Abstract
A new series of six chromone-derived compounds and their Cu(II) complexes have been synthesized and characterized by their physical, spectral and analytical data. The ligands and their Cu(II) complexes were screened for their in vitro antibacterial activity against four Gram-negative (Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Shigella flexneri) and two Gram-positive (Bacillus subtilis, Staphylococcus aureus) bacterial strains by agar-well diffusion method. The ligands were found to exhibit either no or low-to-moderate activities against one or more bacterial species whereas, the Cu(II) complexes exhibited moderate-to-high activity. The ligands which were inactive before complexation became active upon complexation with the Cu(II) metal ion and less active became more active.
Introduction
Metal chelation and its relationship with different biological processes represent a promising area of research in designing novel therapeutic methodologies to deal with global problem of ever increasing “bacterial resistance”. Many metal ions are known to play a significant role in different biological processesCitation1–3. For example, zinc(II) and copper(II) ions are the second- and third-most abundant transition metals present in humans. They are found either at the active sites or as structural components of enzymes or co-enzyme in biochemical processesCitation4.
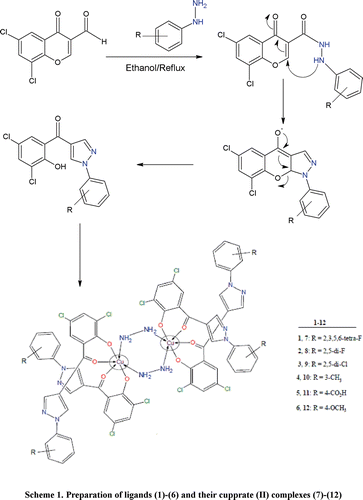
Chromones contain γ-pyrone nucleus fused with benzene ring. Molecules having chromone moiety are versatile compoundsCitation5–10 with a reactive carbonyl group that show a high reactivity towards many nucleophiles allowing synthesis of a wide variety of biologically active compoundsCitation11–14. In view of biological significance and their interesting structural behaviour, a novel class of chromones (1)–(6) and their Cu(II) complexes (7)–(12) have been prepared, characterized and tested for in vitro antibacterial activity against four Gram-negative (Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Shigella flexneri) and two Gram-positive (Bacillus subtilis, Staphylococcus aureus) bacterial strains. The present metal based chromones constitute a new class of antibacterial agents that may serve as good candidates for issues of clinical drug resistance.
Material and methods
Experimental
All reagents and solvents were used as obtained from the supplier and recrystallized/redistilled as necessary. Thin-layer chromatography (TLC) was performed for checking the purity of the compounds. Infrared (IR; KBr disc) was recorded with a Hitachi Model 200-50 Fourier transform infrared spectrophotometer. Nuclear magnetic resonance (NMR) spectra were recorded in dimethyl sulphoxide (DMSO)-d6 with Bruker AM 300 and AM 400 spectrometer (Rheinstetten–Forchheim, Germany) operating at 300 and 400 MHz, respectively. Mass spectra of the ligands were obtained by JEOL MS Route spectrometer using electron ionization (EI) mode. CHN analyses were carried out using Elemental Analyzer Flash EA 1112. Conductance of the metal complexes was measured on conductivity meter 4071, Jenway. Magnetic susceptibility measurements of the metal complexes in the solid state were performed on the Gouy’s balance at room temperature. Melting points were recorded on a Gallenkamp apparatus.
General procedure for the preparation of ligands (1)–(6)
To a stirred solution of 3,5-dichloroformylchromone (0.01 mole) in ethanol (30 ml) was an appropriate solution of hydrazide (0.01 mole) in ethanol (20 ml) was added, according to . The resultant mixture was heated under reflux for 3–4 h. The solid product thus formed during refluxing was collected by suction filtration. Thorough washing with hot ethanol followed by ether furnished the required product in pure form as a single spot on TLC. All compounds were prepared similarly and characterized as below:
(3,5-Dichloro-2-hydroxyphenyl)[1-(2,3,5,6-tetrafluorophenyl)-1H-pyrazol-4-yl]methanone (1)
Yield 68% as a light yellow powder; m.p. 91–93°C; IR (KBr, cm−1): 3440 (dichlorohydroxyphenyl, OH), 1685 (C=O), 1040 (pyrazol, N-N), 620 (C-F); 1H NMR (DMSO-d6, δ ppm): 8.32 (s, 1H, pyrazol), 8.81 (s, 1H, pyrazol), 7.86 (s, 1H, tetrafluorobenzene), 8.01 (s, 1H, dichlorohydroxyphenyl), 8.03 (s, 1H, dichlorohydroxyphenyl), 11.83 (s, 1H, OH); 13C NMR (DMSO-d6, ppm): 106.3, 116.9, 136.6, 136.6, 147.3, 147.4 (tetrafluorobenzene), 107.4, 128.7, 141.8 (pyrazole), 196.3 (C=O), 128.4, 129.3, 135.7, 125.7, 158.4 (C-O), 117.6 (dichlorohydroxyphenyl), EIMS (70 eV) m/z (%): 405.13 (M+, 68%); Anal. Calcd.: for C16H6Cl2F4N2O2 (405.13): C, 47.43; H, 1.49; N, 6.91; Cl, 17.50; F, 18.76; Found: C, 47.35; H, 1.39; N, 6.93, Cl, 17.55%.
