Abstract
Present communication deals with the synthesis of novel 2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one derivatives under phase transfer catalysis (PTC) conditions using benzyl triethyl ammonium chloride (BTEAC) as PTC. It also elicits the studies on in vitro antimicrobial evaluation of synthesized compounds against a representative genera of gram-negative and gram-positive bacteria i.e., Bacillus subtilis, Staphylococcus aureus, Pseudomonas diminuta and Escherichia coli. All the compounds have been found to manifest profound antimicrobial activity. Moreover, extensive quantitative structure-activity relationship (QSAR) studies have been performed to deduce a correlation between molecular descriptors under consideration and the elicited biological activity. A tri-parametric QSAR model has been generated upon rigorous statistical treatment.
Introduction
The fight against bacterial infection is one of the great success challenges of medicinal chemistry. Multiple drug-resistant micro-organismsCitation1,Citation2 are becoming common causes of infections in the acute and long-term care. The emergence of these resistant microorganisms has created a major concern and there exists an urgent need of antibacterial agents in structural classes distinct from known antibacterial agents. A systematic perusal of literature reveals that benzimidazole skeleton has gained a reputation as a drug of privileged sub-structure owing to its extensive use in medicinal chemistryCitation3–6. Interest in benzimidazole containing structures stems from their widespread occurrence in molecules that display a plethora of useful biological properties. They are characterized by a broad spectrum of activity against roundworms (nematodes), an ovicidal effect, and a wide safety margin. Current clinical example includes astemizole, esomeprazole, albendazole, mebendazole, fenbendazole, oxfendazole, oxibendazole, albendazolesulfonamide, thiabendazole, thiophanate, febantel, nelotrimin, and triclabendazole, carbendazim, fuberidazoleCitation7–12. Nowadays, in view of the affinity displayed by them towards a variety of enzymes and protein receptors, compounds containing benzimidazole sub-structure are being recognized as a drug of choice in the current drug design scenario.
The advent of high throughput screening technologies has impacted significantly on the methodologies that are used for the synthesis of a number of medicinal compounds. The implementation in the laboratory of these synthetic technologies to increase the number of molecules generated by chemists is now a pre-requisite to competitive advantage in the field. Several publications describing synthesis of benzimidazole in solution phase and on solid supports have appeared in the literatureCitation13–17. Thus, keeping in view the ever-increasing demand of new antibiotic agents, it was thought of our interest to design the synthesis of a novel series of benzimidazoles i.e., 2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one () under phase transfer catalysis (PTC) conditions.
Figure 1. Structure of synthesized 2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4a–r).
![Figure 1. Structure of synthesized 2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4a–r).](/cms/asset/56a00fe4-e1c0-41a2-9566-681b4454a575/ienz_a_587814_f0001_b.gif)
Moreover, in order to explore the medicinal potential of these synthesized compounds, comprehensive QSAR studies have been performed to establish a correlation between the various physicochemical parameters and the elicited antibacterial behaviour.
Systematic perusal of literature reveals that a number of methods have been reported for the synthesis of different benzimidazole derivatives under variable experimental conditionsCitation18–22. However, most of the existing methods to design benzimidazole skeleton requires the insertion of a carbon into a precursor with ortho heteroatoms on a benzene ring. Moreover, most of the methods have not been found to be quite accessible from the viewpoints of both yield and economics of the reaction. Thus, in order to cater the needs associated with synthetic aspects, herein, we would like to present unique approach to synthesize benzimidazole derivatives under PTC conditions considering insertion-cyclisation sequence onto azo functionality of the precursor.
Thus an important preparative methodology for the synthesis of 2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4a–r) has been delineated incorporating substituted aniline derivatives, urea and ethyl 3-oxobutanoate as the substrates. In this strategy, 1H-benzimidazol-2-amine (1), obtained by the reaction of 1,2-phenylenediamine dihydrochloride with cyanogen bromide, was treated with substituted azo-coupled products i.e., ethyl 3-oxo-2-(phenyldiazenyl)butanoate (2a–r). Furthermore, the obtained precursor compound 2-methyl-3-(phenyldiazenyl) pyrimido[1,2-a] benzimidazol-4(3H)-one (3a–r) was then treated with 3-methylbut-1,2-dien-1-ylidene, generated in situ as per , under PTC conditions to obtain 2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazole-4(3H)-one (4a–r) () as the final product ().
Scheme 2. An overview of synthesis of 2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4a–r).
