Abstract
Mevinolin (MVN) has been used clinically for the treatment of hypercholesterolemia with very good tolerance by patients. Based on epidemiological evidences, MVN was suggested strongly for the treatment of neoplasia. Early experimental trials suggested the mixed apoptotic/necrotic cell death pathway was activated in response to MVN exposure. Herein, the cytotoxic profile of MVN was evaluated, compared to the robust and frequently used anti-cancer drug doxorubicin (DOX), against breast (MCF-7), cervical (HeLa) and liver (HepG2) transformed cell lines. MVN was showed comparable results in cytotoxic profile with DOX in all tested solid tumor cell lines. In addition, the MVN-induced cytotoxicity was inferred to be multi-factorial and not solely dependent on p53 expression. It was concluded that molecular and genetic assessment of MVN-induced cell death would be useful for developing cancer therapeutic treatments.
Introduction
In the developing world mortality rates due to cancer, including solid tumors, have remained constant over more than five decades which has urged intensified efforts to discover local agents for the treatment of cancer.Footnote1 Natural products of plants provide an abundant source of potentially active compounds for the treatments of different disordersCitation1,Citation2. Far East, Middle East, Saharan, and tropical regions were among the richest sources of natural products in the world. The isolation and purification of the active fractions and active ingredients amongst potentially active natural products have received increased scientific and industrial interestCitation2.
Red yeast rice (RYR), a Chinese dietary product made by fermenting ordinary rice with the mould Monascus purpureus, has been widely used as a food condiment and colorant in several Asian countriesCitation3. RYR has been used for centuries without any reports of health hazards or long-term toxicityCitation4. Several medicinally active ingredients were isolated from RYR including monacholin-K, mevinolin (lovastatin), γ-aminobutyric acid, dimerumic acid, sterols (β-sitosterol, campesterol, stigmasterol and sapogenin), isoflavones and monounsaturated fatty acidsCitation3,Citation4.
Mevinolin (MVN) or lovastatin was a potent HMGCo-A reductase enzyme inhibitor that interfered with de novo steroidogenesisCitation5. MVN was used clinically for the treatment of hypercholesterolemia with very good patient tolerance profilesCitation6,Citation7. In the last decade, several epidemiological evidences have drawn attention to possible beneficial roles of HMGCo-A reductase inhibitors (statins), such as MVN, in neoblastic disorders. Some members of the statin group may reduce the recurrence of cancer after radical prostatectomyCitation8. Also, dramatic reduction in the incidence of lipoma was observed for statin treated patientsCitation9. Most interestingly, a negative association was reported between the use of HMGCo-A reductase inhibitors and cancer incidence in veteran populationsCitation10. Many researches focused on the ability of MVN and other statins to sensitize tumor cells for conventional chemotherapeuticsCitation11. Some experimental reports manifested a potential anti-cancer activity of MVN and other HMGCo-A reductase inhibitors per seCitation12. However, the exact signaling mechanism of MVN -induced cell death remain controversial. Few reports attribute the anti-cancer activity of MVN to the induction of apoptosisCitation13, while others negate any role of apoptosis in MVN-induced cell deathCitation14. Whether the apoptosis pathway is involved in MVN-induced cytotoxicity, or not, remained an open issue by 2011. The resolution of the mechanism of MVN might improve understanding of its anti-cancer effects and infer the likelihood of the emergence of resistance among cancer cell lines.
Doxorubicin (DOX) was a cytotoxic anthracycline originally isolated from Streptomyces peucetius which has been used for the past four decades for the treatment of several hematologic as well as solid malignanciesCitation15,Citation16. However, DOX shows unique cardiotoxicity on the top of its regular chemotherapeutic toxicities such as myelosuppression, hyperemesis, diarrhoea, mucositis, impotence and alopeciaCitation17,Citation18.
Herein, the cytotoxic profile of the promising HMGCo-A reductase inhibitor, MVN, was compared to the clinically used chemotherapeutic agent, DOX against breast, cervix and liver cancer cell lines. Further, the expression of markers of apoptosis were compared in response to MVN and DOX treatment in solid tumor-like cell lines.