(3,5-Dichloro-2-hydroxyphenyl)[1-(2,5-difluorophenyl)-1H-pyrazol-4-yl]methanone (2)
Yield 75% as a yellowish powder; m.p. 75–76°C; IR (KBr, cm−1): 3440 (dichlorohydroxyphenyl, OH), 1680 (C=O), 1041 (pyrazol, N-N), 620 (C-F); 1H NMR (DMSO-d6, δ ppm): 8.33 (s, 1H, pyrazol), 8.07 (s, 1H, pyrazol), 7.68 (s, 1H, difluorobenzene), 7.68 (s, 1H, difuorobenzene), 7.71 (s, 1H, difluorobenzene), 7.96 (s, 1H, dichlorohydroxyphenyl), 8.01 (s, 1H, dichlorohydroxyphenyl), 11.82 (s, 1H, OH); 13C NMR (DMSO-d6, ppm): 107.1, 115.4, 118.6, 152.2, 152.4, 130.1 (difluorobenzene), 107.4, 128.7, 141.8 (pyrazol), 196.2 (C=O), 128.4, 129.3, 135.7, 125.7, 158.4 (C-O),117.6 (dichlorohydroxyphenyl); EIMS (70 eV) m/z (%): 369.15 (M+, 88%); Anal. Calcd.: for C16H8Cl2F2N2O2 (369.15): C, 52.01; H, 2.16; N, 7.58; Cl, 19.23. Found: C, 52.11; H, 2.09; N, 7.62; Cl, 19.19%.
(3,5-Dichloro-2-hydroxyphenyl)[1-(2,5-dichlorophenyl)-1H-pyrazol-4-yl]methanone (3)
Yield 72% as a yellowish powder; m.p. 102–103°C; IR (KBr, cm−1): 3435 (dichlorohydroxyphenyl, OH), 1685 (C=O), 1040 (pyrazol, N-N), 620 (C-F); 1H NMR (DMSO-d6, δ ppm): 8.53 (s, 1H, pyrazol), 8.76 (s, 1H, pyrazol), 7.91 (s, 1H, dichlorohydroxyphenyl), 8.02 (s, 1H, dichlorohydroxyphenyl), 7.13 (s, 1H, dichlorobenzene), 7.96 (s, 1H, dichlorobenzene), 8.82 (dichlorobenzene), 11.81 (s, 1H, OH); 13C NMR (DMSO-d6, δ ppm): 117.1, 132.4, 128.6, 131.2, 132.4, 144.1 (dichlorohydroxyphenyl), 107.4, 128.7, 141.8 (pyrazol), 196.3 (C=O), 128.4, 129.3, 135.7, 125.7, 158.3 (C-O), 117.6 (dichlorobenzene); EIMS (70 eV) m/z (%): 402.06 (M+, 79%); Anal. Calcd.: for C16H8Cl4N2O2 (402.06): C, 47.75; H, 1.99; N, 6.96; Cl, 35.31. Found: C, 47.69; H, 2.06; N, 6.95; Cl, 35.35%.
(3,5-Dichloro-2-hydroxyphenyl)[1-(3-methylphenyl)-1H-pyrazol-4-yl]methanone (4)
Yield 75% as a bright yellow powder; m.p. 184–186°C; IR (KBr, cm−1): 3435 (dichlorohydroxyphenyl, OH), 1685 (C=O), 1040 (pyrazol, N-N), 620 (C-F); 1H NMR (DMSO-d6, δ ppm): 2.34 (s, 3H, CH3), 8.47 (s, 1H, pyrazol), 8.22 (s, 1H, pyrazol), 7.91 (s, 1H, dichlorohydroxyphenyl), 8.02 (s, 1H, dichlorohydroxyphenyl), 7. 8.1 (s, 1H, methylbenzene), 7.1 (d, 1H, methylbenzene), 7.22 (m, 1H, methylbenzene), 7.58 (d, 1H, methylbenzene), 11.83 (s, IH, OH); 13C NMR (DMSO-d6, δ ppm): 24.6 (CH3), 117.1, 132.4, 128.6, 131.2, 132.4, 144.1 (methylbenzene), 107.4, 128.7, 141.8 (pyrazol), 196.3 (C=O), 128.4, 129.3, 135.7, 125.7, 158.3 (C-O), 117.6 (dichlorohydroxyphenyl); EIMS (70 eV) m/z (%):347.19 (M+, 62%), Anal. Calcd.: for C17H12Cl2N2O2 (347.19): C, 58.75; H, 3.45; N, 8.06; Cl, 20.44. Found: C, 58.78; H, 3.51; N, 8.11; Cl, 20.52%.