![Scheme 2. An overview of synthesis of 2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4a–r).](/cms/asset/1bfe9c1e-bb95-4dae-84eb-f0170ef96704/ienz_a_587814_f0003_b.gif)
From the viewpoint of mechanistic considerations, the formation of 4a–r could be rationalized by an initial trap of dimethylvinylidene carbene by 2-methyl-3-(phenyldiazenyl)pyrimido[1,2-a] benzimidazol-4(3H)-one at−N=N- to form three membered ring, which is thermally labile compared with the carbocyclic analogue because of much smaller bond dissociation energyCitation23 of N–N bond than C–C bond. Hence, it is rearranged to 2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazole-4(3H)-one (4a–r).
Materials and methods
All the chemicals and other reagents used were of AR grade purity. All the synthesized compounds were characterized by IR, 1H-NMR, 13C NMR, FAB-Mass spectroscopy and elemental analyses. Melting points were determined on an electro-thermal apparatus by open capillary method and are uncorrected. The NMR spectra were recorded on Bruker DRX300 instrument (300 MHz) in CDCl3 using TMS as an internal standard. Chemical shifts are reported in δ (ppm) values. All IR spectra were run on Shimadzu 460 spectrophotometer in KBr Discs; frequencies are reported in cm−1. FAB mass spectra (FAB-MS) were recorded on a JEOL SX 102 Mass Spectrometer. To ascertain the purity of all the synthesized compounds, analytical thin layer chromatography was performed on (E Merck silica gel G0.50 mm plate no. 5700) using acetonitrile, methanol, and water (50:30:20, v/v) as eluting system. Elemental analyses were performed on Elementar Vario EL-III Carlo-Erba-1108 equipment.
Synthesis of 1H-benzimidazol-2-amine (1Citation24)
A solution of 1,2-phenylenediamine dihydrochloride (0.45 g, 2.5 mmol) in 5 mL of water was cooled to 0°C and treated with a solution of cyanogen bromide (0.60 mL, 5 M in acetonitrile, 3.0 mmol) and solid NaHCO3 (0.41 mg, 4.9 mmol). The solution was stirred at ambient temperature for 40–45 h. The mixture was made basic with 1 M aqueous Na2CO3 and the solution was concentrated under reduced pressure. The residue was triturated with hot ethanol, and the ethanolic solution was filtered and concentrated under reduced pressure to obtain the compound 1 in appreciable yield.
1H-benzimidazol-2-amine (1): Yield 85%; mp 135–136°C; Anal. Calcd. for C7H7N3 (R=H): C, 63.14; H, 5.30; N, 31.56%; Found: C, 63.10; H, 5.28; N, 31.53%; IR (υ cm−1): 3045 (C-H, sp2), 3210 (NH, bonded), 3175 (NH, free), 1654 (C=N), 1626, 1586, 1444 (C…C, ring str) 958, 859, 742 (sub. phenyl); 1H-NMR (300 MHz, CDCl3) δ (ppm): 4.0 (s, 2H, NH2), 5.0 (s, NH), 7.6–7.9 (m, 4H, Ar-H); 13C NMR (CDCl3) δ (ppm): 117.41, 124.34, 136.66, 158.62; FAB-MS: 134 (M+H)+.
General procedure for synthesis of ethyl 3-oxo-2-[(E)-phenyldiazenyl]butanoate (2a-rCitation25)
Different substituted coupled products were synthesized as the precursors taking ethyl 3-oxobutanoate as the active methylene compound. Pertinent aniline derivatives (0.01 M) were dissolved in 4 mL HCl and 4 mL distilled water and kept at freezing temperature. To this, an aqueous solution of sodium nitrite (0.69 g, 0.01 M) in 5 mL of distilled water was added dropwise with continuous stirring, keeping the temperature in the vicinity of 0–5°C. Meanwhile, in another beaker ethyl 3-oxobutanoate (1 mL), sodium acetate (7.0 g) and ethyl alcohol (25 mL) were taken and cooled in an ice bath. Now the diazotized solution () was added to this solution dropwise with thorough stirring under temperature-controlled conditions. The reaction mixture was kept for overnight period, filtered through suction, washed well with water and dried in vacuum. Fine yellow crystals of ethyl 3-oxo-2-[(E)-phenyldiazenyl]butanoate (2a–r) were obtained.