Materials and methods
Chemicals and drugs
DOX, sulfarhodamine (SRB) and MVN were purchased from Sigma Chemical Co. (St. Louis, MO, USA). RPMI-164 media, fetal bovine serum and other cell culture materials were purchased from Fisher Scientific Cell Culture(Houston, TX, USA). Other reagents were of the highest analytical grade available.
Cell culture
Human transformed cell lines, from liver (hepatocellular; HepG2), breast (MCF-7) and cervical (HeLa) lines were obtained from Vaccera (Giza, Egypt). Cells were maintained in RPMI-1640 supplemented with 100 µg/mL streptomycin, 100 µg /mL penicillin and 10% (w/v) heat-inactivated fetal bovine serum in a humidified, 5% (v/v) CO2 atmosphere at 37°C.
Cytotoxicity assays
The cytotoxicity of MVN was tested against MCF-7, HeLa and HepG2 cells by the SRB assay as previously describedCitation19. Exponentially growing cells were collected using 0.25% (w/v) Trypsin–EDTA plated in 96-well plates at 1,000–2,000 cells/well. Cells were exposed to each test compound for 72 h and subsequently fixed with TCA (10% (w/v)) for 1 h at 4°C. After several washings, cells were exposed to 0.4% (v/v) SRB solution for 10 min in the dark and subsequently washed with 1% (v/v) glacial acetic acid. After drying overnight, Tris-HCl was used to dissolve the SRB-stained cells and color intensity was measured at 540 nmCitation19.
Data analysis
The dose-response curve of compounds was analyzed using Emax model (Eq. 1).
Where R was the residual unaffected fraction (the resistance fraction), [D] is the drug concentration used, Kd is the drug concentration that produces a 50% reduction of the maximum inhibition rate and m is a Hill-type coefficient. IC50 was defined as the drug concentration required to reduce absorbance to 50% of that of the control (i.e. Kd = IC50 when R = 0 and Emax = 100−R)Citation31.
RNA extraction and real time PCR analysis for gene expression quantification
To assess the effect of MVN and DOX on the apoptosis pathway, total RNA isolation from cells was performed using RNeasy Mini Kit® (Qiagen Inc. Valencia, CA, USA). Reverse transcription was undertaken to construct a cDNA library from different treatments using the High-Capacity cDNA Reverse Transcription Kit™ (Applied Biosystems, Foster City, CA, USA). Real time quantitative PCR reactions were performed as previously described using SYBR green (Fermentas Inc., Glen Burnie, MD, USA) labeled probesCitation20. Primer sequences were as follows; Bcl2 forward primer GGG-TAC-GAT-AAC-CGG-GAG-AT and reverse primer CTG-AAG-AGC-TCC-TCC-ACC-AC; BAX forward primer TCT-GAC-GGC-AAC-TTC-AAC-TG and reverse primer TGG-GTG-TCC-CAA-AGT-AGG-AG; p53 forward primer CCT-CAC-CAT-CAT-CAC-ACT-GG and reverse primer CTG-AGT-CAG-GCC-CTT-CTG-TC. GAPDH was used as reference with forward primer TGC-ACC-ACC-AAC-TGC-TTA-G and reverse primer GAT-GCA-GGG-ATG-ATG-TTCCitation32.
Statistical analysis
Data are presented as mean ± SEM. Analysis of variance (ANOVA) with LSD post hoc test was used for testing the significance using SPSS® for windows, version 17.0.0. p < 0.05 was taken as a cut off value for significance.
Results
Evaluating the anti-cancer effect of DOX and MVN against solid tumor cell lines
SRB-U assay was used to assess the cytotoxicity of DOX and MVN against three different solid tumor cell lines. DOX showed cytotoxicity against the solid tumor cell lines with IC50 that ranged from 0.28 to 0.42 μg/ml. HeLa cells were the most susceptible cell lines to DOX while HepG2 cells were least susceptible (). MVN, showed comparable cytotoxic profiles against the tested solid tumor cell lines with IC50 that ranged from 0.6 to 1.1 μg/ml. HeLa cells were the most susceptible cell line to MVN and HepG2 cells were the least susceptible ().