4-[4-(3,5-Dichloro-2-hydroxybenzoyl)-1H-pyrazol-1-yl]benzoic acid (5)
Yield 72% as a dull yellow powder; m.p. 253–255°C; IR (KBr, cm−1): 3435 (dichlorohydroxyphenyl, OH), 1782 (COOH), 1685 (C=O), 1040 (pyrazol, N-N), 620 (C-F); 1H NMR (DMSO-d6, δ ppm): 8.47 (s, 1H, pyrazol), 8.22 (s, 1H, pyrazol), 7.91 (s, 1H, dichlorohydroxyphenyl), 8.02 (s, 1H, dichlorohydroxyphenyl), 8.3 (s, 1H, benzoic acid), 7.5 (d, 1H, benzoic acid), 8.13 (m, 1H, benzoic acid), 8.73 (d, 1H, benzoic acid), 11.825 (s, 1H, OH), 12.14 (s, IH, COOH); 13C NMR (DMSO-d6, ppm): 125.3, 129.4, 128.2, 131.2, 106.6, 140.1 (benzoic acid), 107.4, 128.7, 141.8 (pyrazol), 196.3 (C=O), 128.4, 129.3, 135.7, 125.7, 158.3 (C-O), 117.6 (dichlorohydroxyphenyl), 170.2 (COOH); EIMS (70 eV) m/z (%):377.18 (M+, 72%), Anal. Calcd.: for C17H10Cl2N2O4 (377.18): C, 54.08; H, 2.65; N, 7.42; Cl, 18.82. Found: C, 54.11; H, 2.69; N, 7.39; Cl, 18.77%.
(3,5-Dichloro-2-hydroxyphenyl)[1-(4-methoxyphenyl)-1H-pyrazol-4-yl]methanone (6)
Yield 70% as a yellowish powder; m.p. 160–162°C; IR (KBr, cm−1): 3435 (dichlorohydroxyphenyl, OH), 1685 (C=O), 1040 (pyrazol, N-N), 620 (C-F); 1H NMR (DMSO-d6, δ ppm): 3.82 (s, 3H, OCH3), 8.46 (s, 1H, pyrazol), 8.25 (s, 1H, pyrazol), 7.91 (s, 1H, dichlorohydroxyphenyl), 8.07 (s, 1H, dichlorohydroxyphenyl), 7.68 (s, 1H, methoxylbenzene), 7.37 (d, 1H, methoxylbenzene), 6.98 (m, 1H, methoxylbenzene), 7.52 (d, 1H, methoxylbenzene), 11.83 (s, IH, OH); 13C NMR (DMSO-d6, δ ppm): 56.3 (OCH3), 113.1, 131.4, 112.2, 161.5, 101.4, 141.4 (methoxylbenzene), 107.4, 128.7, 141.8 (pyrazol), 196.3 (C=O), 128.4, 129.3, 135.7, 125.7, 158.3 (C-O), 117.6 (dichlorohydroxyphenyl); EIMS (70 eV) m/z (%):363.19 (M+, 78%), Anal. Calcd.: for C17H12Cl2N2O3 (363.19): C, 56.16; H, 3.30; N, 7.71; Cl, 19.55. Found: C, 56.09; H, 3.27; N, 7.65; Cl, 19.61%.
General procedure for the preparation of Cu(II) complexes (7)–(12)
To a hot magnetically stirred solution of an appropriate ligand (0.02 mol) in methanol (20 ml) was added a solution of copper(II) chloride (0.01 mol) in warm methanol (10 ml) and resultant mixture refluxed for 1 h. The solid thus formed during reflux was cooled and collected by suction filtration. Thorough washing with hot methanol followed by ether furnished the desired product. It was re-crystallized as the pure product from aqueous-methanol (30:70) by keeping at room temperature for 24 h.
