carbon, which resonances at 59.1 ppm, shows one bond correlation with a doublet at 4.09 ppm. From this correlation, it is revealed that the doublet at 4.09 ppm corresponds to H-1 proton of the cyclohexenone moiety and the 13C signal at 59.1 ppm is assigned to C-1 carbon. The 13C resonance at 123.8/122.5 ppm has correlations with singlet at 6.52 ppm. So the signal at 6.52 ppm is conveniently assigned to H-3 proton and the carbon signal at 123.8/122.5 ppm is assigned to C-3. In the HSQC, the 13C resonances at 159.6/158.9, 169.8/169.7, and 194.7 ppm have no correlations with protons and hence it is due to quaternary carbon C-4, ester C=O at C-1 and C-2 carbons, respectively. Among the quaternary carbons, the 13C resonances at 143.02, 141.00, 140.83, 140.53, 135.54, 134.87 ppm are due to ipso carbons. All the 1H-13C HSQC correlations for compound 16 is given in . Therefore with reference to 1H-1H COSY and 1H-13C HSQC correlations in compound 16, the tentative assignments made for the protons and carbons are confirmed. Based on 1H-1H COSY and 1H-13C HSQC correlations of 16, the 1H and 13C chemical shifts of 16 are assigned unambiguously.
Table 3. H-13C HSQC correlations for 16.
Antibacterial activity of novel bis cyclohexenone ester derivatives 14–19 by disc diffusion method
A series of novel bis cyclohexanone ester derivatives 14–19 are tested for their antimicrobial activity by disc diffusion method against tested bacterial strains. From the zone of inhibitions, it is inferred that compound 14, which have no substitution at the phenyl rings show good zone of inhibition activity against β-H. streptococcus, M. luteus, and S. flexneri whereas it shows moderate zone of inhibition activity against B. subtilis, E. coli, P. aeruginosa, and K. pneumonia and shows poor activity against V. cholerae even at a very higher concentration of 500 ppm. Compound 15, which has electron withdrawing fluoro substituent at the para position of the two phenyl rings, exhibits excellent zone of inhibition antibacterial activity against all the tested bacterial strains and shows moderate activity against S. typhii. It shows poor zone of inhibition activity against S. flexneri even at a very higher concentration of 500 ppm. Compound 16, which has electron donating methyl substituent at the para position of the two phenyl rings, possesses good zone of inhibition antibacterial activity against S. aureus, β-H. streptococcus, and P. aeruginosa whereas it possesses modest activity against bacterial strains namely M. luteus, B. subtilis, S. flexneri, V. cholerae, and E. coli. Compound 17, which has electron withdrawing chloro functional group present at the para positions of the two phenyl rings, exerts moderate zone of inhibition activity against S. aureus, β-H. streptococcus, and B. subtilis and exhibits good activities against S. typhii, S. flexneri, V. cholerae, E. coli, P. aeruginosa. Electron withdrawing bulky bromo substituted compound 18 reveals good antibacterial activity against S. aureus, M. luteus, B. subtilis, S. typhii, and K. pneumonia, and exhibits moderate activity against β-H. streptococcus, S. flexneri and V. cholerae. Moreover, it exerts very poor zone of inhibition activity against E. coli even at a very higher concentration of 500 ppm. Electron donating methoxy substituted compound 19 possesses good zone of inhibition antibacterial activity against S. aureus, β-H. streptococcus, M. luteus, and K. pneumonia, whereas it reveals moderate activity against S. typhii, S. flexneri, E. coli, and P. aeruginosa.