Ethyl 3-oxo-2-[(E)-phenyldiazenyl]butanoate (2a): Yield 82%; mp 145–146°C; Anal. Calcd. for C12H14N2O3: C, 62.11; H, 5.76; N, 19.06%; Found: C, 62.08; H, 5.71; N, 19.01%; IR (υ cm−1): 3050 (C-H, sp2), 2945 (C-H, sp3), 2010 (N=N), 1750 (C=O, ester), 1618, 1580, 1449 (C…C, ring str), 1118 (C-O), 958, 859, 742 (sub. phenyl); 1H-NMR (300 MHz, CDCl3) δ (ppm): 1.1 (t, 3H, CH2CH3, J = 6.5 Hz), 2.0 (s, 3H, CH3), 5.4 (q, 2H, CH2CH3, J = 6.5 Hz), 6.1 (s, CH), 7.2-7.6 (m, 5H, Ar-H); 13C NMR (CDCl3) δ (ppm): 21.24, 27.42, 86.14, 122.62, 125.10, 129.25, 150.20, 172.02; FAB-MS: 235 (M+H)+.
General procedure for synthesis of 2-methyl-3-(phenyldiazenyl)pyrimido[1,2-a]benzimidazol-4(3H)-one (3a–r)
An equimolar (0.01 M) quantity of 1H-benzimidazol-2-amine (1) and ethyl 3-oxo-2-[(E)-phenyldiazenyl] butanoate (2a–r) was taken in a 250 cc RB flask and 10 mL freshly prepared sodium ethoxide was added to this. The mixture was heated under reflux condition for 30 min. The solid product thus formed was cooled, dehydrated by anhydrous sodium sulphate, filtered and recrystallized from ethanol. The obtained yield was 70–86%.
2-methyl-3-(phenyldiazenyl) pyrimido[1,2-a] benzimidazol-4(3H)-one (3a): Yield 78%; mp 163–164°C; Anal. Calcd. for C17H13N5O: C, 67.32; H, 4.32; N, 32.09%; Found: C, 67.26; H, 4.28; N, 23.05%; IR (υ cm−1): 3055 (C-H, sp2), 2940 (C-H, sp3), 2051 (N=N), 1621 (C=C/C=N), 1620, 1532, 1445 (C…C, ring str), 955, 850, 745 (sub. phenyl); 1H-NMR (300 MHz, CDCl3) δ (ppm): 2.4 (s, 3H, CH3), 5.6 (s, CH), 7.30 -7.61 (m, 9H, Ar-H); 13C NMR (CDCl3) δ (ppm): 20.20, 70.62, 114.20, 115.24, 122.64, 123.25, 125.20, 130.18, 137.02, 139.56, 141.54, 159.22, 165.50; FAB-MS: 304 (M+H)+.
General procedure for synthesis of 2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4a–rCitation26)
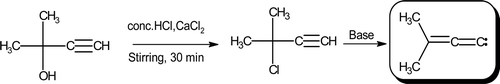
In a 100 mL three-necked bolt-head flask, fitted with a dropping funnel and a mechanical stirrer, a mixture of 50% of aqueous potassium hydroxide (15 mL), benzyltriethylammonium chloride (BTEAC) (0.57 g, 2.5 mmol) and benzene (5 mL) was taken and stirred thoroughly for 30 min. To this, a pertinent 2-methyl-3-(phenyldiazenyl)pyrimido[1,2-a] benzimidazol-4(3H)-one (3a–r) (2.5 mmol) was added slowly and stirred for further 5–7 h under nitrogen atmosphere. While stirring was going on, 3-chloro-3-methyl-1-butyne (25 mmol) () in benzene (5 mL) was added slowly to the mixture. The contents were diluted with water (120 mL), followed by extraction with ether (120 mL) to afford crude product. It was purified on an alumina column (benzene as eluent) so as to finally obtain 2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4a–r). Their purity was further ascertained upon performing TLC resolution on E Merck silica gel-G plates using acetonitrile, methanol, and water (50:30:20, v/v) as eluting system. An overview of all the synthetic steps is depicted in .
2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4a): Yield 76%; mp 122–123°C; Anal. Calcd. for C22H19N5O: C, 71.53; H, 5.18; N, 18.96%; Found: C, 71.50; H, 5.15; N, 18.96%; IR (υ cm−1): 3085 (C-H, sp2), 2952 (C-H, sp3), 1720 (C=O), 1631 (C=C/C=N), 1620, 1522, 1440 (C…C, ring str), 952, 853, 741 (sub. phenyl); 1H-NMR (300 MHz, CDCl3) δ (ppm): 1.85 (s, 6H, 2 × CH3, isopropenyl), 2.02 (s, 3H, CH3), 4.54 (s, CH, pyrimidine), 5.13 (s, CH, methine), 7.27 (m, 4H, H11, H12, H21, H22), 7.66 (m, 4H, H10, H13, H20, H23); 13C NMR (CDCl3) δ (ppm): 17.44 (C24), 20.25 (C28), 24.27 (C27), 65.82 (C3), 110.02, 114.12, 115.28, 123.02, 123.26, 130.74, 134.28, 138.91, 139.24 (aromatic ring), 141.64 (C6, C16), 144.66 (C26), 165.16 (C4), 169.57 (C2); FAB-MS: 370 (M+H)+.