Copper(II) complex of (3,5-dichloro-2-hydroxyphenyl)[1-(2,3,5,6-tetrafluorophenyl)-1H-pyrazol-4-yl]metha-none (7)
Yield 63% as a brownish powder; m.p. (decomp.) 194–196°C; IR (KBr, cm−1): 1696 (C=O), 1335 (C-O), 1040 (pyrazol, N-N), 620 (C-F), 485 (M-O of C=O), 455 (M-O of deprotonated OH); UV (DMSO): λmax (cm−1); 12985; µeff 2.36 B.M.; molar conductance (34 Ohm−1 cm2 mol−1); water content, 5.92%; Anal. Calcd.: for C64H28Cl8Cu2F16N12O8 (1807.67): C, 42.52; H, 1.56; N, 9.30; Cl, 15.71. Found: C, 42.63; H, 1.65; N, 9.43; Cl, 15.69%.
Copper(II) complex of (3,5-dichloro-2-hydroxyphenyl)[1-(2,5-difluorophenyl)-1H-pyrazol-4-yl]methanone (8)
Yield 59% as a dark green powder; m.p. (decomp.) 188–190°C; IR (KBr, cm−1): 1695 (C=O), 1338 (C-O), 1041 (pyrazol, N-N), 620 (C-F), 490 (M-O of C=O), 465 (M-O of deprotonated OH); UV (DMSO): λmax (cm−1); 14150; µeff 2.42 B.M.; molar conductance (32 Ohm−1 cm2 mol−1); water content, 0.49%; Anal. Calcd.: for C64H36Cl8Cu2F8N12O8 (1663.75): C, 46.20; H, 2.18; N, 10.10; Cl, 17.08 Found.: C, 46.41; H, 2.16; N, 11.24; Cl, 17.12%.
Copper(II) complex of (3,5-dichloro-2-hydroxyphenyl)[1-(2,5-dichlorophenyl)-1H-pyrazol-4-yl]methanone (9)
Yield 62% as a reddish brown powder; m.p. (decomp.) 214–217°C; IR (KBr, cm−1): 1695 (C=O), 1335 (C-O), 1040 (pyrazol, N-N), 620 (C-F), 470 (M-O of C=O), 465 (M-O of deprotonated OH); UV (DMSO): λmax (cm−1); 16810; µeff 1.92 B.M.; molar conductance (36 Ohm−1 cm2 mol−1); water content, 0.53%; Anal. Calcd.: for C64 H36Cl16Cu2 N12O8 (1795.38): C, 42.81; H, 2.02; N, 9.36; Cl, 31.64 Found: C, 42.78; H, 2.00; N, 9.35; Cl, 31.59%.
Copper(II) complex of (3,5-dichloro-2-hydroxyphenyl)[1-(3-methylphenyl)-1H-pyrazol-4-yl]methanone (10)
Yield 64% as a brown powder; m.p. (decomp.) 310–312°C; IR (KBr, cm−1): 1695 (C=O), 1335 (C-O), 1040 (pyrazol, N-N), 620 (C-F), 465 (M-O of C=O), 460 (M-O of deprotonated OH); UV (DMSO): λmax (cm−1); 11760; µeff 2.05 B.M.; molar conductance (35 Ohm−1 cm2 mol−1); water content, 0.98%; Anal. Calcd.: for C68H52Cl8Cu2N12O8 (1575.93): C, 51.83; H, 3.33; N, 10.67; Cl, 18.03 Found: C, 51.80; H, 3.30; N, 10.66; Cl, 17.99%.
Copper (II) complex of 4-[4-(3,5-dichloro-2-hydroxybenzoyl)-1H-pyrazol-1-yl]benzoic acid (11)
Yield 62% as a brownish powder; m.p. (decomp.) 240–243°C; IR (KBr, cm−1): 1695 (C=O), 1337(C-O), 1040 (pyrazol, N-N), 620 (C-F), 460 (M-O of C=O), 445 (M-O of deptotonated OH); UV (DMSO): λmax (cm−1); 14620; µeff 1.95 B.M.; molar conductance (34 Ohm−1 cm2 mol−1); water content, 0.01%; Anal. Calcd.: for C68H44Cl8Cu2N12O16 (1695.86): C, 48.16; H, 2.62; N, 9.91; Cl, 16.75. Found: C, 48.14; H, 2.59; N, 9.91; Cl, 16.81%.
Copper (II) complex of (3,5-dichloro-2-hydroxyphenyl)[1-(4-methoxyphenyl)-1H-pyrazol-4-yl]methanone (12)
Yield 60% as a dark green powder; m.p. (decomp.) 309–311°C; IR (KBr, cm−1): 1695 (C=O), 1335 (C-O), 1040 (pyrazol, N-N), 620 (C-F), 455 (M-O of C=O), 465 (M-O of deprotonated OH); UV (DMSO): λmax (cm−1); 12700; µeff 1.98 B.M.; Molar conductance (36 Ohm−1 cm2 mol−1); water content, 0.01%; Anal. Calcd.: for C68H52Cl8Cu2N12O12 (1639.93): C, 49.80; H, 3.20; N, 10.25; Cl, 17.32. Found: C, 49.68; H, 3.27; N, 10.34; Cl, 17.40%.