Antibacterial activity of novel bis cyclohexenone ester derivatives 14–19 by two-fold serial dilution method
In vitro antibacterial activity results by two-fold serial dilution method of novel bis cyclohexanone ester derivatives 14–19 is shown in . Ciprofloxacin is used as standard drug. All the tested compounds 14–19 exhibit good antibacterial activity at an MIC value range of 200–6.25 µg/ml. But Compound 14 against V. cholerae, 15 against S. flexneri, 18 against E. coli do not show any activity even at a maximum concentration of 200 µg/ml. Compound 14 exhibits excellent antibacterial activity against β-H. streptococcus and S. flexneri at an MIC value of 6.25 µg/ml, whereas it shows good activity against M. luteus at an MIC value of 12.5 µg/ml. Compound 15 exhibits excellent antibacterial activity against S. aureus, β-H. streptococcus, M. luteus, E. coli, and K. pneumonia at an MIC value of 6.25 µg/ml, whereas it shows promising activity against B. subtilis, V. cholerae, and P. aeruginosa at an MIC value of 12.5 µg/ml. Compound 16 against S. aureus and P. aeruginosa displays superior activity at an MIC value of 6.25 µg/ml, whereas it reveals fine activities against β-H. streptococcus at an MIC value of 12.5 µg/ml. Compound 17 displays good antibacterial activity against S. typhii and S. flexneri at an MIC value of 6.25 µg/ml and shows good activities against V. cholerae, E. coli, and P. aeruginosa at an MIC value of 12.5 µg/ml. Poor antibacterial activity at an MIC value of 200 µg/ml is noted against K. pneumonia by compound 17. Compound 18 exhibits good microbial activity against S. aureus, S. typhii, and K. pneumonia at an MIC value of 6.25 µg/ml, whereas it reveals fine activities against M. luteus and B. subtilis at an MIC value of 12.5 µg/ml. Likewise, compound 19 against S. aureus and β-H. streptococcus shows activity at an MIC value of 12.5 µg/ml, whereas excellent antibacterial activity is shown against M. luteus and K. pneumonia at an MIC value of 6.25 µg/ml. Electron withdrawing substituted fluoro compound 15 against S. aureus, β-H. streptococcus, M. luteus, E. coli, K. pneumonia, M. gypseum, chloro substituted compound 17 against S. typhii, S. flexneri, A. niger, M. gypseum, bulky bromo substituted compound 18 against S. aureus, S. typhii, K. pneumonia, M. indicus, M. gypseum exhibit excellent antibacterial activity at an MIC value of 6.25 µg/ml. Compound 15 against B. subtilis, V. cholerae, P. aeruginosa, 17 against V. cholerae, E. coli, P. aeruginosa, 18 against M. luteus and B. subtilis exhibits good antibacterial activity at an MIC value of 12.5 µg/ml.
Table 4. In vitro antibacterial activities of compounds 14–19 by two-fold serial dilution method.
Antifungal activity of novel bis cyclohexenone ester derivatives 14–19 by disc diffusion method
Novel bis cyclohexanone ester derivatives 14–19 are tested for their antifungal activity by disc diffusion method against tested fungal strains. From the zone of inhibitions, it is inferred that compound 14 shows good zone of inhibition activity against A. niger, whereas it exhibits moderate activity against A. flavus and M. gypseum. Compound 14 against R. arrhizus reveals poor activity even at a higher concentration of 500 ppm. Compound 15 exhibits fine zone of inhibition activity against M. gypseum, whereas it shows modest activity against A. flavus, A. niger, and M. indicus. Excellent antifungal zone of inhibition is noted for compound 16 against the fungal strains A. flavus and M. indicus, whereas it exhibits moderate activity against A. niger and R. arrhizus. Compound 17 displays superior zone of inhibition activity against A. flavus A. niger, and M. gypseum and displays moderate activity against M. indicus. Compound 18 possesses superior zone of inhibitions activity against M. indicus and M. gypseum and displays modest zone of inhibition activity against A. flavus, A. niger, and R. arrhizus. Compound 19 displays excellent antifungal zone of inhibition activity against A. flavus, A. niger, and M. gypseum and exerts moderate activity against M. indicus.
Antifungal activity of novel bis cyclohexenone ester derivatives 14–19 by two-fold serial dilution method
In vitro antifungal activity results by two-fold serial dilution method () of novel bis cyclohexanone ester derivatives 14–19 show that compound 14 displays admirable activities against A. niger at an MIC value of 12.5 µg/ ml, whereas it displays low activity against A. flavus and M. gypseum at an MIC value of 50 µg/ml. Fluconazole is used as a standard drug. Compound 15 displays good activity against M. gypseum at an MIC value of 6.25 µg/ ml and possesses modest activity against A. niger and M. indicus at an MIC value of 25 µg/ml. It displays low activity against A. flavus at an MIC value of 50 µg/ ml. Compound 16 exhibits excellent antifungal activity against A. flavus and M. indicus at an MIC value of 6.25 µg/ml and displays poor activity against M. gypseum at an MIC value of 200 µg/ml. Compound 17 displays fine activity against A. flavus at an MIC value of 12.5 µg/ml, whereas it shows superior activity against A. niger and M. gypseum at an MIC value of 6.25 µg/ml. Excellent antifungal activity is noted against M. indicus and M. gypseum by compound 18 at an MIC value of 6.25 µg/ml. Compound 19 displays good activity against A. flavus and A. niger at an MIC value of 12.5 µg/ml and displays excellent antifungal activity against M. gypseum at an MIC value of 6.25 µg/ml. From the above observed antifungal activity, it is known that electron withdrawing substituted compound 15 against M. gypseum, compound 17 against A. niger, M. gypseum, compound 18 against M. indicus, M. gypseum, exhibit excellent antifungal activity at an MIC value of 6.25 µg/ ml, whereas compound 17 against A. flavus reveals good antifungal activity at an MIC value of 12.5 µg/ml.