2-methyl-3-[4-Chloro-2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4f): Yield 79%; mp 126–127°C; Anal. Calcd. for C22H18N5 ClO: C, 65.43; H, 4.49; N, 17.34%; Found: C, 65.40; H, 4.46; N, 17.31%; IR (υ cm−1): 3052 (C-H, sp2), 2950 (C-H, sp3), 1725 (C=O), 1621 (C=C/C=N), 1605, 1550, 1440 (C—C, ring str), 952, 840, 755 (sub.phenyl), 6.3 (C-Cl); 1H-NMR (300 MHz, CDCl3) δ (ppm): 1.82 (s, 6H, 2 × CH3, isopropenyl), 1.99 (s, 3H, CH3), 4.62 (s, CH, pyrimidine), 5.82 (s, CH, methine), 7.18 (d, 1H, H21), 7.24 (t, 1H, H22), 7.24 (d, 2H, H11, H12), 7.62 (d, 2H, H10, H13), 7.48 (d, 1H, H23); 13C NMR (CDCl3) δ (ppm): 17.46 (C24), 19.22 (C28), 25.29 (C27), 65.34 (C3), 113.35, 114.13, 115.28, 120.74, 123.07, 124.12, 124.49, 130.75, 138.32, 138.90, 140.37 (aromatic ring), 141.50 (C6, C16), 143.52 (C26), 164.74 (C4), 167.88 (C2); FAB-MS: 405 (M+H)+.
2-methyl-3-[4-methyl-2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4i): Yield 84%; mp 122–123°C; Anal. Calcd. for C23H21N5O: C, 72.04; H, 5.52; N, 18.26%; Found: C, 72.00; H, 5.49; N, 18.22%; IR (υ cm−1): 3062 (C-H, sp2), 2971 (C-H, sp3), 1726 (C=O), 1629 (C=C/C=N), 1612, 1525, 1437 (C…C, ring str), 954, 849, 743 (sub. phenyl); 1H-NMR (300 MHz, CDCl3) δ (ppm): 1.80 (s, 6H, 2 × CH3, isopropenyl), 1.95 (s, 3H, CH3), 2.12 (s, 3H, ArCH3), 4.52 (s, CH, pyrimidine), 5.75 (s, CH, methine), 7.17 (t, 1H, H22), 7.44 (d, 1H, H23), 7.56 (d, 1H, H21), 7.24 (d, 2H, H11, H12), 7.68 (d, 2H, H10, H13); 13C NMR (CDCl3) δ (ppm): 17.02 (Ar-CH3), 18.06 (C24), 20.12 (C28), 24.89 (C27), 66.14 (C3), 112.25, 114.13, 115.22, 122.95, 123.04, 124.18, 126.24, 130.77, 138.82, 139.08, 140.37 (aromatic ring), 142.33 (C6, C16), 144.11 (C26), 165.64 (C4), 168.28(C2); FAB-MS, 384 (M+H)+.
2-methyl-3-[4-hydroxy-2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4j): Yield 83%; mp 120–121°C; Anal. Calcd. for C22H19N5O2: C, 68.56; H, 4.97; N, 18.17%; Found: C, 68.53; H, 4.95; N, 18.15%; IR (υ cm−1): 3345 (O-H), 3056 (C-H, sp2), 2925 (C-H, sp3), 1718 (C=O), 1620 (C=C/C=N), 1595, 1521, 1450 (C…C, ring str), 950, 852, 744 (sub. phenyl); 1H-NMR (300 MHz, CDCl3) δ (ppm): 1.85 (s, 6H, 2 × CH3, isopropenyl), 1.97 (s, 3H, CH3), 4.67 (s, CH, pyrimidine), 5.78 (s, CH, methine), 6.25 (s, 1H, OH), 6.95(d,1H, H21), 7.08 (t, 1H, H22), 7.15 (d, 1H, H23), 7.29 (d, 2H, H11, H12), 7.68 (d, 2H, H10, H13). 13C NMR (CDCl3) δ (ppm): 17.48 (C24), 19.22 (C28), 25.25 (C27), 66.18 (C3), 105.62, 107.84, 121.82, 123.05, 123.18, 114.15, 115.28, 130.74, 138.91, 140.39, 145.08 (aromatic ring), 141.58 (C6, C16), 143.53 (C26), 164.70 (C4), 168.22 (C2); FAB-MS: 386 (M+H)+.