Antibacterial bioassay (in vitro)
Preliminary screening
The synthesized ligands (1)–(6) and their copper(II) complexes (7)–(12) were screened in vitro for their antibacterial activity against four Gram-negative (E. coli, P. aeruginosa, S. typhi and S. flexneri) and two Gram-positive (B. subtilis and S. aureus) bacterial strains by the agar-well diffusion methodCitation15,Citation16. The wells (6 mm in diameter) were dug in the media with the help of a sterile metallic borer with centres at least 24 mm apart. Two- to eight-hours-old bacterial inocula containing approximately 104–106 colony-forming units (CFU/ml) were spread over the surface of the nutrient agar using a sterile cotton swab. Appropriate amount of the test sample (1 mg ml−1 in DMSO) was introduced into the respective wells. Other wells supplemented with DMSO and reference antibacterial drug, imipenam, served as negative and positive controls, respectively. The plates were incubated immediately at 37°C for 24 h. Activity was determined by measuring the diameter (mm) of zones showing complete inhibition. In order to clarify any participating role of DMSO in the biological screening, separate studies were carried out with pure DMSO and they showed no activity against any bacterial strains.
Minimum inhibitory concentration
Compounds exhibiting most significant antibacterial activity (greater than 16 mm) were selected for minimum inhibitory concentration (MIC) determinations by use of disc diffusion technique by employing discs containing 5, 10, 25, 50 and 100 μg ml−1 of the compound under investigation and applying the reported protocolCitation17.
Results and discussion
Chemistry
The ligands (1)–(6), were prepared by refluxing 3,5-dichloroformylchromone in equimolar quantities with the respective hydrazide for 3–4 h in ethanol (30–40 ml) as shown in . All synthesized ligands were characterized by their physical, spectroscopic (IR and 1H NMR), mass spectral and elemental analyses data. The metal complexes (7)–(12) were all air stable and prepared by the stoichiometric reaction of the copper(II) chloride with the ligand in a molar ratio (metal:ligand) of 1:2. The complexes were intensely coloured and amorphous solids which decomposed without melting. They were insoluble in common organic solvents such as ethanol, methanol, chloroform or acetone and soluble in aqueous-methanol, DMSO and N,N-dimethylformamide (DMF). Molar conductance (32–36 Ohm−1 cm2 mol−1) of the complexes (7)–(12) in DMF (10−3M solution at 25°C), indicated that they are non-electrolytic in natureCitation18. The elemental analysis data of the Cu(II) complexes agree well with the proposed composition of the compounds, which were found to be dimeric in nature with two hydrazine molecules bridging the two copper atoms through coordination. Efforts to grow suitable crystals of the metal complexes for x-ray diffraction studies were unsuccessful due to their poor solubility in common organic solvents.
IR, NMR and mass spectra
In the IR spectra of the ligands, the absence of characteristic bands at 1670 and 3315 cm−1 assigned to formylo (HC=O) and amino (-NH2) moieties, respectively, indicatedCitation19 the coordination of the starting material. The IR spectrum of all the ligands displayed ν(C=O) and ν(OH) at 1650–1685 and 3450 cm−1. The IR spectrum of the ligand (5) exhibited ν(COOH) in the region at 2785 cm−1, respectively. The comparison of the IR spectra of the ligands (1)–(6) with their metal complexes (7)–(12) revealed that the compounds are bidentately coordinated to the metal ions. In all the complexes, the band appearing at 1650–1670 cm−1 due to the νC=O vibrations is shifted to higher frequency by 17–25 cm−1, which is indicative of the involvement of the C=O in chelation. In addition to this, the band at 3450 cm−1 attributed to ν(OH) in the ligands shifted to lower frequency at 1345 cm−1 by 15–25 cm−1 in its metal complexes indicating the deprotonation and coordination of the oxygen atom to the metal atom. These new bands were not present in the spectra of their corresponding ligands. Further conclusive evidence of the coordination of the ligands with the metal ions was established by the far IR spectra in which new bands appearing in the spectra of the metal complexes and not in the spectra of the ligands at 455–470 and 420–440 cm−1 assigned to M-O (of carbonyl)Citation20 and M-O (of dichlorohydroxyphenyl) were observed. Moreover, bands at 615 cm−1 were assigned to C-Cl in the spectra of all compounds.