Figure 1. The effect of MVN and DOX on different solid tumor cell lines. Dose-response curves of DOX (A) and MVN (B) in solid tumor cell line cultures of HeLa (•) HepG2 (○) and MCF-7 (▾) cells. Cells were exposed to serial dilutions of DOX or MVN for 72 h. Cell viability was determined using SRB-U assay and data are expressed as mean ± S.D. (n = 3).
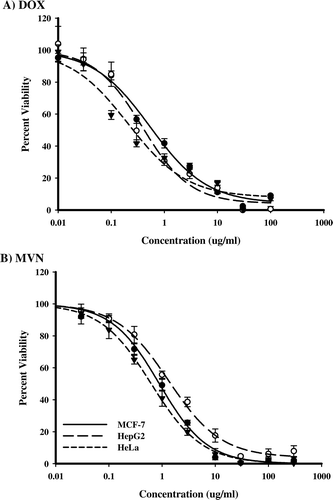
Comparting treatments with the IC50 of DOX against HepG2 and MCF-7 were 0.42 ± 0.05 and 0.42 ± 0.11 μg/ml, respectively. Despite the fact that HeLa cells were the most susceptible cell lines to DOX (IC50 = 0.28 ± 0.07 μg/ml), they showed the highest occurrence of resistance amongst the cell lines (7.7 ± 0.5%). HepG2 and MCF-7 showed resistance at 5.3 ± 2.2% and 2.6 ± 0.9%. The IC50 of MVN against both MCF-7 and HeLa were very similar (at 0.67 ± 0.15 and 0.61 ± 0.02 μg/ml, respectively). Also the frequency of resistance of both MCF-7 and HeLa cells were similarly low (1.03 ± 0.15 and 0.33 ± 0.09%, respectively). In contrast to DOX, HepG2 cells were the most resistant in terms of IC50 (1.1 ± 0.12 μg/ml) and resistant frequency (5.1 ± 0.82%) among all tested cell lines. In general, MVN showed about two fold less potency than DOX in all the tested cell lines. However, resistance to MVN was much lower than to DOX (2–20 fold ().
Table 1. The cytotoxicity parameters of MVN and DOX in different solid tumor cell lines.
Assessment of apoptosis in solid tumor cell lines after treatment with DOX and MVN
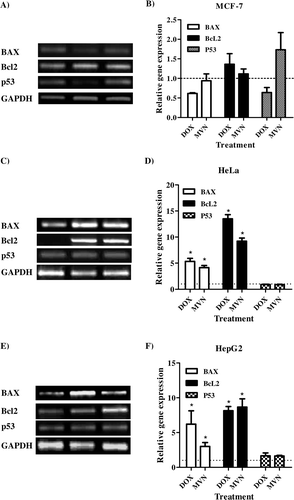
In order to examine the effect of DOX and MVN on the apoptosis pathways in the solid tumor cell lines, the transcript abundance of a pro-apoptotic gene (BAX), an anti-apoptotic gene (BcL2) and the key gene of apoptosis (p53) were quantified using RT-PCT technique in cells treated for 72 h with the IC50 of DOX and MVN. In MCF-7, DOX increased BcL2 transcript abundance but decreased BAX transcript abundance. In contrast, MVN treatment did not change the transcript abundance of BAX, but did decrease BcL2 transcript abundance. Consequently, the p53 transcript was increased in MVN treated cells and decreased in DOX treated cells ( and ). Both DOX and MVN dramatically increased the transcript abundances of BAX and BcL2 in HeLa cells (up to 4–5 fold for BAX and up to 10–15 fold for BcL2). The effective apoptosis marker gene, p53 was not changed in transcript abundance due to either treatment with DOX or MVN in the HeLa cell lines ( and ). Similar to HeLa cell lines, DOX and MVN dramatically increased the transcript abundance of BAX and BcL2 in HepG2 cells (up to 2–5 fold in BAX and up to 7–10 fold in BcL2). However, the transcript abundance of p53 the gene was increased by both DOX and MVN treatments ( and ).