2-methyl-3-[6-methyl-2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4k): Yield 76%; mp 121–22°C; Anal. Calcd. for C23H21N5O: C, 72.04; H, 5.52; N, 18.26%; Found: C, 72.02; H, 5.49; N, 18.25%; IR (υ cm−1): 3068 (C-H, sp2), 2979 (C-H, sp3), 1721 (C=O), 1625 (C=C/C=N), 1615, 1528, 1441 (C…C, ring str), 952, 847, 746 (sub. phenyl); 1H-NMR (300 MHz, CDCl3) δ (ppm): 1.85 (s, 6H, 2 × CH3, isopropenyl), 1.98 (s, 3H, CH3), 2.32 (s, 3H, ArCH3), 4.64 (s, CH, pyrimidine), 5.83 (s, CH, methine), 7.24 (d, 2H, H11, H12), 7.43 (s, 1H, H23), 7.49 (d, 1H, H21), 7.58 (d, 1H, H21), 7.68 (d, 2H, H10, H13); 13C NMR (CDCl3) δ (ppm): 18.06 (C24), 21.42 (Ar-CH3), 20.24 (C28), 24.96 (C27), 66.92 (C3), 114.13, 115.12, 115.34, 123.04, 126.04, 130.74, 131.22, 132.84, 138.82, 138.88, 140.37 (aromatic ring), 141.84 (C6, C16), 143.78 (C26), 165.25 (C4), 169.28(C2); FAB-MS: 384 (M+H)+.
2-methyl-3-[4-nitro-2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4m): Yield 73%; mp 124–25°C; Anal. Calcd. for C22H19N6O3: C, 63.76; H, 4.38; N, 20.28%; Found: C, 63.73; H, 4.35; N, 20.25 %; IR (υ cm−1): 3080 (C-H, sp2), 2959 (C-H, sp3), 1725 (C=O), 1625 (C=C/C=N), 1612, 1520, 1440 (C…C, ring str), 1331 (NO2) 952, 855, 745 (sub. phenyl); 1H-NMR (300 MHz, CDCl3) δ (ppm): 1.80(s, 6H, 2 × CH3, isopropenyl), 1.94 (s, 3H, CH3), 4.64 (s, CH, pyrimidine), 5.23 (s, CH, methine), 7.23 (d, 2H, H11, H12), 7.45 (t, 1H, H22), 7.62 (d, 2H, H10,H13), 7.95 (d, 1H, H23), 8.18(d,1H, H21); 13C NMR (CDCl3) δ (ppm): 17.43 (C24), 19.28 (C28), 25.27 (C27), 64.88 (C3), 114.23, 115.26, 118.65, 121.32, 123.02, 123.95, 130.75, 133.08, 137.06, 138.90, 139.87 (aromatic ring), 141.53 (C6, C16), 143.53 (C26), 164.66 (C4), 166.28 (C2); FAB-MS: 415 (M+H)+.
In vitro antibacterial assay
Broth micro-dilution assay
The newly synthesized compounds were screened for their antibacterial activity against Pseudomonas diminuta, Escherichia coli, Bacillus subtilis and Staphylococcus aureus using broth micro-dilution method. Approximately 109 cells of respective bacterial cells were inoculated in 10 mL Mueller-Hinton broth. Each synthesized compound (200 µg) was added and mixed well by shaking at 200 rpm and the plates are incubated at 37°C for 48–72 h. After every 6-h interval, 1 mL of cell culture were withdrawn and subjected to serial dilution (10−1–10−6) and spread on nutrient agar plates and the plates were incubated at 37°C for 24 h. The bacterial colonies were counted by colony form unit (CFU) in each dilution to find out the antibacterial activity of the tested compounds.
The MIC (µg/mL) of a compound was determined according to the lowest concentration that inhibited visible growth of bacteria after incubation at 37°C for 24 h. IC50 (µg/mL) represents that inhibitory concentration of the compound, at which the 50% inhibition of the growth of bacteria occurred in vitro. IC50 is also converted to the pIC50 scale (-log IC50), in which higher values indicate exponentially greater potency. Mueller-Hinton broth was used as the test medium. Each experiment in the antibacterial assays was replicated twice in order to define the MIC and IC50 values. Ampicillin was used as standard antibacterial agent.