Based on the experimental evidences available through this study, the complexes are proposed to have binuclear structures as shown below in . Interestingly, the presence of hydrazine molecule (N2H4) is shown by thermal analysis data that acts as a bridging link between the two copper atoms. Construction of molecular model reveals that the proposed structure for the complexes is justifiable. The 1H and C13 NMR spectral data of ligands (1)–(6) along with their possible assignments is reported in the experimental part and all the protons and carbons were found in their expected region. These studies are well supported by their IR and 1H NMR spectra.
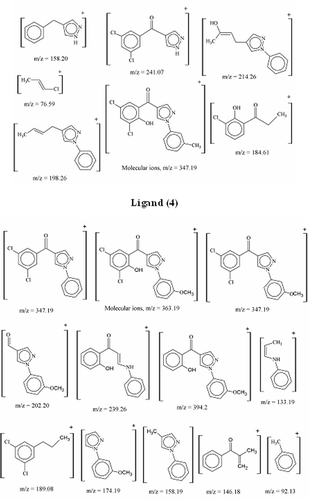
Mass spectral data along with the fragments of the ligands under study are given. The molecular ion peaks (M+) was visible in this spectra. This data clearly indicate the formation of the ligands having the proposed structures. Fragmentation pattern of the representative ligands (4) and (6) are reproduced in .
Magnetic susceptibility measurements
The room temperature magnetic moments of the solid Cu(II) complexes were found in the range 1.92–2.42 B.M. The values of complexes (7)–(12) were higher than the normal values (~1.63 B.M.) for Cu(II) ion, suggestingCitation21,Citation22 that these complexes are not mononuclear. Thus the magnetic moment values support the proposed molecular structure of the complexes as binuclearCitation23.
Conductivity measurements
The conductance values in DMF at the concentration 10−3 mole dm−3 fall in the range 32–36 Ohm−1 cm2 mol−1. The conductance values indicate the non-electrolytic nature of the complexes as there are no anions present in the latticeCitation24. However, slightly higher conductivity values are because of the binuclear nature of the complexes.
Water content and thermal analysis
Water contents of the Cu(II) complexes were determined by the Karl–Fischer titration method. The values do not equate with any whole number of water molecules suggesting that the water content is just the lattice water and the complexes are not completely dry.
The TG, DSC and DTA thermograms of respective Cu(II) complexes are shown as below. The relevant data obtained from the thermograms of all the metal complexes is given in . The TG-DTA curves of all the complexes showed three to five steps in their decomposition. In the first step of each complex, one out of two hydrazine molecules was lost between 100–156°C. The complexes begin to lose weight around 200–500°C; a sharp decrease in weight shows the loss of one of the ligands from the complexes. The DTA curves show different peaks in the range of 210–390°C. The endothermic peaks in these complexes in the range of 210–375°C are assigned to the loss of the ligands. Some of the representative thermograms are shown in Supplementary Figure S1
Table 1. Thermal analysis data of Cu(II) complexes (7)–(12).
Electronic absorption spectra
The electronic absorption bands of the complexes under investigation are listed in experimental data. The d-d spectra are consistent with the distorted octahedral geometry of the complexes. In the absorption spectra, there is an intense broad band observed at 11,760–16,810 cm−1 which is assigned to a 2eg→2t2g transitionCitation25. Although three transitions are expected in this case, but they are very close in energy and often appear in the form of one broad envelopCitation26. The values of the electronic transitions for the Cu(II) complexes are specific to an axially deformed octahedral geometryCitation27. The band around 22,220 cm−1 may be assigned to Cu-Cu linkage or bridgingCitation28.
Biological activity
Antibacterial studies of the ligands (1)–(6) and their copper(II) complexes (7)–(12)
Antibacterial activities of the synthesized ligands (1)–(6) and their corresponding Cu(II) complexes (7)–(12) were determined against four Gram-negative (Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi and Shigella flexneri) and two Gram-positive (Bacillus subtilis and Staphylococcus aureus) bacterial strains. The results of all the synthesized compounds were compared with those of the standard drug imipenam (). The ligands either exhibited no activity or have varying degree of inhibitory effects (low-to-moderate) against different tested strains. Among all the tested compounds, (1), (4), (5), (7), (8), (10), (11) and (12) were found to be active against Gram-negative and Gram-positive bacterial strains. In comparison to the ligands, the activity of the Cu(II) complexes against all the Gram-negative and Gram-positive species increased on coordination of the ligands with the Cu(II) except the compounds (3), (4), (6) and (9). Interestingly, five complexes (7), (8) and (10)–(12) were found to be more active than the ligands. These results substantiate our own findings and the findings of some other workersCitation29–31 that biologically inactive compounds become active and less active compounds become more active upon coordination/complexation. Such induction or enhancement in activity of the metal complexes can be explained on the basis of Overtone’s concept and chelation theory. According to Overtone’s concept of cell permeability, the lipid membrane that surrounds the cell favours the passage of only lipid soluble materials due to which liposolubility is an important factor that controls antimicrobial activity. On chelation, the polarity of the metal ion is reduced to a greater extent due to the overlap of the ligand orbital and partial sharing of the positive charge of the metal ion with donor groups. Further, it increases the delocalization of π-electrons over the whole chelate ring and enhances the lipophilicity of the complex. This increased lipophilicity in turn enhances the penetration of the complexes into lipid membranes and blocking of metal binding sites on the enzymes of the microorganismsCitation32. The metal complex may also be a vehicle for activation of the ligand as the cytotoxic agent. Moreover, coordination may lead to significant reduction of drug resistanceCitation33–35. Apart from this, other factors such as solubility, conductivity and dipole moment as influenced by the presence of metal ions may also be among the possible reasons causing enhancement of the bactericidal activity of the metal complexes as compared to the uncomplexed compoundsCitation36–39.