Table 5. In vitro antifungal activities of compounds 14–19 by two-fold serial dilution method.
Conclusion
Synthesis and their spectral characterization of novel diethyl 6,6′-(1,4-phenylene)bis(4-aryl-2-oxo-cyclohex-3-enecarboxylates), a new bis cyclohexanone ester derivative is described. This reaction may have wide applicability in building a variety of heterocycles by choosing bis cyclohexanone esters as synthon, which has three versatile functional groups, that is ketone, olefin, and ester for the synthesis of structurally diverse organic compounds. The microbiological screening studies carried out to evaluate the antibacterial and antifungal potencies of the synthesized novel bis cyclohexanone ester derivatives 14–19 are clearly known from and . Compound 14 against β-H. streptococcus, S. flexneri, compound 15 against S. aureus, β-H. streptococcus, M. luteus, E. coli, K. pneumonia, M. gypseum, compound 16 against S. aureus, P. aeruginosa, A. flavus, M. indicus, compound 17 against S. typhii, S. flexneri, A. niger, M. gypseum, compound 18 against S. aureus, S. typhii, K. pneumonia, M. indicus, M. gypseum, compound 19 against M. luteus, K. pneumonia, and M. gypseum exhibits excellent antimicrobial activity at an MIC value of 6.25 µg/ml. A close inspection of the in vitro antibacterial and antifungal activity profile in differently electron donating (CH3 and OCH3) and electron withdrawing –F, -Cl, Br) functional group substituted phenyl rings of novel target compounds exerted strong antibacterial and antifungal activity against all the tested bacterial strains. Among the all tested compounds, electron withdrawing substituted compounds 15, 17, and 18 exerted potent antimicrobial activity, since electron withdrawing substitutent increases the lipophilicity due to the strong electron withdrawing capabilityCitation27. To improve metabolic stability, bioavailability, and protein ligand interactionsCitation28, fluorine substitution is commonly used in contemporary medicinal chemistry. The methods of action of these compounds were unknown. These observations may promote a further development of our research in this field. Further development of this group of cyclohexanone ester derivatives may lead to compounds with better pharmacological profile than standard antibacterial and antifungal drugs.
Acknowledgements
Authors are thankful to NMR Research Centre, Indian Institute of Science, Bangalore and Sophisticated Instruments Facility, Indian Institute of Technology, Chennai for recording spectra.
Declaration of interest
V.Kanagarajan is grateful to Council of Scientific and Industrial Research (CSIR), New Delhi, India, for providing financial support in the form of CSIR-Senior Research Fellowship (SRF) in Organic Chemistry. M.R. Ezhilarasi is thankful to Cavin Kare Research Centre, Chennai, for providing financial support in the form of Junior Research Fellowship.
References
- House HO. Modern Synthetic Reactions. Benjamin WA, Menlo Park, 1972.
- Jung ME. In: Trost BM, Fleming I (eds), Comprehensive Organic Synthesis. Pergamon, Oxford, 1991.
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V. Synthesis, spectral analysis, antibacterial and antifungal activities of some 4,6-diaryl-4,5-dihydro-3-hydroxy-2[H]-indazole-a novel fused indazole derivative. J Enzyme Inhib Med Chem 2008;23:974–979.
- Reddy DB, Reddy AS, Padmavathi V. Synthesis of annelated 1,2,3-selena- or -thiadiazoles. J Chem Res S 1998;784–785.
- Padmavathi V, Sharmila K, Reddy AS, Reddy DB. Synthesis of spirocyclohexanones. Ind J Chem Sect B 2001;40:11–14.
- Hayakawa K, Ohsuki S, Kanematsu K. General approach for the stereocontrolled synthesis of tricyclic lactones via allene intramolecular cycloaddition. An application to the synthesis of (±)-platyphyllide. Tetrahedron Lett 1986;27:947–950.