Results and discussion
In order to screen these synthesized benzimidazoles as more potent lead compounds, QSAR study was performed on substituted 2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4a–r) derivatives. The structure of more active member of the series is shown in . Fifty percent inhibitory concentration [IC50] of the substituted 2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4a–r) was calculated and converted to the negative logarithmic scale (pIC50) (). A number of physicochemical parameters viz., Connolly accessible area (CAA), Connolly molecular area (CMA), molar refractivity (MR), dipole-dipole energy (DDENE), non-1,4 VDW energy (NVDW), VDW 1,4 energy (VDW) were calculated by the Chem office 6.0 using Chem. 3D Pro packageCitation27. Geometry optimization was done by PM3 method incorporated in MOPACCitation28 server of the ChemOffice 6.0 program. Single point energies calculation for the lead compounds was done by RHF (Restricted Hartree-Fock: closed shell) wave function. HyperChem. Release 8.0.3 Molecular Modeling SystemCitation29 was used for the calculation of van der Waal surface volume (WVOL), van der Waal surface area (WSA), surface area grid (SAG), hydrophobicity (logP), molecular refractivity (REF), molecular polarizability (P), and molecular orbital energies (HOMO and LUMO). Initial energy minimizations were done by employing molecular mechanics methods. These initially optimized geometries were optimized by PM3 method employing Fletcher-Reeves (conjugate gradient) algorithm with a termination RMS gradient of 0.0001 kcal/(Å mol).
Table 1. The in vitro antimicrobial inhibition activities of 2-methyl-3-[2-(2-methylprop-1-en-1-yl)-1H-benzimidazol-1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one (4a–r).
Figure 2. PM3 optimized geometry of more active 2-methyl-3-[2-(2-methylprop-1-en-1-yl)- 1H-benzimidazol -1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one.
![Figure 2. PM3 optimized geometry of more active 2-methyl-3-[2-(2-methylprop-1-en-1-yl)- 1H-benzimidazol -1-yl]pyrimido[1,2-a]benzimidazol-4(3H)-one.](/cms/asset/95e656fe-bf19-4377-bc8b-febdbab7445d/ienz_a_587814_f0004_b.gif)
All the statistical analyses were carried out by the computer program ValstatCitation30. Correlation analysis between molecular descriptors (calculated by ChemOffice and HyperChem packages), and biological activity was performed. The dataset were divided into test set and training set containing three and fifteen compounds, respectively. All possible combinations of parameters were considered for the QSAR study. Multiple regression analysis was carried out using in vitro antimicrobial inhibition activities as the dependent variable and all molecular descriptors as independent variables in all possible combinations. The statistical quality of the regression equations was justified on the basis of significant statistical parameters viz., percentage of explained variance (% EV), variance ratio (F), and standard error of estimate (SEE). All the final equations have regression coefficients, intercepts and variance ratio (F) significant to more than 95% confidence level. Use of more than one variable in the multivariate equation was justified by an autocorrelation study. The predictive powers of the equations were validated by the leave one out (LOO) cross validation method. Predicted residual sum of square (PRESS), total sum of squares (SSY) and cross-validated r2 (r2CV) were considered for the validation of the models.
Tri-parametric QSAR model for activity against P. diminuta
n = 18, F = 273.089, PRESS = 0.311, SSY = 5.406, PRESS/SSY = 0.058, r2CV = 0.942, % EV = 93.805, SEE = 0.144, r2bsp = 0.989, Bootstrapping std = 0.006, Chance < 0.001, Q2 = 0.967, SPRESS = 0.121, SDEP = 0.104
Contribution of parameters to model is MR: CMA: DDENE:: 85.306: 12.958: 1.000. In the above equation % EV, F, SEE, PRESS, SSY, r2CV, r2bsp, SPRESS are percentage of explained variance, ratio between the variances of observed and calculated activities, standard error of estimate, predicted residual sum of squares, total sum of squares, cross validated r2, Bootstrapping r2 and standard deviation error of prediction, respectively.
Equation (1) explains up to 93.805% of the variation of the activities. However, MR is the key factor to control biological activity, which can be attributed to steric interaction occurring in polar species. It is related to molar volume (V) and the refractivity index (η) according to the relation:
which reveals that MR is a measure of bulk due to its relation with molar volume. However, it also contains a polarizability component η. Hence, the positive coefficient of MR corresponds to higher activity of these compounds. On the other hand, negative coefficient of the CMA signifies the importance of Connolly molecular area in the activity. Lower value of this index corresponds to higher activity of these compounds.