Table 2. Antibacterial activity data of the prepared ligands (1)–(6) and their Cu(II) complexes (7)–(12).
Minimum inhibitory concentration
The data obtained after preliminary antibacterial screening showed that compounds (7), (8), (9), (10), (11) and (12) were the most active (above 80%) and their average inhibition values were 18.83, 19.00, 17.66, 19.5, 19.33 and 20.66 mm, respectively. These compounds were therefore, selected for MIC studies (). The MIC of these compounds was in the range 7.136 × 10−8 to 1.282 × 10−6 M. The compound (8) proved to be the most active. It inhibited the growth of B. subtilis at 1.282 × 10−6 M.
Table 3. Minimum inhibitory concentration (M/ml) of the Selected Compounds (7), (8), (9), (10), (11) and (12) against selected bacterial strains.
Declaration of interest
The authors report no conflict of interests and are responsible for the contents of the paper.
Supplementary Material
Download PDF (467.7 KB)References
- Ming LJ. Structure and function of metalloantibiotics. Med Res Rev 2003;23:697–762.
- Louie AY. Metal complexes as enzyme inhibitors. Med Chem Rev 1999;99:2711–2734.
- Brown DH, Smith WE, Teape JW, Lewis AJ. Antiinflammatory effects of some copper complexes. J Med Chem 1980;23:729–734.
- Castillo-Blum SE, Barba-Behrens N. Coordination chemistry of some biologically active ligands. Coord Chem Rev 2000;196:3–30.
- Madje BR, Shindalkar SS, Ware MN and Shingare MS. An efficient condensation of 4-oxo-4H-benzopyran-3-carbaldehydes with 3-methyl-1-phenyl-1H-pyrazol-5 (4H)-one. ARKIVOC. 2005;14:82–86.
- Ellis GP. Chromenes, Chromanones and Chromones. John Wiley and Sons, New York, (1977) p. 1
- Staunton J., In: Barton D, Ollis WD, eds. Comprehensive Organic Chemistry. Vol 4. Oxford: Pergamon Press 1974, 659.
- Miao H, Yang Z. Regiospecific carbonylative annulation of iodophenol acetates and acetylenes to construct the flavones by a new catalyst of palladium-thiourea-dppp complex. Org Lett 2000;2:1765–1768.
- Hadjeri M, Barbier M, Ronot X, Mariotte AM, Boumendjel A, Boutonnat J. Modulation of P-glycoprotein-mediated multidrug resistance by flavonoid derivatives and analogues. J Med Chem 2003;46:2125–2131.
- Gerwick WH, Lopez A, Duyne GD, Clardy J, Ortiz W, Buez A. Tetrahedron Lett 1986;270:1979–1982.
- Chohan ZH, Shad HA, Youssoufi MH, Ben Hadda T. Some new biologically active metal-based sulfonamide. Eur J Med Chem 2010;45:2893–2901.
- Chohan ZH, Sumrra SH, Youssoufi MH, Hadda TB. Metal based biologically active compounds: design, synthesis, and antibacterial/antifungal/cytotoxic properties of triazole-derived Schiff bases and their oxovanadium(IV) complexes. Eur J Med Chem 2010;45:2739–2747.
- Chohan ZH, Youssoufi MH, Jarrahpour A, Ben Hadda T. Identification of antibacterial and antifungal pharmacophore sites for potent bacteria and fungi inhibition: indolenyl sulfonamide derivatives. Eur J Med Chem 2010;45:1189–1199.
- Chohan ZH, Iqbal MS and Aftab SK. Design, synthesis, characterization and antibacterial properties of copper(II) complexes with chromone-derived compounds. Appl Organomet Chem 2010;24:47–56.