Figure 2. Effects of MVN and DOX on the apoptosis pathway in solid tumor cells. Transcript abundances of BAX, Bcl2 and p53 using RT-PCR in MCF-7 (A and B), HeLa (C and D) and HepG2 (E and F) cells after treatment with DOX or MVN. Data were expressed as means ± S.D.s (n = 3). The * indicated significant differences from controls at p < 0.05.
Discussion
The slow advances in modifying the epidemiological mortality of solid tumors has warranted wider screening for new drugs with potential anti-cancer activityCitation1,Citation2. Rather than searching for new chemical moieties, the discovery of new applications for drugs with known clinical and toxicological profiles would cut down the time required for scaling-up to clinical stages. MVN was shown to be a clinically safe drug of natural origin that is known to inhibit the HMGCo-A reductase activity and interfere with steroidogenesisCitation4. Here the cytotoxic profile of MVN was compared to DOX in three solid tumor-like cell lines, MCF-7, HeLa and HepG2. In addition, the transcript abundance of some key apoptosis markers was measured.
Steroidogenesis and cholesterol transport were suggested to be essential for the growth and proliferation of tumor cellsCitation21. Steroidogenesis inhibition and the disruption of geranylgeranyl pyrophosphate-dependent survival pathways were attributed to the anti-proliferative effects of simvastatin, another HMGCo-A reductase inhibitorCitation22. Additionally, the association between the statins in general and the low incidence of carcinogenesis supported this hypothesisCitation10. The interference with mevalonate pathway (prenylation) was known with its complexity to be affecting several apoptotic signaling pathwaysCitation23. Moreover, MNV and other statins have shown to affect cell viability via mixed apoptosis and necrosis pathways in the same timeCitation24. This explains the observed low resistant cell fractions in all cell types treated with MNV compared to DOX (). Similar efficacy of MVN against all the cell lines might be partly attributed the multiplicity of its target signaling pathways ().
In comparison with DOX, which was well-known as an anti-cancer agent and used clinically, MVN showed comparable cytotoxicity and less potential to allow the development of resistance (). Additionally, DOX-induced toxicity was a major limitation in patient compliance and chemotherapeutic course completionCitation16,Citation17. The safety profile of MVN both in experimental and clinical stages were encouraging for further clinical trials for the treatment of various types of tumors. The dose of MVN suggested for anti-cancer treatments was believed to be clinically safeCitation6,Citation7. Therefore, very high doses of MVN administered every four hours to patients have been found tolerableCitation23. Consequently, MVN and other natural statins were believed to be better treatment option for cancer than synthetic statins by many factorsCitation26.
Apoptosis has been receiving great attention as a major mechanism of cell death in normal as well as tumor cells. However, the programmed cell death might be interrupted due to defective signaling pathway nonetheless in tumor cells with higher rate of mutationCitation27. Defective apoptosis has been reflected in the form of cell resistance to apoptotic inducing agents and, consequently, treatment failure. MVN has been suggested to induce cell death via multiple apoptoticCitation13, necroticCitation24 and autophagic pathwaysCitation14. In the current work, MCF-7 seemed to undergo apoptosis by the p53-dependent pathway; however both Bcl2 and BAX were not significantly affected (). That agrees with previous work of Lee and coworkers showing ameliorated cytotoxic effects of simvastatin in p53 knockdown clones of HCT116 colon cancer cell linesCitation26. On the other hand, HeLa cell lines showed no significant change in p53 transcript abundance after MVN treatment, despite the very high BAX transcript abundance (). However, the anti-apoptotic marker Bcl2 was also increased in transcript abundance in HeLa cells after MVN and DOX treatments which might explain the unchanged p53 transcript abundance. Similarly, MVN cytotoxic effects in more than one cancer cell lines was p53 independent in natureCitation29,Citation30. In contrast to DOX, there was very low resistant fraction of HeLa cells to MVN indicative of proceeding via non-apoptotic cell death pathway such as necrosis or autophagy. This may also explain the ability of MVN to overcome K-Ras mutation in human non-small lung cancerCitation31. In HepG2 cells, both apoptotic (BAX) and anti-apoptotic (Bcl2) signals were increased in transcript abundance. However, p53 was marginally increased in transcript abundance as well (). This may explain the relatively higher fraction of resistant cells in HepG2 cells treated with MVN. In future, gene silencing studies would be recommended to understand the exact molecular mechanism of MVN and other statins-induced cytotoxicity in tumor cells.