Further, PRESS is a cross-validation parameter whose value less than SSY (sum of the squares of response value) points out that the model predicts better than Chance and can be considered statistically important. To be a reasonable QSAR model, PRESS/SSY ratio should be smaller than 0.4 and its value smaller than 0.11 indicates an excellent model. The data indicate that for all the proposed models, this ratio is < 0.1 suggesting all of them to be excellent models. Furthermore, the value of cross-validated correlation coefficient (r2CV) and Bootstrapping r2 are further supporting the predictive power of these explaining models.
Moreover, the compounds have also been screened against E. coli, B. subtilis and S. aureus bacteria and the tri-parametric QSAR models are presented in the equation 2, 3, and 4, respectively.
n = 18, F = 295.238, PRESS = 0.057, SSY = 4.670, PRESS/SSY = 0.012, r2CV = 0.988, % EV = 98.679, SEE = 0.062, r2bsp = 0.989, Bootstrapping std = 0.007, Chance < 0.001, Q2 = 0.971, SPRESS = 0.111, SDEP = 0.095
Contribution of parameters to model is MR: CMA: DDENE:: 83.414: 11.981: 1.000.
n = 18, F = 327.158, PRESS = 0.052, SSY = 4.677, PRESS/SSY = 0.011, r2CV = 0.989, % EV = 98.801, SEE = 0.059, r2bsp = 0.990, Bootstrapping std = 0.006, Chance < 0.001, Q2 = 0.969, SPRESS = 0.115, SDEP = 0.099
Contribution of parameters to model is MR: CMA: DDENE:: 85.306: 12.958: 1.000.
n = 18, F = 282.885, PRESS = 0.054, SSY = 4.630, PRESS/SSY = 0.012, r2CV = 0.988, % EV = 98.736, SEE = 0.060, r2bsp = 0.990, Bootstrapping std = 0.006, Chance < 0.001, Q2 = 0.964, SPRESS = 0.123, SDEP = 0.106
Contribution of parameters to model is MR: CMA: DDENE:: 89.055: 12.034: 1.000.
In all the above models, MR term has significant contribution as supported by statistics of the analysis. Thus, it is revealed from all the proposed models deduced through equations 1-4 that the MR is the main governing factor. Moreover, increase in the percentage contribution of CMA also signifies the importance of Connolly molecular area (CMA).
Conclusion
A variety of benzimidazole derivatives have been successfully synthesized in appreciable yields and screened in vitro for their antimicrobial activities against both strains of gram-positive and gram-negative bacteria. Moreover, quantitative structure-activity relationship studies have been performed to explore their inherent antimicrobial potential. All the synthesized compounds have shown excellent antimicrobial activities. It is evident from the developed QSAR model that MR, CMA, and DDENE parameters were the main governing physicochemical factors for the displayed antimicrobial activities. Such a QSAR evaluation would open up future perspectives to use these compounds as new lead compounds in drug discovery processes.
Declaration of interest
We are thankful to the Defence Research Development Organization (DRDO), New Delhi (India), and Central Drugs Research Institute (CDRI), Lucknow (India), for providing financial assistance and spectro-analytical facilities, respectively.
References
- Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000;406:775–781.
- Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med 1998;157:531–539.
- Velík J, Baliharová V, Fink-Gremmels J, Bull S, Lamka J, Skálová L. Benzimidazole drugs and modulation of biotransformation enzymes. Res Vet Sci 2004;76:95–108.
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev 1999;12:40–79.
- Tunçbilek M, Göker H, Ertan R, Eryigit R, Kendi E, Altanlar N. Synthesis and antimicrobial activity of some new anilino benzimidazoles. Arch Pharm (Weinheim) 1997;330:372–376.
- Habib NS, Soliman R, Ashour FA, el-Taiebi M. Synthesis and antimicrobial testing of novel oxadiazolylbenzimidazole derivatives. Pharmazie 1997;52:746–749.
- Gudmundsson KS, Freeman GA, Drach JC, Townsend LB. Synthesis of fluorosugar analogues of 2,5,6-trichloro-1-(beta-D-ribofuranosyl)benzimidazole as antivirals with potentially increased glycosidic bond stability. J Med Chem 2000;43:2473–2478.
- Richards ML, Lio SC, Sinha A, Tieu KK, Sircar JC. Novel 2-(substituted phenyl)benzimidazole derivatives with potent activity against IgE, cytokines, and CD23 for the treatment of allergy and asthma. J Med Chem 2004;47:6451–6454.