- Mori K, Kato M. Synthesis and absolute configuration of (+)-hernandulcin. A new sesquiterpene with intensely sweet taste. Tetrahedron Lett 1986;27:981–982.
- Bringmann G, Lang G, Mühlbacher J, Schaumann K, Steffens S, Rytik PG et al. Sorbicillactone A: a structurally unprecedented bioactive novel-type alkaloid from a sponge-derived fungus. Prog Mol Subcell Biol 2003;37:231–253.
- Bringmann G, Lang G, Muhlbacher J, Schaumann K, Steffens S, Muller WEG. Method for producing sorbicillactone A. German Patent Application. 2002; DE102 38 257.3.
- Zhi L, Martinborought E. Selective androgen receptor modulators (SARMs). Ann Rep Med Chem 2001;36:169–180.
- Kong C, Xu X, Zhou B, Hu F, Zhang C, Zhang M. Two compounds from allelopathic rice accession and their inhibitory activity on weeds and fungal pathogens. Phytochemistry 2004;65:1123–1128.
- Li JY, Strobel GA. Jesterone and hydroxy-jesterone antioomycete cyclohexenone epoxides from the endophytic fungus Pestalotiopsis jesteri. Phytochemistry 2001;57:261–265.
- Stang PJ, Treptow WL. Synthesis and antitumor activity of simple vinyl and alpha-methylene-gamma-butyrolactone sulfonate esters and silyl enol ethers. J Med Chem 1981;24:468–472.
- Nagarajan K, David J, Shah RK. Central nervous system active 5-oxo-1,4,5,6,7,8-hexahydrocinnolines. J Med Chem 1976;19:508–511.
- Eddington ND, Cox DS, Roberts RR, Stables JP, Powell CB, Scott KR. Enaminones-versatile therapeutic pharmacophores. Further advances. Curr Med Chem 2000;7:417–436.
- Kachhadia VV, Popat KH, Nimavat KS, Joshi HS. Synthesis, antitubercular and antimicrobial activity of 6-carbethoxy-5-aryl-3-[p-(3′-chloro-2′-benzo [b]thiophenoylamino)phenyl]-2-cyclohexenones. J Ind Chem Soc 2004;81:694–695.
- Luu B, Aguilar JLGD, Junges CG. Cyclohexenonic longchain fatty alcohols as neuronal growth stimulators. Molecules 2000;5:1439–1460.
- Li JY, Harper JK, Grant DM, Tombe BO, Bashyal B, Hess WM et al. Ambuic acid, a highly functionalized cyclohexenone with antifungal activity from Pestalotiopsis spp. and Monochaetia sp. Phytochemistry 2001;56:463–468.
- Ghoneim KM, Badran MM, Shaaban MA, El-Meligie S. Novel thienyl substituted pyridine and pyridine derivatives. Egypt J Pharm Sci 1989;30:369–377.
- Roman G. Cyclohexenones through addition of ethyl acetoacetate to chalcones derived from 2-acetyl thiphene. Acta Chim Slov 2004;51:537–544.
- Gopalakrishnan M, Thanusu J, Kanagarajan V, Govindaraju R. Synthesis, antibacterial and antifungal activities of biolabile (E)-1-4-morpholinophenyl)-3-aryl-prop-2-en-1-ones. Med Chem Res 2009;18:341–350.
- Gopalakrishnan M, Thanusu J, Kanagarajan V. Design, synthesis, spectral analysis and in vitro microbiological evaluation of 2-phenyl-3-(4,6-diarylpyrimidin-2-yl)thiazolidin-4-ones. J Enzyme Inhib Med Chem 2009;24:1088–1094.
- Thanusu J, Kanagarajan V, Gopalakrishnan M. Synthesis, spectral analysis and in vitro microbiological evaluation of 3-(3-alkyl-2,6-diarylpiperin-4-ylidene)-2-thioxoimidazolidin-4-ones as a new class of antibacterial and antifungal agents. Bioorg Med Chem Lett 2010;20:713–717.
- Guthrie W, Wang XP. The aldol condensation of acetophenone with acetone. Can J Chem 1991;69:339–344.
- Maruzella JC, Percival AH. Antibacterial activity of essential oil vapors. J Am Pharm Assoc 1958;47:471–473.
- Dhar ML, Dhar MM, Dhawan BN, Mehrotra BN, Ray C. Screening of Indian plants for biological activity: I. Indian J Exp Biol 1968;6:232–247.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliver Rev 1997;23:3–25.
- Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chem Soc Rev 2008;37:320–330.