Conclusion
In conclusion, MVN showed promising cytotoxic profile comparable to DOX in breast (MCF-7), cervix (HeLa) and liver (HepG2) solid tumor cell lines. MVN-induced cytotoxicity was inferred to be multi-factorial and not solely dependent on p53 molecules.
Acknowledgement
This research was fully funded by grants from the National CFIDS Foundations Inc, Needham, MA 02492–3931, USA. We would like to thank Mr. Abdel-Hay G. Abu-Hussein, Department of biotechnology, Faculty of Agriculture Research Park, Cairo University, Cairo, Egypt, for his technical support.
Declaration of interest
The authors report no conflicts of interest.
Notes
1Cancer remains the leading cause of death over 50 years (Source: 1950 mortality data - CDC/NCHS, NVSS, mortality Revised. 2002 mortality Data-NVSR-Death final Data 2002- Volume 53, No. 5. Cost data from american cancer Society Cancer & Figures 2005).
References
- El-Shemy HA, Aboul-Enein AM, Aboul-Enein KM, Fujita K. Willow leaves’ extracts contain anti-tumor agents effective against three cell types. Plos one 2007;2:e178.
- Nassr-Allah AA, Aboul-Enein AM, Aboul-Enein KM, Lightfoot DA, Cocchetto A., El-Shemy HA. Anti-cancer and anti-oxidant activity of some Egyptian medicinal plants. J Med Plants Res 2009; 3: 799–808.
- Hong MY, Seeram NP, Zhang Y, Heber D. Anticancer effects of Chinese red yeast rice versus monacolin K alone on colon cancer cells. j Nutr Biochem 2008;19:448–458.
- Kumari HP, Naidu KA, Vishwanatha S, Narasimhamurthy K, Vijayalakshmi G. Safety evaluation of Monascus purpureus red mould rice in albino rats. Food Chem Toxicol 2009;47:1739–1746.
- Folkers K, Langsjoen P, Willis R, Richardson P, Xia LJ, Ye CQ et al. Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci usa 1990;87:8931–8934.
- Yang L, Wang Y, Lv TJ, Zhou LQ, Jin J. [Effects of clinically effective dose of lovastatin on prostate cancer PC3 cells]. Beijing Da Xue Xue Bao 2010;42:391–395.
- Yao CJ, Lai GM, Chan CF, Cheng AL, Yang YY, Chuang SE. Dramatic synergistic anticancer effect of clinically achievable doses of lovastatin and troglitazone. Int j Cancer 2006;118:773–779.
- Hamilton RJ, Banez LL, Aronson WJ, Terris MK, Platz EA, Kane CJ et al. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer 2010;116:3389–3398.
- Self TH, Akins D. Dramatic reduction in lipoma associated with statin therapy. j Am Acad Dermatol 2008;58:S30–S31.
- Farwell WR, Scranton RE, Lawler EV, Lew RA, Brophy MT, Fiore LD et al. The association between statins and cancer incidence in a veterans population. j Natl Cancer Inst 2008;100:134–139.
- Riganti C, Doublier S, Costamagna C, Aldieri E, Pescarmona G, Ghigo D et al. Activation of nuclear factor-kappa B pathway by simvastatin and RhoA silencing increases doxorubicin cytotoxicity in human colon cancer HT29 cells. Mol Pharmacol 2008;74:476–484.
- Perchellet JP, Perchellet EM, Crow KR, Buszek KR, Brown N, Ellappan S et al. Novel synthetic inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase activity that inhibit tumor cell proliferation and are structurally unrelated to existing statins. Int j Mol Med 2009;24:633–643.
- Klawitter J, Shokati T, Moll V, Christians U, Klawitter J. Effects of lovastatin on breast cancer cells: a proteo-metabonomic study. Breast Cancer Res 2010;12:R16.