- Li YF, Wang GF, He PL, Huang WG, Zhu FH, Gao HY et al. Synthesis and anti-hepatitis B virus activity of novel benzimidazole derivatives. J Med Chem 2006;49:4790–4794.
- Katiyar SK, Gordon VR, McLaughlin GL, Edlind TD. Antiprotozoal activities of benzimidazoles and correlations with beta-tubulin sequence. Antimicrob Agents Chemother 1994;38:2086–2090.
- Navarrete-Vázquez G, Cedillo R, Hernández-Campos A, Yépez L, Hernädez-Luis F, Valdez J et al. Synthesis and antiparasitic activity of 2-(trifluoromethyl)-benzimidazole derivatives. Bioorg Med Chem Lett 2001;11:187–190.
- Stefanska JZ, Gralewska R, Starosciak BJ, Kazimierczuk Z. Antimicrobial activity of substituted azoles and their nucleosides. Pharmazie 1999;54:879–884.
- Cee VJ, Downing NS. A one-pot method for the synthesis of 2-aminobenzimidazoles and related heterocycles. Tetrahedron Lett 2006;47:3747–3750.
- Seth PP, Robinson DE, Jefferson EA, Swayze EE. Efficient solution phase synthesis of 2-(N-acyl)-aminobenzimidazoles. Tetrahedron Lett 2002;43:7303–7306.
- Huang KT, Sun CM. Liquid-phase combinatorial synthesis of aminobenzimidazoles. Bioorg Med Chem Lett 2002;12:1001–1003.
- Ozden S, Atabey D, Yildiz S, Göker H. Synthesis, potent anti-staphylococcal activity and QSARs of some novel 2-anilinobenzazoles. Eur J Med Chem 2008;43:1390–1402.
- Wu Z, Ede NJ, Mathieu MN. Solid-phase synthesis of benzimidazole N-oxides on SynPhase™ Lanterns. Tetrahedron Lett 2003;44:2293–2296.
- Göker H, Alp M, Yildiz S. Synthesis and potent antimicrobial activity of some novel N-(alkyl)-2-phenyl-1H-benzimidazole-5-carboxamidines. Molecules 2005;10:1377–1386.
- Ng RA, Guan J, Alford VC Jr, Lanter JC, Allan GF, Sbriscia T et al. Synthesis and SAR of potent and selective androgen receptor antagonists: 5,6-Dichloro-benzimidazole derivatives. Bioorg Med Chem Lett 2007;17:784–788.
- Sharma P, Mandloi M, Pritimani S. Synthesis of new 2-(substituted benzothiazolylcarbamoyl)benzimidazoles as potential CNS depressants. Indian J Chem 1999;38B:1289–1294.
- El-Marsry AH, Fahmy HH, Abdelwahed SHA. Synthesis and antimicrobial activity of some new benzimidazole derivatives. Molecules 2000;5:1429–1438.
- Jae-SangY Kyongtae, K. Synthesis of novel tetra- and pentacyclic fused benzimidazole derivatives. Heterocycles 2002;57:2267–2277.
- Tarama K. In: Handbook of organic structural analysis; Yukawa Y (ed.), Benjamin WA. Inc New York 1965, 537–547.
- Leonard NJ, Curtin DY, Peck KM. Sulfonate salts of substituted benzimidazoles. J Am Chem Soc 1947;69:2459–2461.
- Bandyopadhyay P, Guha L, Seenivasagan T, Sathe M, Sharma P, Parashar BD et al. Synthesis and bio-evaluation of aryl hydrazono esters for oviposition responses in Aedes albopictus. Bioorg Med Chem Lett 2011;21:794–797.
- Sharma P, Kumar A, Sharma M. Synthesis of 4-[2-(2-methylprop-1-enylidene)-2,3-dihydro-1H-benzimidazole-1-yl]-1-napthol via azo group insertion of dimethylvinylidenecarbene under phase transfers catalysis conditions. Cat Comm 2006;7:6.
- Software CHEM 3D-6.0, CambridgeSoft Corporation, 100 Cambridge Park, MA 02140–2317, USA.
- Stewart JJP, MOPAC 6.0, QCPE 455, Indiana University, Bloomington, IN 47405, 1990.
- Hypercube, Inc. Hyperchem Professional Release 8.0 Hypercube Inc., Gainesville.
- VALSTAT software developed at the Department of Pharmacy, SGSITS, 23, Park Road, Indore, India (available on request).