- Carron RA, Maran JM, Montero L, Fernanddozalgo A, Dominguez A. Plants Medicinates et Phytother. 1987;21:195
- Atta-ur-Rahman Choudhary, MI, Thomsen WJ. Bioassay Techniques for Drug Development. Amsterdam: Harward Academic Press 2001, 16.
- Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 1966;45:493–496.
- Geary WJ. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord Chem Rev 1971;7:81–122.
- Nakamoto K. Infrared Spectra of Inorganic and Coordination Compounds, 2nd edn. New York: Wiley Interscience 1970.
- Ferraro JR. Low Frequency Vibrations of Inorganic and Coordination Compounds. 2nd edn. New York: John Wiley 1971.
- Dutta RL, Syamal A. Elements of Magnetochemistry. 2nd edn. New Delhi: Elsevier 1992.
- Subramanian PS, Srinivas D. Polyhedron. 1996;15:985–989.
- Yasodhai S, Sivakumar T, Govindarajan S. Preparation, characterisation and thermal reactivity of transition metal complexes of hydrazine with citric acid. Thermochimica Acta 1999;338:57–65.
- Yasodhai S, Govindarajan S. Preparation and characterisation of glutarate and adipate complexes of metals with neutral hydrazine. Synthesis and Reactivity in Inorganic Metal-Organic and Nano-Metal Chem 1999;29:919–934.
- Raman N, Kulandaisamy A, Jeyasubramanian K. Synthesis, spectroscopic, characterization, redox and biological screening studies of some Schiff base transition metals (II) complexes derived from salicylidene-4-aminoantipyrine and 2-aminophenol/2-aminothiophenol. Synth React Inorg Met Org Chem. 2001;31:1249–1270.
- Khar VK, Sharma RS, Sharma RC. Predominant tuberculosis spoligotypes. Proc Natl Acad Sci India 2000;70:441.
- Raman N, Kulandaisamy A, Jeyasubramanian K. Synthesis, spectral, redox and biological studies of some Schiff base copper(II)and nickel(II) complexes. Indian J Chem 2002; 41A: 942; Chem Abstr. 2002; 137: 162801m.
- Dubicki L, Martin RL. The pi-system of binuclear copper (II) and chromium (II) acetates. Inorg Chem 1966;5:2203–2209.
- Li-June M. Structure and function of metallo-antibiotics. Med Resear Rev 2003;23:697–762.
- Afanas’eva IB, Ostrakhovitch EA, Mikhal’chik EV, Ibragimova GA, Korkina LG. Enhancement of antioxidant and anti-inflammatory activities of bioflavonoid rutin by complexation with transition metals. Biochem Pharmacol 2001;61:677–684.
- Clark MJ, Nonrasca DRF. Platinum chemotherapeutic metallopharmaceutical. Chem Rev 1999;99:2511–2534.
- Raman N, Muthuraj V, Ravichandran S, Kulandaisamy A. Synthesis, characterisation and electrochemical behaviour of Cu(II), Co(II), Ni(II) and Zn(II) complexes derived from acetylacetone and p-anisidine and their antimicrobial activity. Proc Indian Acad Sci 2003;115:161–167.
- West DX, Padhye SB, Sonawane PB. Structure and Bonding. Vol 76 New York: Springer-Verlag 1991, 1.
- Chohan ZH, Supuran CT, Ben Hadda T, Nasim FU, Khan KM. Metal based isatin-derived sulfonamides: their synthesis, characterization, coordination behavior and biological activity. J Enzyme Inhib Med Chem 2009;24:859–870.
- Chohan ZH, Supuran CT. Structure and biological properties of first row d-transition metal complexes with N-substituted sulfonamides. J Enzyme Inhib Med Chem 2008;23:240–251.
- Chohan ZH, Supuran CT. Structure and biological properties of first row d-transition metal complexes with N-substituted sulfonamides. J Enzyme Inhib Med Chem 2008;23:240–251.
- Chohan ZH. Synthesis of organometallic-based biologically active compounds: In vitro antibacterial, antifungal and cytotoxic properties of some sulfonamide incorporated ferrocences. J Enzyme Inhib Med Chem 2009;24:169–175.
- Chohan ZH. Synthesis and biological properties of Cu (II) complexes with 1,1′-disubstituted ferrocenes. Trans Met Chem 2009;34:153–161.
- Chohan ZH, Shaikh AU, Rauf A, Supuran CT. Antibacterial, antifungal and cytotoxic properties of novel N-substituted sulfonamides from 4-hydroxycoumarin. J Enzyme Inhib Med Chem 2006;21:741–748.