- Sane KM, Mynderse M, Lalonde DT, Dean IS, Wojtkowiak JW, Fouad F et al. A novel geranylgeranyl transferase inhibitor in combination with lovastatin inhibits proliferation and induces autophagy in STS-26T MPNST cells. J Pharmacol Exp Ther 2010;333:23–33.
- Arcamone F, Cassinelli G, Fantini G, Grein A, Orezzi P, Pol C et al. Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol Bioeng 1969;11:1101–1110.
- Hortobágyi GN. Anthracyclines in the treatment of cancer. An overview. Drugs 1997;54 Suppl 4:1–7.
- Von Hoff DD, Layard MW, Basa P, Davis HL Jr, Von Hoff AL, Rozencweig M et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 1979;91:710–717.
- Licata S, Saponiero A, Mordente A, Minotti G. Doxorubicin metabolism and toxicity in human myocardium: role of cytoplasmic deglycosidation and carbonyl reduction. Chem Res Toxicol 2000;13:414–420.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990;82:1107–1112.
- Longo MC, Berninger MS, Hartley JL. Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene 1990;93:125–128.
- Hong MY, Seeram NP, Zhang Y, Heber D. Chinese red yeast rice versus lovastatin effects on prostate cancer cells with and without androgen receptor overexpression. J Med Food 2008;11:657–666.
- Fuchs D, Berges C, Opelz G, Daniel V, Naujokat C. HMG-CoA reductase inhibitor simvastatin overcomes bortezomib-induced apoptosis resistance by disrupting a geranylgeranyl pyrophosphate-dependent survival pathway. Biochem Biophys Res Commun 2008;374:309–314.
- Mo H, Elson CE. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp Biol Med (Maywood) 2004;229:567–585.
- Sánchez CA, Rodríguez E, Varela E, Zapata E, Páez A, Massó FA et al. Statin-induced inhibition of MCF-7 breast cancer cell proliferation is related to cell cycle arrest and apoptotic and necrotic cell death mediated by an enhanced oxidative stress. Cancer Invest 2008;26:698–707.
- Holstein SA, Knapp HR, Clamon GH, Murry DJ, Hohl RJ. Pharmacodynamic effects of high dose lovastatin in subjects with advanced malignancies. Cancer Chemother Pharmacol 2006;57:155–164.
- Ahn KS, Sethi G, Aggarwal BB. Reversal of chemoresistance and enhancement of apoptosis by statins through down-regulation of the NF-kappaB pathway. Biochem Pharmacol 2008;75:907–913.
- Tomiyama N, Matzno S, Kitada C, Nishiguchi E, Okamura N, Matsuyama K. The possibility of simvastatin as a chemotherapeutic agent for all-trans retinoic acid-resistant promyelocytic leukemia. Biol Pharm Bull 2008;31:369–374.
- Lee SK, Kim YC, Song SB, Kim YS. Stabilization and translocation of p53 to mitochondria is linked to Bax translocation to mitochondria in simvastatin-induced apoptosis. Biochem Biophys Res Commun 2010;391:1592–1597.
- Milkevitch M, Jeitner TM, Beardsley NJ, Delikatny EJ. Lovastatin enhances phenylbutyrate-induced MR-visible glycerophosphocholine but not apoptosis in DU145 prostate cells. Biochim Biophys Acta 2007;1771:1166–1176.
- Martirosyan A, Clendening JW, Goard CA, Penn LZ. Lovastatin induces apoptosis of ovarian cancer cells and synergizes with doxorubicin: potential therapeutic relevance. BMC Cancer 2010;10:103.
- Park IH, Kim JY, Jung JI, Han JY. Lovastatin overcomes gefitinib resistance in human non-small cell lung cancer cells with K-Ras mutations. Invest New Drugs 2010;28:791–799.
- Al-Abd AM, Lee JH, Kim SY, Kun N, Kuh HJ. Novel application of multicellular layers culture for in situ evaluation of cytotoxicity and penetration of paclitaxel. Cancer Sci 2008;99:423–431.